Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Francesco Scaglione and Version 3 by Camila Xu.
Carbocysteine (R-2-amino-3[(carboxymethyl)thiol] propionic acid) is a biologically active dibasic amino acid. The carbocysteine molecule is characterized by the presence of a bound sulfhydrilic group. Carbocysteine can increase cilia beating in airway epithelial cells, thus improving the function of the mucociliary escalator and its function of removing harmful particles, viruses, and bacteria from the airway surface.
- carbocysteine
- carbocysteine pharmacology
- carbocysteine human diseases
1. Molecular Effects of Carbocysteine
Carbocysteine (R-2-amino-3[(carboxymethyl)thiol] propionic acid) is a biologically active dibasic amino acid. The carbocysteine molecule (Figure 12) is characterized by the presence of a bound sulfhydrilic group.
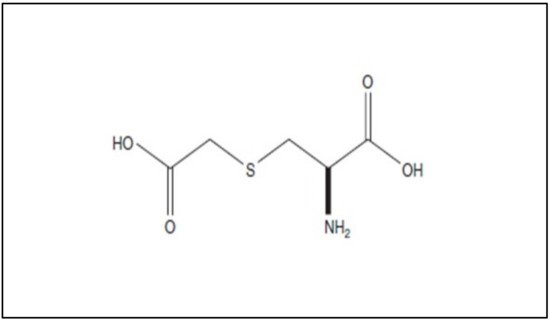
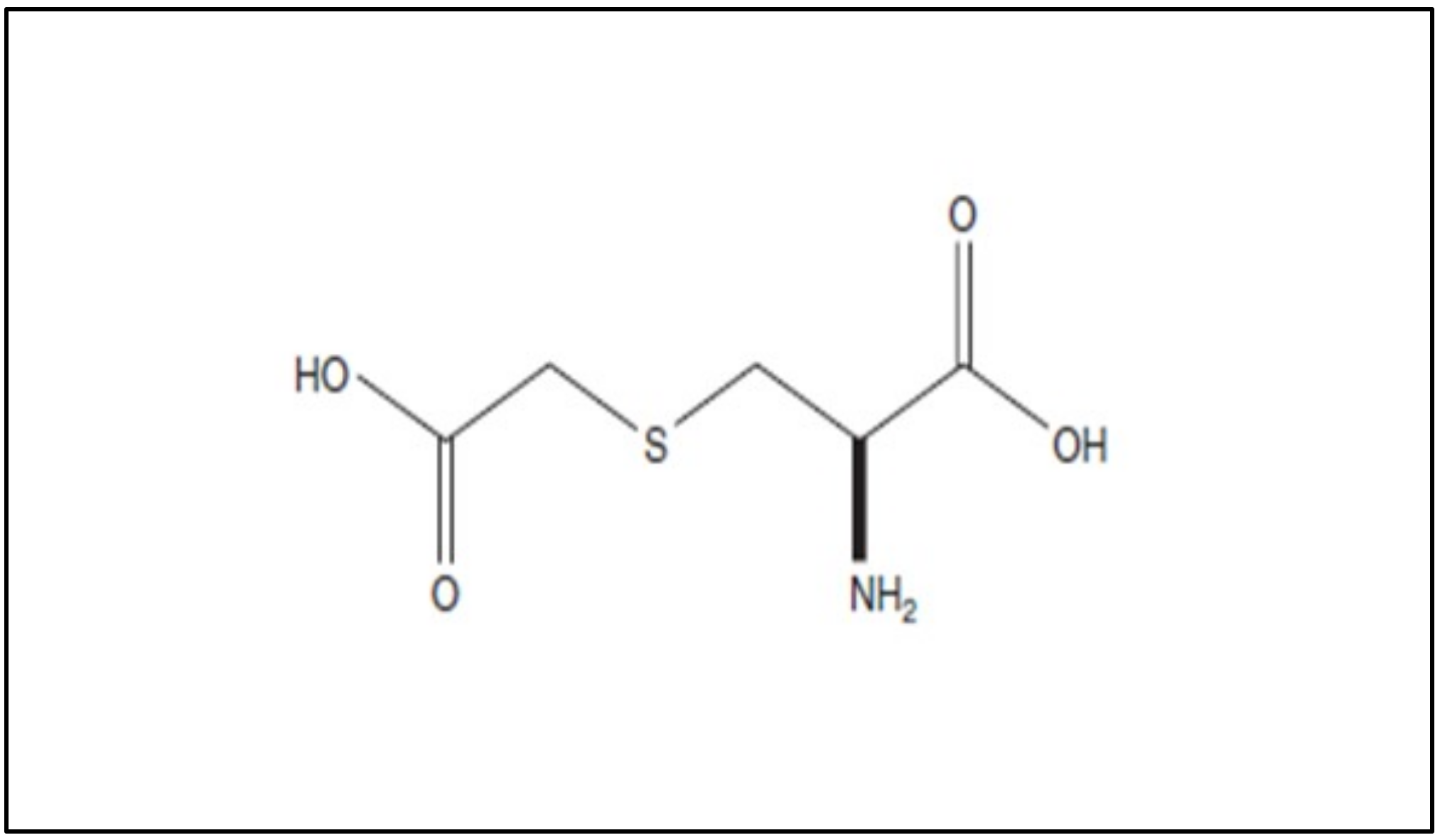
Figure 12. Chemical structure of carbocysteine.
