Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by JuneSung Bae.
The cancer burden is rapidly increasing in most countries, and thus, new anticancer drugs for effective cancer therapy must be developed. Cancer model systems that recapitulate the biological processes of human cancers are one of the cores of the drug development process. PDCO has emerged as a unique model that preserves the genetic, physiological, and histologic characteristics of original cancer, including inter- and intratumoral heterogeneities.
- patient-derived cancer organoid
- cancer
- cancer medicine
1. Introduction
Cancer is the leading cause of death among noninfectious diseases in most countries, and its burden is growing rapidly [1] because of population growth and aging, living environmental pollution, and lifestyle changes [2,3,4][2][3][4]. Despite the advances in cancer prevention and treatment modalities including surgery, radiation therapy, chemotherapy, targeted therapy, and immune therapy, cancer incidence and mortality are still increasing; thus, accelerated programs to develop more effective anticancer therapeutics are required [5,6,7][5][6][7].
In anticancer therapeutic development, well-characterized model systems representing human cancers have been widely used as an essential element. However, the success rate of anticancer drugs was only 3.4% from 2000 to 2015 [8,9,10][8][9][10]. One of the major causes of drug discovery failures is the incompleteness of cancer model systems to recapitulate the biological processes of cancers in the human body. In other words, cancer drug candidates that may be highly developed in the existing in vitro human or in vivo non-human model systems will not be applied to the human body [11,12][11][12]. Accordingly, a humanized model system that can provide human physiological characteristics from the development stage of cancer treatment has emerged. Furthermore, drug responses to human cancers highly vary based on an individual patient due to intertumoral heterogeneity. Therefore, newly presented models derived from patients with cancer, including patient-derived cancer cell lines (PDCs), patient-derived xenografts (PDXs), and patient-derived cancer organoids (PDCOs), have been used to discover anticancer drugs. Those patient-derived cancer models have been reported to partly recapitulate human cancers based on cellular heterogeneity (PDC, PDX, and PDCO), drug response (PDC, PDX, and PDCO), and tissue structure (PDX and PDCO) of human cancer [13,14,15][13][14][15].
2. Possible Applications Using Patient-Derived Cancer Organoids for Cancer Research
Organoids are generally formed through lineage development and differentiation from stem cells (adult, embryonic, and induced pluripotent stem cells) and have the characteristics of self-renewal and self-organization [36,37,38,39,40,41,42][16][17][18][19][20][21][22]. They can be formed from biopsies directly isolated from diseased patient tissues, including cancer, and researchers have succeeded in culturing cancer organoids from various cancer types [43,44,45,46,47,48,49,50,51,52,53,54,55][23][24][25][26][27][28][29][30][31][32][33][34][35]. These PDCOs mimic tissue architecture as well as gene expression profiles and genomic alterations of primary cancers, and these properties remain stable in long-term cultures [43,44,45,46,47,48,49,50,51,52,53,54,55][23][24][25][26][27][28][29][30][31][32][33][34][35]. PCDO is evaluated as an in vitro human cancer model system that can efficiently conduct studies to evaluate anticancer drug efficacy. Previous co-clinical studies have reported that the drug response of PDCO can accurately reflect the clinical outcome of patients with PDCO-matched cancer [56,57,58][36][37][38]. The PDCO characteristics are illustrated in Figure 1.