The structure and mechanism of action of carbocysteine differ from those of other commonly available mucolytic drugs. For instance, N-acetylcysteine (NAC) and erdosteine bear free sulfhydryl (thiol) groups that allow them to split glycoprotein bonds in mucus [1]. Conversely, a study using animal models demonstrated that carbocysteine increases chloride transport across the airway epithelium, which may also contribute to its mucoregulatory action [2].
2. In Vitro and In Vivo Studies of Carbocysteine’s Effects
2.1. In Vitro Studies of Carbocysteine’s Effects on Different Cell Lines
Carbocysteine counteracts some pro-inflammatory CSE-mediated effects in a human bronchial epithelial cell line (16-HBE). In CSE-stimulated bronchial epithelial cells, carbocysteine has been demonstrated to play a crucial role in the reduction in TLR4 expression and lipopolysaccharide (LPS) binding, IL-8 mRNA, and IL-8 release due to IL-1 stimulation and neutrophil chemotactic activity [3]. Similar anti-inflammatory effects of carbocysteine were observed in nasal epithelial cells exposed to CSE [4], providing compelling evidence that carbocysteine may also be considered a promising therapeutic strategy in chronic inflammatory nasal diseases. The effects of carbocysteine on neutrophils have also been confirmed by other observations. Ishii et al. showed that carbocysteine can reduce neutrophil chemotaxis not only by inhibiting N-formylmethionyl-leucyl-phenylalanine (fMLP)-mediated neutrophil adherence