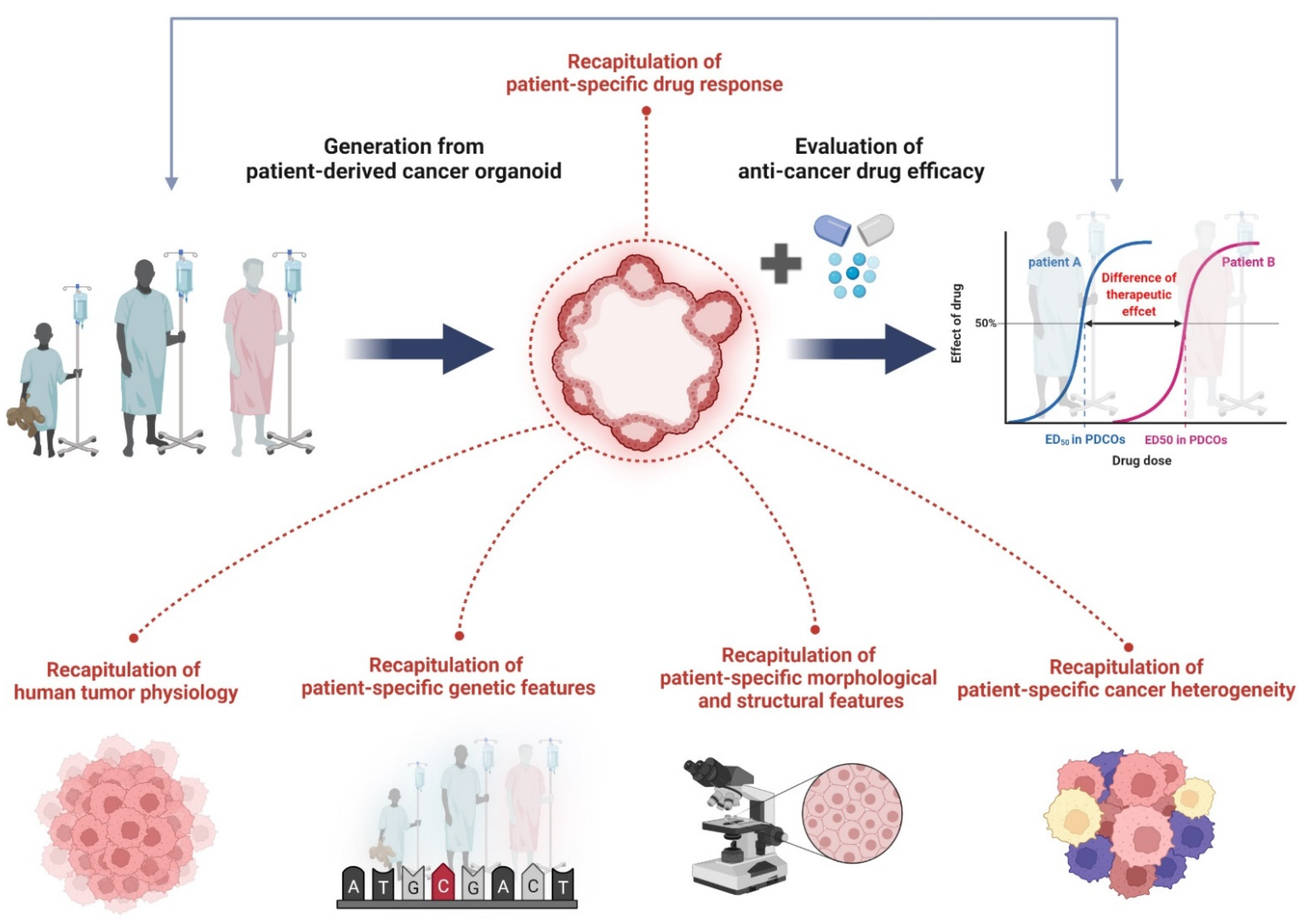
Figure 1. The characteristics of PDCOs in terms of recapitulation of human cancer biology. Organoids derived directly from human tumor tissue have been shown to accurately recapitulate tumor physiology, genetic features, histological properties, and cancer heterogeneity. Specifically, drug responses of PDCOs can exactly reflect clinical outcomes of PDCO-matched cancer patients. As a result, PDCOs are a promising tool to evaluate anticancer drug efficacy studies in clinic. Figure was created using BioRender.com (accessed on 31 March 2022).
2.1. PDCOatient-Derived Cancer Organoids Biobank
Compared to PDX, PDCO can efficiently propagate tumor cells ex vivo in a short time with little cost and effort. Additionally, a PDCO biobank can be established from individual PDCOs classified based on the clinical criteria and/or genetic and mutational profiles. Then, it is possible to validate therapeutic agents in PDCOs with specific genomic characteristics and clinical information. Recently, efforts have been made to build such a living PDCO biobank. Corro et al. [11] and LeSavage et al. [59][39] reviewed the current status of PDCO biobanks built for different cancer types in each organ. PDCOs stored in the biobank are expected to be very useful not only to discover and evaluate an anticancer drug but also for the developing of precision medicine tailored to the patient (Figure 32). Accumulation of drug sensitivity data in PDCOs with known clinical and genomic information can be used to discover new cancer drugs to meet unmet clinical needs [51][31], predict patient clinical outcomes, and design drug combination therapeutic strategies [50,58,60][30][38][40].