to pulmonary vascular endothelial cells, but also by decreasing the production of inositol 1,4,5-triphosphate (IP3) and diacylglycerol in neutrophils [5]. Other than reducing neutrophil chemotactic molecule expression/release, adhesion, and chemotaxis of neutrophils, Nogawa et al. stated that carbocysteine decreases rat neutrophils. Moreover, it shows scavenging effects on H2O2, HOCl, •OH, and peroxynitrite (ONOO-), thus reducing further pro-inflammatory responses (e.g., IL-8 and IL-6 release) [6]. In vitro models of distal airways (A549 cells) cultured with or without H2O2 [7] provide evidence that carbocysteine counteracts the effects of H2O2 by increasing cell viability, decreasing lactate dehydrogenase (LDH), IL-6, and IL-8 in cell supernatants, and attenuating the activation of extracellular signal-regulated kinase 1/2 (ERK1/2) and NF-κB. In the same in vitro model, Wang et al. demonstrated that carbocysteine, administered to the cells 24 h before or after TNF stimulation, can regulate the release of IL-6 and IL-8, as well as the activation of ERK1/2 and NF-κB [8]. The main molecular events promoted in vitro in airway cellular models by carbocysteine are reported in Figure 23.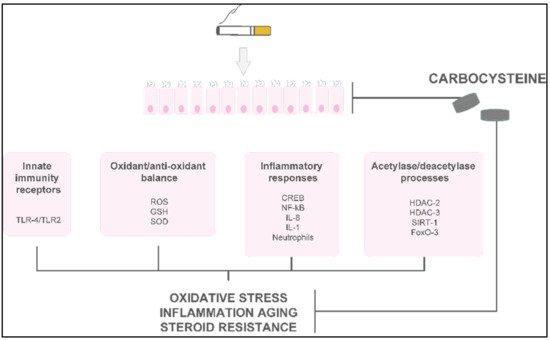
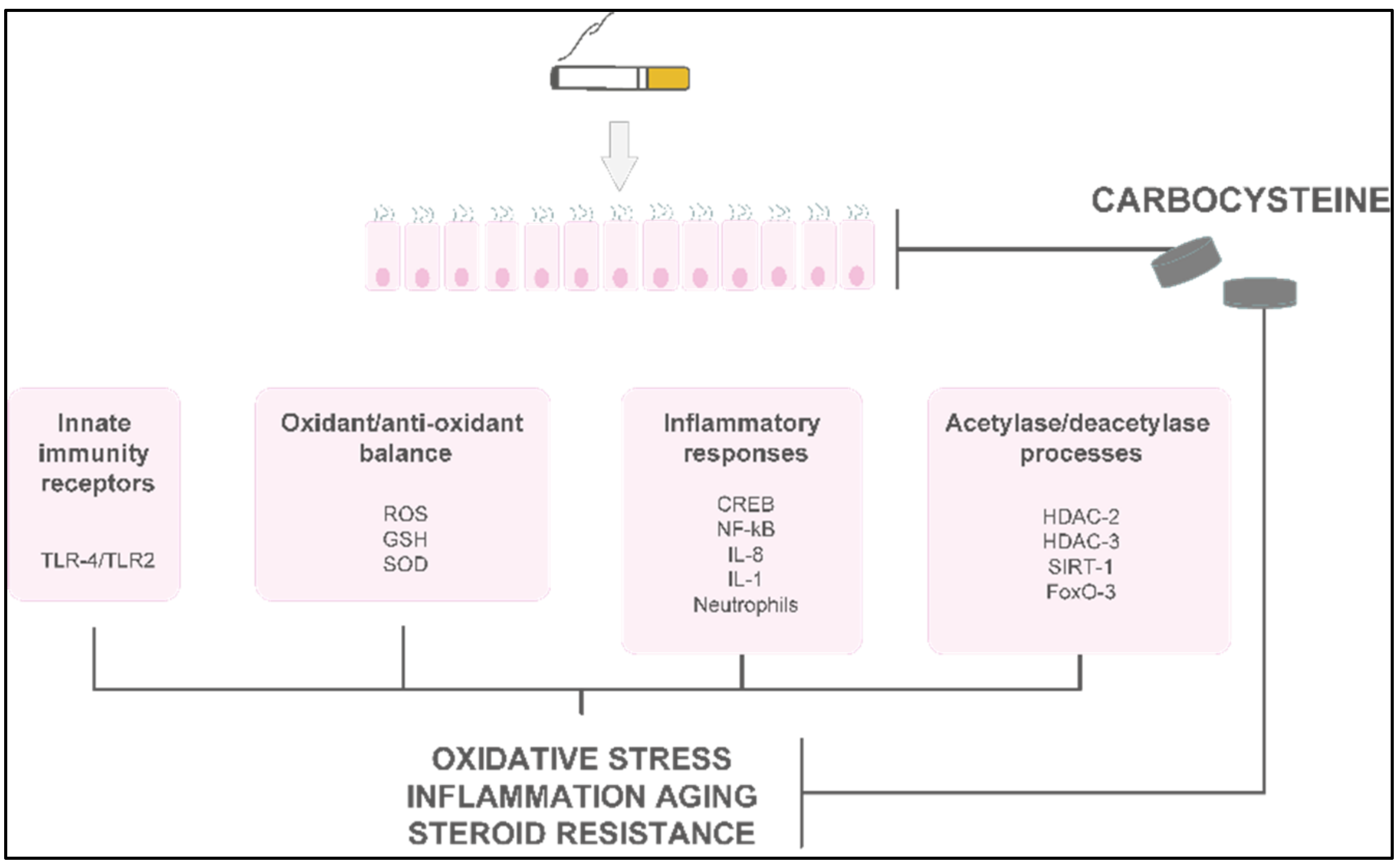
Figure 23. The main molecular mechanisms and effects of carbocysteine in smoke-injured airway epithelial cells.
The main molecular mechanisms and effects of carbocysteine in smoke-injured airway epithelial cells.