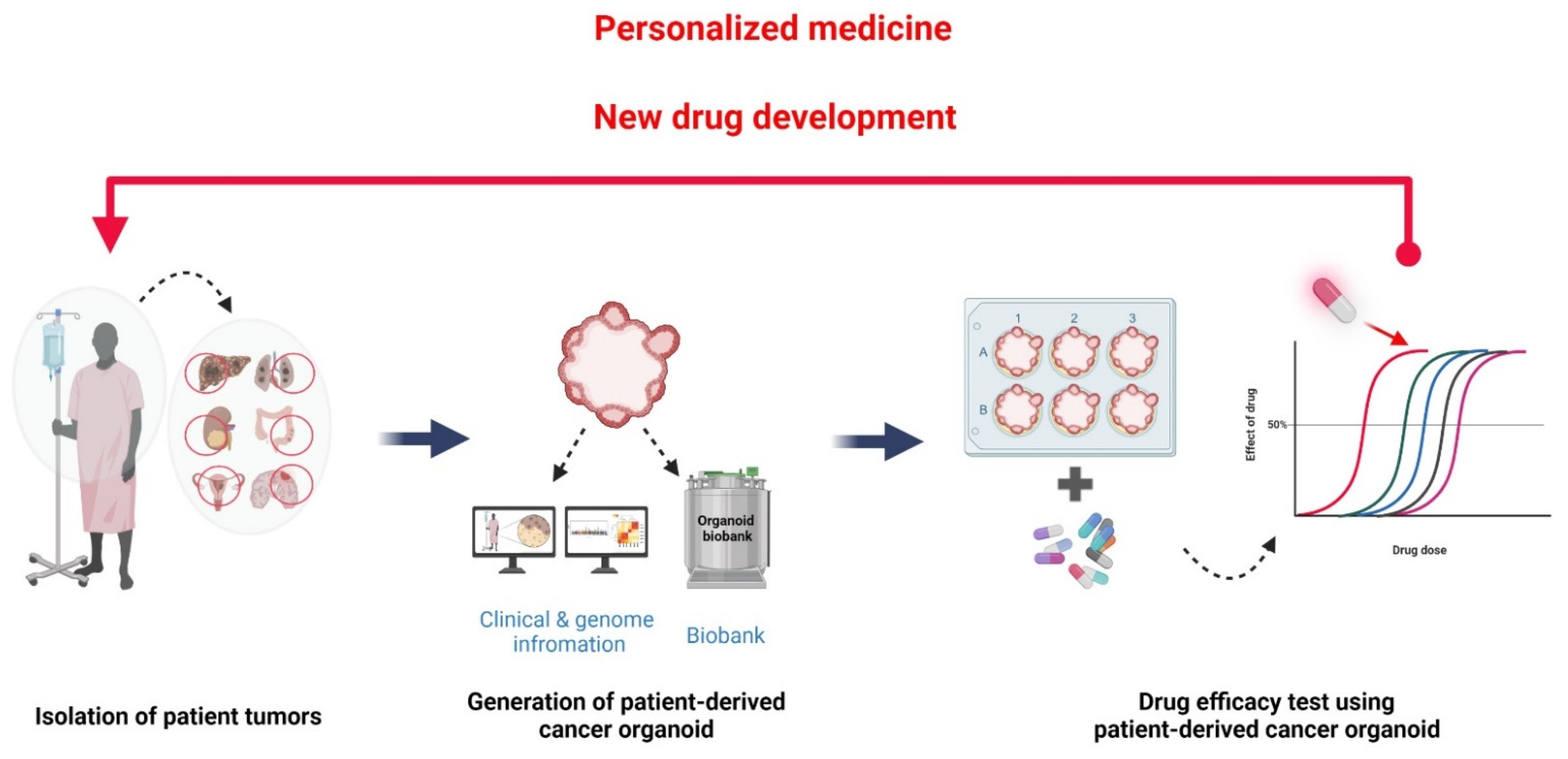
Figure 32. Construction of living PDCO biobank with clinical and genomic information. PDCOs are efficiently propagated from a patient’s tumor biopsy. This property is an advantage for using PDCOs as a living biobank. PDCO biobanks can be classified using a patient’s information, including medical history, cancer type, medication, and surgery. Genomic information, which might inform the utilization of targeted therapies, also can be used as a criterion in PDCOs classification. Discovering the specific drug sensitivities of PDCOs with known clinical and genomic information are essential for the discovery and evaluation of anticancer drugs as well as personalized medicine. Figure was created using BioRender.com (accessed on 31 March 2022).
2.2. Drug Efficacy Study and High-Throughput Screening
PDCs and animal models are limited in their use to predict patient drug response because these models cannot fully mimic human cancer biology, are costly to use, and have animal ethics issues [61,62,63][41][42][43]. Conversely, PDCOs have not only represented the primary tumor but are also relatively simple culture methods and they avoid ethical issues, making them a suitable model for drug screening. Multiple studies have shown that a preclinical model PDCO has the potential to predict patient drug response in high-throughput screening systems [50,51,58,60,64,65][30][31][38][40][44][45]. The PDCO model is further developed to reduce the labor power and time consumption required for high-throughput screening. The ordinary organoid culture, in which dissociated cells and sticky ECM hydrogel are mixed and dispensed and a culture medium is added, is more difficult and time-consuming than the culture of 2D monolayer cells. The liquid handling robotic systems can culture large amounts of PDCO with smaller efforts and can minimize inter- and intra-personal variability, enabling drug screening with high reproducibility [66,67,68][46][47][48]. Furthermore, large-scale drug response data analysis can be automated by measuring image data, such as changes in the organoid size or morphologic characteristics of PDCO after drug treatment [69,70,71][49][50][51]. The ultimate goal in this field is to automate the entire high-throughput screening process, including organoid cultures, seeding of dissociated cells, drug treatment, and drug response analysis. With this physiologically relevant cancer model, automated culture and large-scale data analysis on the high-throughput platforms will be effectively used to identify novel drugs for cancer treatment.
2.3. Precision Cancer Medicine
Because a measurable amount of PDCO can be cultured in a short time from a small tumor sample, it is a well-suited model for screening drugs to be administered. In the PDCO model, the patient’s drug response can be predicted within 3 months, depending on the tumor sample types or the number of drugs to be tested [72][52]. Several studies have shown that drug screening results using PDCO are very similar to patient clinical outcomes [49,50,54,58,65,72,73,74][29][30][34][38][45][52][53][54]. The genetic information of PDCOs is also a powerful tool to predict patient clinical outcomes. For example, breast cancer organoids with the BRCA1/2-associated mutational signature were highly responsive to poly(ADP-ribose)polymerase inhibitors (PARPi). Conversely, the immunohistological analysis of protein biomarkers for tumor samples, a basis of the current precision medicine, did not completely match the drug response. Drugs targeting the HER signaling pathway in breast cancer organoids may not exhibit the expected reactivity depending on the immunological staining status of HER [50][30]. Therefore, the PDCO model, which accurately reproduces the genetic profile and drug responsiveness of a patient’s primary tumor, is expected to be actively used for cancer medicine with the current pathological biomarker analysis of tumors
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Turner, M.C.; Andersen, Z.J.; Baccarelli, A.; Diver, W.R.; Gapstur, S.M.; Pope, C.A., 3rd; Prada, D.; Samet, J.; Thurston, G.; Cohen, A. Outdoor air pollution and cancer: An overview of the current evidence and public health recommendations. CA Cancer J. Clin. 2020, 70, 460–479.
- Vieira, V.M.; VoPham, T.; Bertrand, K.A.; James, P.; DuPre, N.; Tamimi, R.M.; Laden, F.; Hart, J.E. Contribution of socioeconomic and environmental factors to geographic disparities in breast cancer risk in the Nurses’ Health Study II. Environ. Epidemiol. 2020, 4, e080.
- Boffetta, P.; Nyberg, F. Contribution of environmental factors to cancer risk. Br. Med. Bull. 2003, 68, 71–94.