2.2. In Vivo Studies of Carbocysteine’s Effects in Animal Models
Asti et al. demonstrated the efficacy of carbocysteine administered either orally or by inhalation to reduce airway hyper-reactivity and inflammation promoted by smoke exposure, using in vivo models [9]. In mice treated with intratracheal instillation of LPS, carbocysteine decreased neutrophil numbers by increasing the binding of apoptotic neutrophils to alveolar macrophages, and by promoting phagocytosis of neutrophils [10]. Song generated a murine model of COPD by instilling LPS and cigarette smoke exposure to study carbocysteine’s effects. He stated that carbocysteine significantly restored the MUC5B/MUC5AC ratio, together with decreased neutrophil counts, keratocyte-derived cytokine and IL-6 levels, and TNF-α mRNA expression in the studied mice. Furthermore, carbocysteine significantly improved lung function, as reflected by airway resistance and dynamic compliance [11].3. In Vivo Studies of Carbocysteine’s Effects in COPD Patients
Limited evidence demonstrates the in vivo anti-inflammatory or antioxidant activities of carbocysteine in COPD or asthma patients (Figure 34).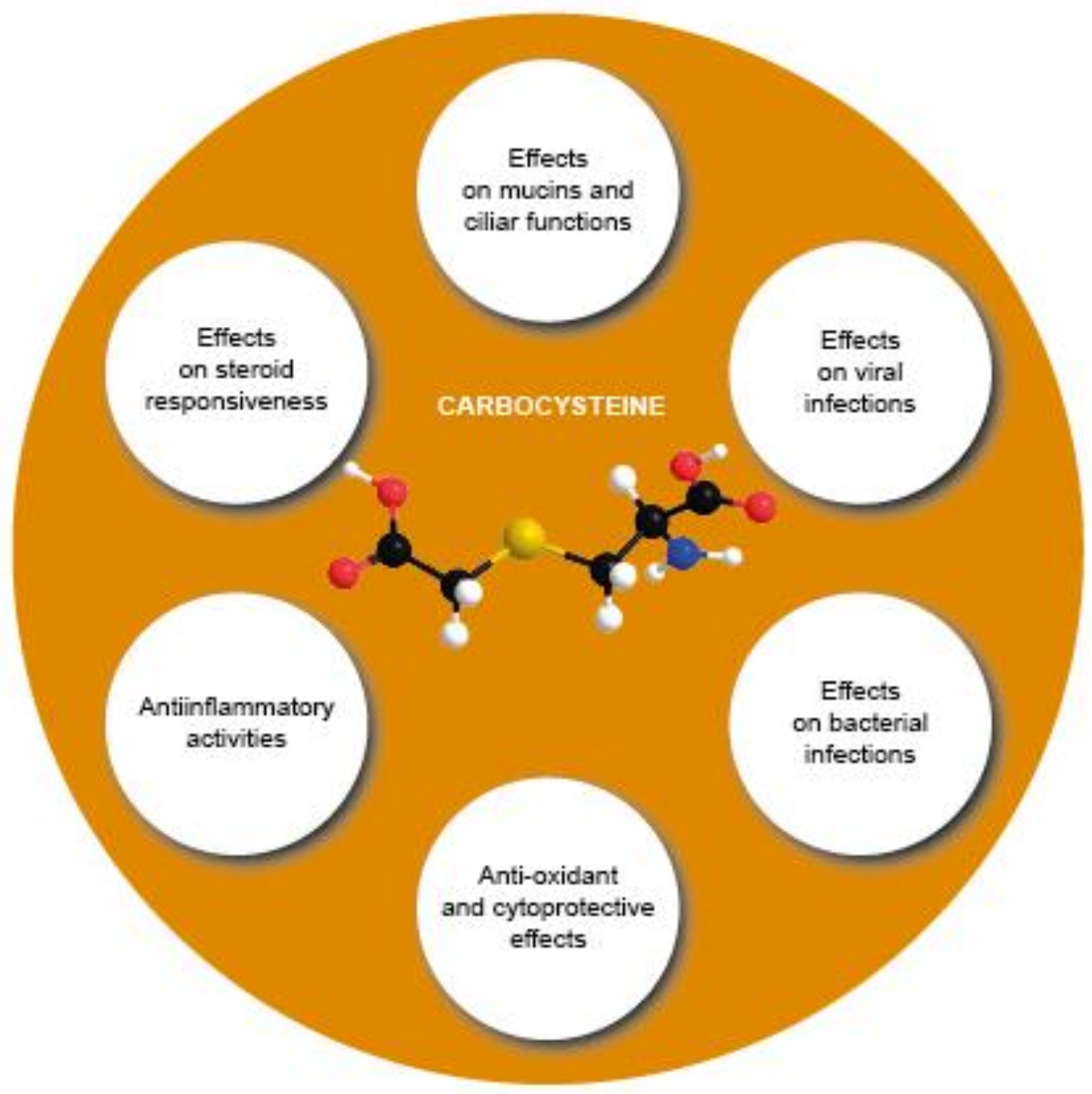
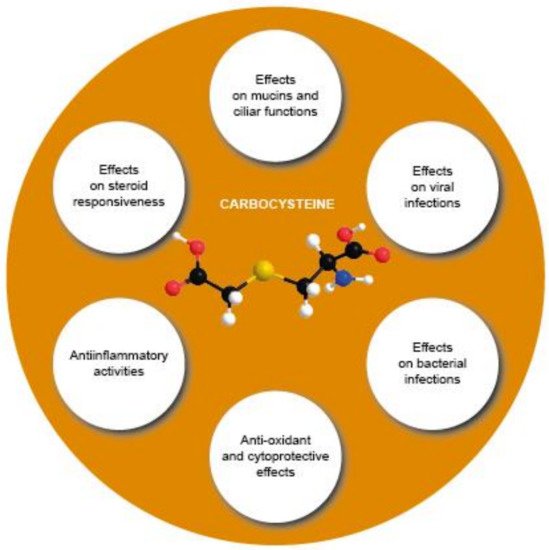
Figure 34. Overview of the various effects of carbocysteine with a positive impact in COPD.
Overview of the various effects of carbocysteine with a positive impact in COPD.
Clinical Studies of Carbocysteine’s Effects in COPD
To date, few clinical studies regarding carbocysteine’s effects in COPD patients are available. In the UK, before 1990, small-scale studies investigated the effects of daily administration of carbocysteine (2.25–3 g) versus (vs.) placebo in chronic bronchitis patients. The results were rather heterogeneous in terms of FEV1 and peak flow rate, but the outcomes regarding more subjective parameters—such as cough, dyspnea, and increased sputum clearance—were consistently positive [14][15][16][17] (Figure 45 and Table 1). An earlier, more accurate double–blind, parallel-group study developed in the UK compared the treatment effects after administration of 750 mg of carbocysteine three times daily vs. placebo in 109 patients with chronic bronchitis over 6 winter months. The results suggested no significant differences in the exacerbation rate, and an increase in the peak flow from baseline in both the placebo and intervention groups [18] (Figure 45 and Table 1). On the other hand, two RCTs conducted in Tokyo compared 1.5 g of daily carbocysteine with placebo in 156 COPD patients over 12 months. This researchtudy disclosed a significant reduction in the number of common colds, along with a lower exacerbation rate in COPD patients [19]. None of the patients received ICSs or oral corticosteroids during the researchtudy. Moreover, an Italian multicenter, prospective, double-blind RCT involving 662 COPD patients reported no significant differences in baseline FEV1, but a significantly prolonged mean time until the first exacerbation episode in patients treated with 2.7 g of SCMC–Lys once daily for 6 months compared to placebo [20] (Figure 45 and Table 1). The PEACE study was the first clinical trial to closely follow a reliable research design to clarify whether COPD patients could benefit from protracted mucolytic therapy [21]. This researchtudy was classified as a multicenter (22 centers in China), randomized, double-blind, placebo-controlled, parallel-group study. To be enrolled in this researchstudy, patients had to have a history of at least two COPD exacerbations within the previous 2 years, and to be considered clinically stable for over 4 weeks before the researchstudy. The PEACE study enrolled more