- Emmons, K.M.; Colditz, G.A. Realizing the Potential of Cancer Prevention—The Role of Implementation Science. N. Engl. J. Med. 2017, 376, 986–990.
- Colditz, G.A.; Wolin, K.Y.; Gehlert, S. Applying what we know to accelerate cancer prevention. Sci. Transl. Med. 2012, 4, 127rv124.
- Advancing Cancer Therapy. Nat. Cancer 2021, 2, 245–246.
- Paul, S.M.; Mytelka, D.S.; Dunwiddie, C.T.; Persinger, C.C.; Munos, B.H.; Lindborg, S.R.; Schacht, A.L. How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010, 9, 203–214.
- Scannell, J.W.; Blanckley, A.; Boldon, H.; Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 2012, 11, 191–200.
- Wong, C.H.; Siah, K.W.; Lo, A.W. Estimation of clinical trial success rates and related parameters. Biostatistics 2019, 20, 273–286.
- Corro, C.; Novellasdemunt, L.; Li, V.S.W. A brief history of organoids. Am. J. Physiol. Cell Physiol. 2020, 319, C151–C165.
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Margolin, A.A.; Kim, S.; Wilson, C.J.; Lehar, J.; Kryukov, G.V.; Sonkin, D.; et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012, 483, 603–607.
- Lee, J.K.; Liu, Z.; Sa, J.K.; Shin, S.; Wang, J.; Bordyuh, M.; Cho, H.J.; Elliott, O.; Chu, T.; Choi, S.W.; et al. Pharmacogenomic landscape of patient-derived tumor cells informs precision oncology therapy. Nat. Genet. 2018, 50, 1399–1411.
- Invrea, F.; Rovito, R.; Torchiaro, E.; Petti, C.; Isella, C.; Medico, E. Patient-derived xenografts (PDXs) as model systems for human cancer. Curr. Opin. Biotechnol. 2020, 63, 151–156.
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125.
- Takebe, T.; Zhang, R.R.; Koike, H.; Kimura, M.; Yoshizawa, E.; Enomura, M.; Koike, N.; Sekine, K.; Taniguchi, H. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat. Protoc. 2014, 9, 396–409.
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.R.; Ueno, Y.; Zheng, Y.W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484.
- Hu, H.; Gehart, H.; Artegiani, B.; Löpez-Iglesias, C.; Dekkers, F.; Basak, O.; Van Es, J.; Chuva de Sousa Lopes, S.M.; Begthel, H.; Korving, J.; et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell 2018, 175, 1591–1606.e19.
- Takasato, M.; Er, P.X.; Chiu, H.S.; Maier, B.; Baillie, G.J.; Ferguson, C.; Parton, R.G.; Wolvetang, E.J.; Roost, M.S.; Chuva de Sousa Lopes, S.M.; et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 2015, 526, 564–568.
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379.
- Fujii, M.; Matano, M.; Toshimitsu, K.; Takano, A.; Mikami, Y.; Nishikori, S.; Sugimoto, S.; Sato, T. Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition. Cell Stem Cell 2018, 23, 787–793.e6.
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265.
- van de Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; van Houdt, W.; van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015, 161, 933–945.
- Kim, M.; Mun, H.; Sung, C.O.; Cho, E.J.; Jeon, H.J.; Chun, S.M.; Jung, D.J.; Shin, T.H.; Jeong, G.S.; Kim, D.K.; et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun. 2019, 10, 3991.
- Sachs, N.; Papaspyropoulos, A.; Zomer-van Ommen, D.D.; Heo, I.; Bottinger, L.; Klay, D.; Weeber, F.; Huelsz-Prince, G.; Iakobachvili, N.; Amatngalim, G.D.; et al. Long-term expanding human airway organoids for disease modeling. EMBO J. 2019, 38, e100300.
- Boretto, M.; Maenhoudt, N.; Luo, X.; Hennes, A.; Boeckx, B.; Bui, B.; Heremans, R.; Perneel, L.; Kobayashi, H.; Van Zundert, I.; et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat. Cell Biol. 2019, 21, 1041–1051.
- Li, X.; Francies, H.E.; Secrier, M.; Perner, J.; Miremadi, A.; Galeano-Dalmau, N.; Barendt, W.J.; Letchford, L.; Leyden, G.M.; Goffin, E.K.; et al. Organoid cultures recapitulate esophageal adenocarcinoma heterogeneity providing a model for clonality studies and precision therapeutics. Nat. Commun. 2018, 9, 2983.