than 700 COPD patients, who were followed for 1 year. The cumulative number of exacerbations for the whole year was 325 in the carbocysteine group and 439 in the placebo group, corresponding to 1.01 (SE 0.06) exacerbations per patient/year with carbocysteine treatment vs. 1.35 (SE 0.06) with placebo. The risk ratio of exacerbation was 0.75 (95% CI 0.62–0.92, p = 0.004). Interestingly, the number of acute exacerbations in COPD patients who received carbocysteine (1500 mg/day for a year) decreased by 24% compared to the placebo group. Moreover, carbocysteine demonstrated a better rate of prevention in patients who suffered frequent exacerbation events, but these preventive effects were found only in patients treated with carbocysteine for 6 months or more [21] (Figure 45 and Table 1). These results may lead to the conclusion that the anti-inflammatory and antioxidant properties of carbocysteine in COPD patients require time to be effective. Thus, the longer the carbocysteine administration, the better the preventive effects against recurrent exacerbation. Non-significant interaction was found between the preventive effects and COPD severity, smoking, and concomitant therapy. The advantage of carbocysteine over placebo in the prevention of exacerbations was noteworthy even after adjustment for concomitant use of ICSs. This finding has been to the object of debate, particularly because it differs from the conclusions of the BRONCUS study [22], in which a significant reduction in exacerbation rate by N-acetylcysteine was shown only in patients without concomitant use of ICSs. The research euthors of the PEACE study themselves provide some reasonable explanations for such differences. First of all, the response to treatment could be associated with patients’ ethnicity, as the PEACE study’s patients were Chinese. Secondly, PEACE patients were receiving ICSs at a lower percentage and a smaller dose compared with BRONCUS participants. As such, the effects of carbocysteine would be more readily identified in Chinese patients with modest use of concomitant ICSs, as opposed to the BRONCUS study.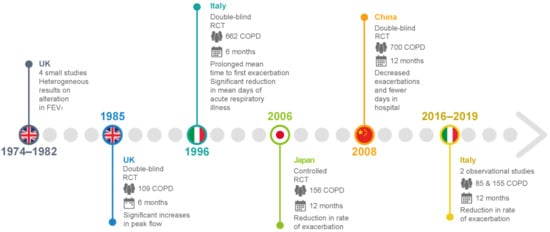
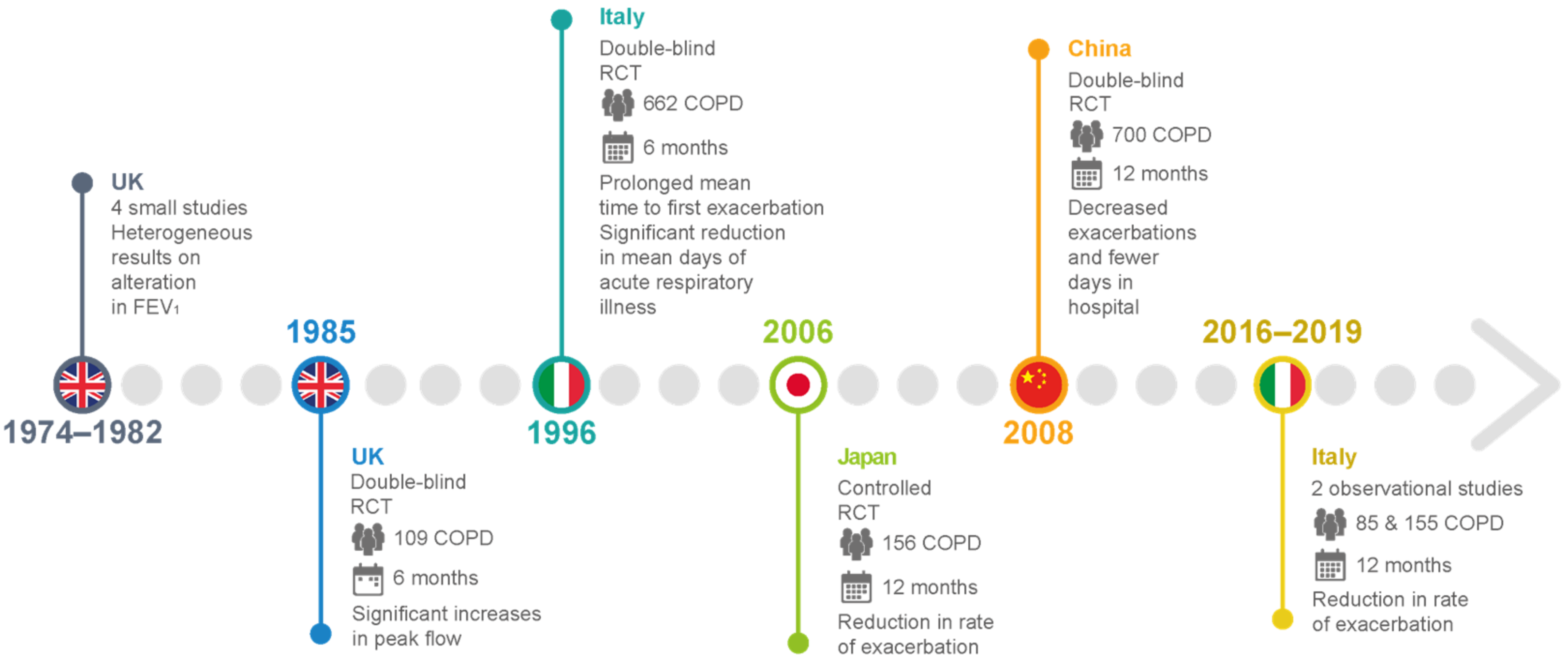
Figure 45.
Main clinical studies of carbocysteine’s effects in COPD patients
.
Table 1.
Clinical studies on the efficacy of carbocysteine treatment in COPD patients.