- Seidlitz, T.; Merker, S.R.; Rothe, A.; Zakrzewski, F.; von Neubeck, C.; Grutzmann, K.; Sommer, U.; Schweitzer, C.; Scholch, S.; Uhlemann, H.; et al. Human gastric cancer modelling using organoids. Gut 2019, 68, 207–217.
- Lee, S.H.; Hu, W.; Matulay, J.T.; Silva, M.V.; Owczarek, T.B.; Kim, K.; Chua, C.W.; Barlow, L.J.; Kandoth, C.; Williams, A.B.; et al. Tumor Evolution and Drug Response in Patient-Derived Organoid Models of Bladder Cancer. Cell 2018, 173, 515–528.e17.
- Sachs, N.; de Ligt, J.; Kopper, O.; Gogola, E.; Bounova, G.; Weeber, F.; Balgobind, A.V.; Wind, K.; Gracanin, A.; Begthel, H.; et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 2018, 172, 373–386.e10.
- Broutier, L.; Mastrogiovanni, G.; Verstegen, M.M.; Francies, H.E.; Gavarro, L.M.; Bradshaw, C.R.; Allen, G.E.; Arnes-Benito, R.; Sidorova, O.; Gaspersz, M.P.; et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 2017, 23, 1424–1435.
- Boj, S.F.; Hwang, C.-I.; Baker, L.A.; Chio, I.I.C.; Engle, D.D.; Corbo, V.; Jager, M.; Ponz-Sarvise, M.; Tiriac, H.; Spector, M.S.; et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 2015, 160, 324–338.
- Gao, D.; Vela, I.; Sboner, A.; Iaquinta, P.J.; Karthaus, W.R.; Gopalan, A.; Dowling, C.; Wanjala, J.N.; Undvall, E.A.; Arora, V.K.; et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014, 159, 176–187.
- Jacob, F.; Salinas, R.D.; Zhang, D.Y.; Nguyen, P.T.T.; Schnoll, J.G.; Wong, S.Z.H.; Thokala, R.; Sheikh, S.; Saxena, D.; Prokop, S.; et al. A Patient-Derived Glioblastoma Organoid Model and Biobank Recapitulates Inter- and Intra-tumoral Heterogeneity. Cell 2020, 180, 188–204.e22.
- Cho, E.J.; Kim, M.; Jo, D.; Kim, J.; Oh, J.H.; Chung, H.C.; Lee, S.H.; Kim, D.; Chun, S.M.; Kim, J.; et al. Immuno-genomic classification of colorectal cancer organoids reveals cancer cells with intrinsic immunogenic properties associated with patient survival. J. Exp. Clin. Cancer Res. 2021, 40, 230.
- Liu, L.; Yu, L.; Li, Z.; Li, W.; Huang, W. Patient-derived organoid (PDO) platforms to facilitate clinical decision making. J. Transl. Med. 2021, 19, 40.
- Gomez, G.A.; Oksdath, M.; Brown, M.P.; Ebert, L.M. New approaches to model glioblastoma in vitro using brain organoids: Implications for precision oncology. Transl. Cancer Res. 2019, 8, S606–S611.
- Vlachogiannis, G.; Hedayat, S.; Vatsiou, A.; Jamin, Y.; Fernandez-Mateos, J.; Khan, K.; Lampis, A.; Eason, K.; Huntingford, I.; Burke, R.; et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 2018, 359, 920–926.
- LeSavage, B.L.; Suhar, R.A.; Broguiere, N.; Lutolf, M.P.; Heilshorn, S.C. Next-generation cancer organoids. Nat. Mater. 2022, 21, 143–159.
- Pauli, C.; Hopkins, B.D.; Prandi, D.; Shaw, R.; Fedrizzi, T.; Sboner, A.; Sailer, V.; Augello, M.; Puca, L.; Rosati, R.; et al. Personalized In Vitro and In Vivo Cancer Models to Guide Precision Medicine. Cancer Discov. 2017, 7, 462–477.