| Clinical Trial | Study Design and Enrolled Patients | Therapeutic Regimen | Outcome | ||
|---|---|---|---|---|---|
| [14][15][16][17] | Small-scale UK studies on patients with chronic bronchitis | 2.25–3.00 g carbocysteine daily vs. placebo | Heterogeneous results for alterations in FEV | 1 | , peak flow rate, and dyspnea scores |
| [18] | A double-blind, parallel-group study in the UK of 109 patients with chronic bronchitis over 6 winter months | 750 mg carbocysteine three times daily compared with placebo in terms of peak flow and exacerbation rate. |
No significant difference in exacerbation rate. Significant increases in peak flow from baseline in both placebo and intervention groups |
||
| [19] | Placebo-controlled RCTs in Tokyo for 156 patients with COPD over 12 months | 1.5 g carbocysteine daily with placebo | Significant reduction in the number of common colds and reduction in the rate of exacerbation | ||
| [20] | An Italian multicenter, prospective, double-blind RCT involving 662 outpatients with chronic bronchitis | 2.7 g SCMC–Lys once daily for 6 months in COPD patients | No significant difference in baseline FEV | 1 | between the groups. Mean time to first exacerbation was significantly prolonged, and significant reduction in mean days of acute respiratory illness per patient. |
| [21] | Multicenter, randomized, double-blind, placebo-controlled, parallel-group study in China involving more than 700 COPD patients with a history of at least two COPD exacerbations within the previous 2 years | 1500 mg/day carbocysteine for one year | Long-term (one year) use of carbocysteine produced a reduction in the numbers of exacerbations in patients with COPD. Decreased exacerbations and fewer days in the hospital. No loss of lung function, and improvement in health-related quality of life. |
||
| [23] | An Italian observational and prospective study including 85 COPD outpatients with a history of at least 1 COPD exacerbation within the previous year. | 2.7 g of carbocysteine daily for one year | Reduction in the exacerbation rate after 12 months of therapy, completely independent of the use of ICSs. Statistically significant improvement in the quality of life assessed (decrease in SGRQ score) and the distance walked (6MWT), with a significant reduction in the BODE index. No significant improvement in lung function (FEV1, FVC, FEV1/FVC). |
||
| [25] | Observational prospective study of 155 consecutively enrolled COPD patients with a history of at least 1 COPD exacerbation within the previous year. | 2.7 g of carbocysteine daily for one year | Reduction in the number of exacerbations at 1-year evaluation, irrespective of treatment with or without ICSs. |
References
- Mitchell, S.C.; Steventon, G.B. S-carboxymethyl-L-cysteine. Drug Metab. Rev. 2012, 44, 129–147.
- Colombo, B.; Turconi, P.; Daffonchio, L.; Fedele, G.; Omini, C.; Cremaschi, D. Stimulation of Cl- secretion by the mucoactive drug S-carboxy-methylcysteinelysine salt in the isolated rabbit trachea. Eur. Respir. J. 1994, 7, 1622–1628.
- Pace, E.; Ferraro, M.; Siena, L.; Scafidi, V.; Gerbino, S.; Di Vincenzo, S.; Gallina, S.; Lanata, L.; Gjomarkaj, M. Carbocysteine regulates innate immune responses and senescence processes in cigarette smoke stimulated bronchial epithelial cells. Toxicol. Lett. 2013, 223, 198–204.
- Pace, E.; Ferraro, M.; Di Vincenzo, S.; Gerbino, S.; Bruno, A.; Lanata, L.; Gjomarkaj, M. Oxidative stress and innate immunity responses in cigarette smoke stimulated nasal epithelial cells. Toxicol. Vitr. 2014, 28, 292–299.
- Ishii, Y.; Kimura, T.; Morishima, Y.; Mochizuki, M.; Nomura, A.; Sakamoto, T.; Uchida, Y.; Sekizawa, K. S-carboxymethyl cysteine inhibits neutrophil activation mediated by N-formyl-methionyl-leucyl-phenylalanine. Eur. J. Pharmacol. 2002, 449, 183–189.
- Nogawa, H.; Ishibashi, Y.; Ogawa, A.; Masuda, K.; Tsubuki, T.; Kameda, T.; Matsuzawa, S. Carbocysteine can scavenge reactive oxygen species in vitro. Respirology 2009, 14, 53–59.
- Wang, W.; Zheng, J.P.; Zhu, S.X.; Guan, W.J.; Chen, M.; Zhong, N.S. Carbocysteine attenuates hydrogen peroxide-induced inflammatory injury in A549 cells via NF-κB and ERK1/2 MAPK pathways. Int. Immunopharmacol. 2015, 24, 306–313.
- Wang, W.; Guan, W.J.; Huang, R.Q.; Xie, Y.Q.; Zheng, J.P.; Zhu, S.X.; Chen, M.; Zhong, N.S. Carbocysteine attenuates TNF-α-induced inflammation in human alveolar epithelial cells in vitro through suppressing NF-κB and ERK1/2 MAPK signaling pathways. Acta Pharmacol. Sin. 2016, 37, 629–636.
- Asti, C.; Melillo, G.; Caselli, G.F.; Daffonchio, L.; Hernandez, A.; Clavenna, G.; Omini, C. Effectiveness of carbocysteine lysine salt monohydrate on models of airway inflammation and hyperresponsiveness. Pharmacol. Res. 1995, 31, 387–392.
- Inoue, M.; Ishibashi, Y.; Nogawa, H.; Yasue, T. Carbocysteine promotes phagocytosis of apoptotic cells by alveolar macrophages. Eur. J. Pharmacol. 2012, 677, 173–179.
- Song, Y.; Wang, W.; Xie, Y.; Xiang, B.; Huang, X.; Guan, W.; Zheng, J. Carbocysteine inhibits the expression of Muc5b in COPD mouse model. Drug Des. Devel. Ther. 2019, 13, 3259–3268.