- Clohessy, J.G.; Pandolfi, P.P. Mouse hospital and co-clinical trial project--from bench to bedside. Nat. Rev. Clin. Oncol. 2015, 12, 491–498.
- Voest, E.E.; Bernards, R. DNA-Guided Precision Medicine for Cancer: A Case of Irrational Exuberance? Cancer Discov. 2016, 6, 130–132.
- Zehir, A.; Benayed, R.; Shah, R.H.; Syed, A.; Middha, S.; Kim, H.R.; Srinivasan, P.; Gao, J.; Chakravarty, D.; Devlin, S.M.; et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017, 23, 703–713.
- Kopper, O.; de Witte, C.J.; Lohmussaar, K.; Valle-Inclan, J.E.; Hami, N.; Kester, L.; Balgobind, A.V.; Korving, J.; Proost, N.; Begthel, H.; et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat. Med. 2019, 25, 838–849.
- Tiriac, H.; Belleau, P.; Engle, D.D.; Plenker, D.; Deschenes, A.; Somerville, T.D.D.; Froeling, F.E.M.; Burkhart, R.A.; Denroche, R.E.; Jang, G.H.; et al. Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer. Cancer Discov. 2018, 8, 1112–1129.
- Francies, H.E.; Barthorpe, A.; McLaren-Douglas, A.; Barendt, W.J.; Garnett, M.J. Drug Sensitivity Assays of Human Cancer Organoid Cultures. Methods Mol. Biol. 2019, 1576, 339–351.
- Du, Y.; Li, X.; Niu, Q.; Mo, X.; Qui, M.; Ma, T.; Kuo, C.J.; Fu, H. Development of a miniaturized 3D organoid culture platform for ultra-high-throughput screening. J. Mol. Cell Biol. 2020, 12, 630–643.
- Yan, H.H.N.; Siu, H.C.; Law, S.; Ho, S.L.; Yue, S.S.K.; Tsui, W.Y.; Chan, D.; Chan, A.S.; Ma, S.; Lam, K.O.; et al. A Comprehensive Human Gastric Cancer Organoid Biobank Captures Tumor Subtype Heterogeneity and Enables Therapeutic Screening. Cell Stem Cell 2018, 23, 882–897.e11.
- Lee, S.; Chang, J.; Kang, S.M.; Parigoris, E.; Lee, J.H.; Huh, Y.S.; Takayama, S. High-throughput formation and image-based analysis of basal-in mammary organoids in 384-well plates. Sci. Rep. 2022, 12, 317.
- Brandenberg, N.; Hoehnel, S.; Kuttler, F.; Homicsko, K.; Ceroni, C.; Ringel, T.; Gjorevski, N.; Schwank, G.; Coukos, G.; Turcatti, G.; et al. High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat. Biomed. Eng. 2020, 4, 863–874.
- Czerniecki, S.M.; Cruz, N.M.; Harder, J.L.; Menon, R.; Annis, J.; Otto, E.A.; Gulieva, R.E.; Islas, L.V.; Kim, Y.K.; Tran, L.M.; et al. High-Throughput Screening Enhances Kidney Organoid Differentiation from Human Pluripotent Stem Cells and Enables Automated Multidimensional Phenotyping. Cell Stem Cell 2018, 22, 929–940.e4.
- Driehuis, E.; Kretzschmar, K.; Clevers, H. Establishment of patient-derived cancer organoids for drug-screening applications. Nat. Protoc. 2020, 15, 3380–3409.
- Golebiewska, A.; Hau, A.C.; Oudin, A.; Stieber, D.; Yabo, Y.A.; Baus, V.; Barthelemy, V.; Klein, E.; Bougnaud, S.; Keunen, O.; et al. Patient-derived organoids and orthotopic xenografts of primary and recurrent gliomas represent relevant patient avatars for precision oncology. Acta Neuropathol. 2020, 140, 919–949.
- Driehuis, E.; van Hoeck, A.; Moore, K.; Kolders, S.; Francies, H.E.; Gulersonmez, M.C.; Stigter, E.C.A.; Burgering, B.; Geurts, V.; Gracanin, A.; et al. Pancreatic cancer organoids recapitulate disease and allow personalized drug screening. Proc. Natl. Acad. Sci. USA 2019, 116, 26580–26590.
More