- Carpagnano, G.E.; Resta, O.; Foschino-Barbaro, M.P.; Spanevello, A.; Stefano, A.; Di Gioia, G.; Serviddio, G.; Gramiccioni, E. Exhaled Interleukine-6 and 8-isoprostane in chronic obstructive pulmonary disease: Effect of carbocysteine lysine salt monohydrate (SCMC-Lys). Eur. J. Pharmacol. 2004, 505, 169–175.
- Ferraro, M.; Di Vincenzo, S.; Sangiorgi, C.; Leto Barone, S.; Gangemi, S.; Lanata, L.; Pace, E. Carbocysteine Modifies Circulating miR-21, IL-8, sRAGE, and fAGEs Levels in Mild Acute Exacerbated COPD Patients: A Pilot Study. Pharmaceuticals 2022, 15, 218.
- Aylward, M. An assessment of S-carboxymethyl cysteine in the treatment of chronic bronchitis. Curr. Med. Res. Opin. 1974, 2, 387–394.
- Edwards, G.F.; Steel, A.E.; Scott, J.K.; Jordanz, J.W. S-carboxymethyl cysteine in the fluidification of sputum and treatment of chronic airway obstruction. Chest 1976, 70, 506–513.
- Miskoviti, G.; Szule, P.; Mescaros, K. Double blind study of carbocysteine against placebo in chronic bronchitis; Mucoregulation in respiratory tract disorders. Proc. R. Soc. Med. 1982, 5, 1–3.
- Puchelle, E.; Girard, F.; Zahm, J.M. Rheology of bronchial secretions and mucociliary transport. Bull. Eur. Physiopathol. Respir. 1976, 12, 771–779.
- Grillage, M.; Barnard-Jones, K. Long-term oral carbocysteine therapy in patients with chronic bronchitis. A double-blind trial with placebo control. Br. J. Clin. Pract. 1985, 39, 395–398.
- Yasuda, H.; Yamaya, M.; Sasaki, T.; Inoue, D.; Nakayama, K.; Yamada, M.; Asada, M.; Yoshida, M.; Suzuki, T.; Nishimura, H.; et al. Carbocisteine inhibits rhinovirus infection in human tracheal epithelial cells. Eur. Respir. J. 2006, 28, 51–58.
- Allegra, L.; Cordaro, C.I.; Grassi, C. Prevention of acute exacerbations of chronic obstructive bronchitis with carbocysteine lysine salt monohydrate: A multicenter, double-blind, placebo-controlled trial. Respiration 1996, 63, 174–180.
- Zheng, J.P.; Kang, J.; Huang, S.G.; Chen, P.; Yao, W.Z.; Yang, L.; Bai, C.X.; Wang, C.Z.; Wang, C.; Chen, B.Y.; et al. Effect of carbocysteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): A randomised placebo-controlled study. Lancet 2008, 371, 2013–2018.
- Decramer, M.; Rutten-van Molken, M.; Dekhuijzen, P.N.; Troosters, T.; van Herwaarden, C.; Pellegrino, R.; van Schayck, C.P.O.; Olivieri, D.; Del Donno, M.; De Backer, W.; et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): A randomised placebo-controlled trial. Lancet 2005, 365, 1552–1560.
- Esposito, A.; Valentino, M.R.; Bruzzese, D.; Bocchino, M.; Ponticiello, A.; Stanziola, A.; Sanduzzi, A. Effect of Carbocysteine in the prevention of exacerbation of chronic obstructive pulmonary disease (CAPRI study): An observational study. Pulm. Pharmacol. Ther. 2016, 37, 85–88.
- Celli, B.R.; Cote, C.G.; Marin, J.M.; Casanova, C.; de Oca, M.M.; Mendez, R.A.; Plata, V.P.; Cabral, H.J. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N. Eng. J. Med. 2004, 350, 1005–1012.
- Paone, G.; Lanata, L.; Saibene, F.; Toti, S.; Palermo, P.; Graziani, C.; Flore, M.C.; Ramaccia, M.; Puglisi, G. A prospective study of the effects of carbocysteine lysine salt on frequency of exacerbations in COPD patients treated with or without inhaled steroids. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2627–2635.
- Vogelmeier, C.F.; Román Rodríguez, M.; Singh, D.; Han, M.K.; Rodríguez-Roisin, R.; Ferguson, G.T. Goals of COPD treatment: Focus on symptoms and exacerbations. Respir. Med. 2020, 166, 105938.
- Zeng, Z.; Yang, D.; Huang, X.; Xiao, Z. Effects of carbocysteine on patients with COPD: A systematic review and meta-analysis. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 2277–2283.
More
