Invasive candidiasis (IC) is a systemic life-threatening infection of immunocompromised humans, but remains a relatively neglected disease among public health authorities. Ongoing assessments of disease epidemiology are needed to identify and map trends of importance that may necessitate improvements in disease management and patient care. Well-established incidence increases, largely due to expanding populations of patients with pre-disposing risk factors, has led to increased clinical use and pressures on antifungal drugs. This has been exacerbated by a lack of fast, accurate diagnostics that have led treatment guidelines to often recommend preventative strategies in the absence of proven infection, resulting in unnecessary antifungal use in many instances. The consequences of this are multifactorial, but a contribution to emerging drug resistance is of primary concern, with high levels of antifungal use heavily implicated in global shifts to more resistant Candida strains.
- Candida
- invasive candidiasis
- candidemia
- epidemiology
- antifungal
- diagnostics
1. Introduction
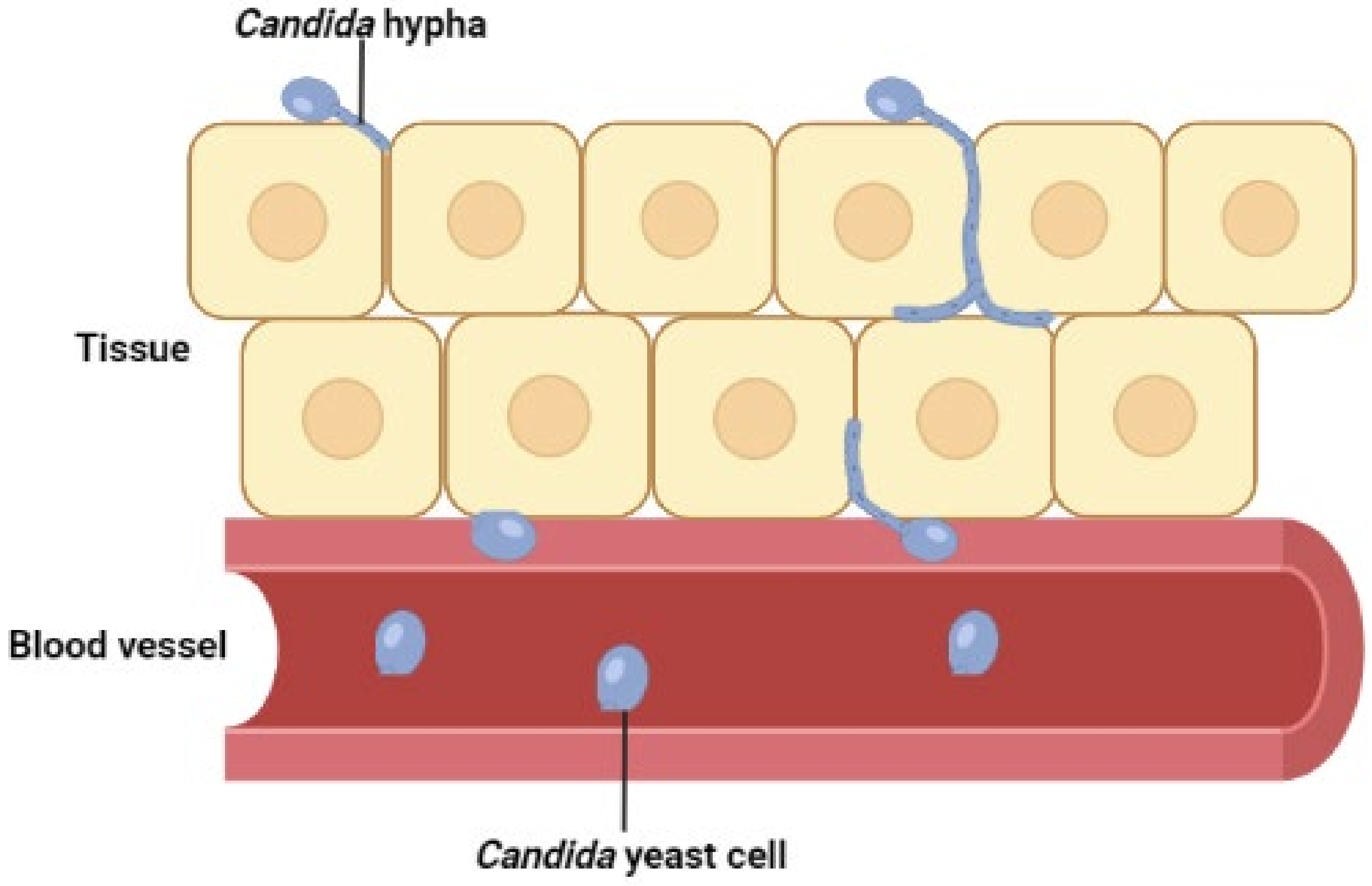
2. Species Distribution and Antifungal Susceptibilities
2.1. Influence of Pre-Disposing Risk Factors
2.2. Geographical Trends
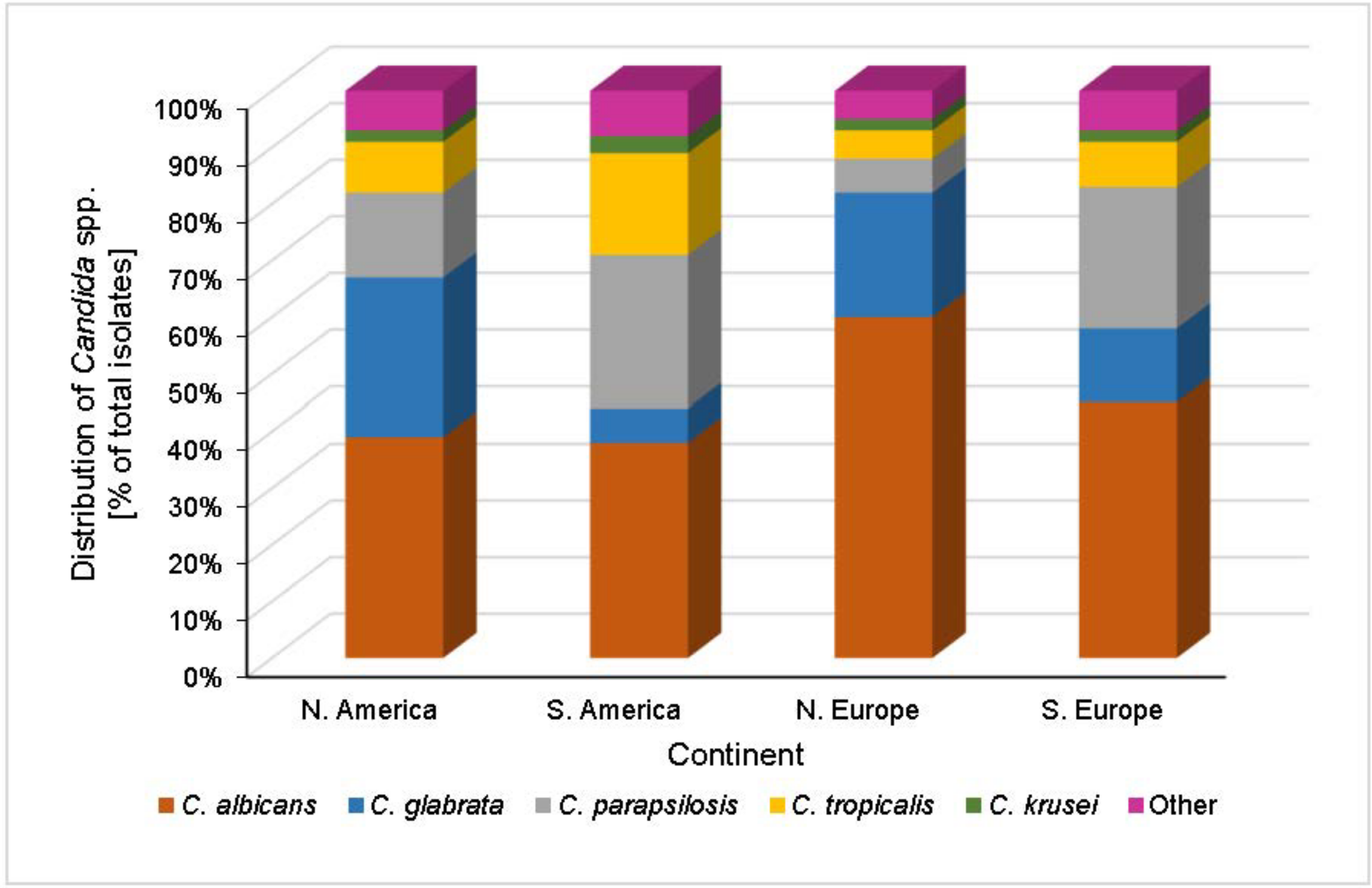
2.2.1. United States
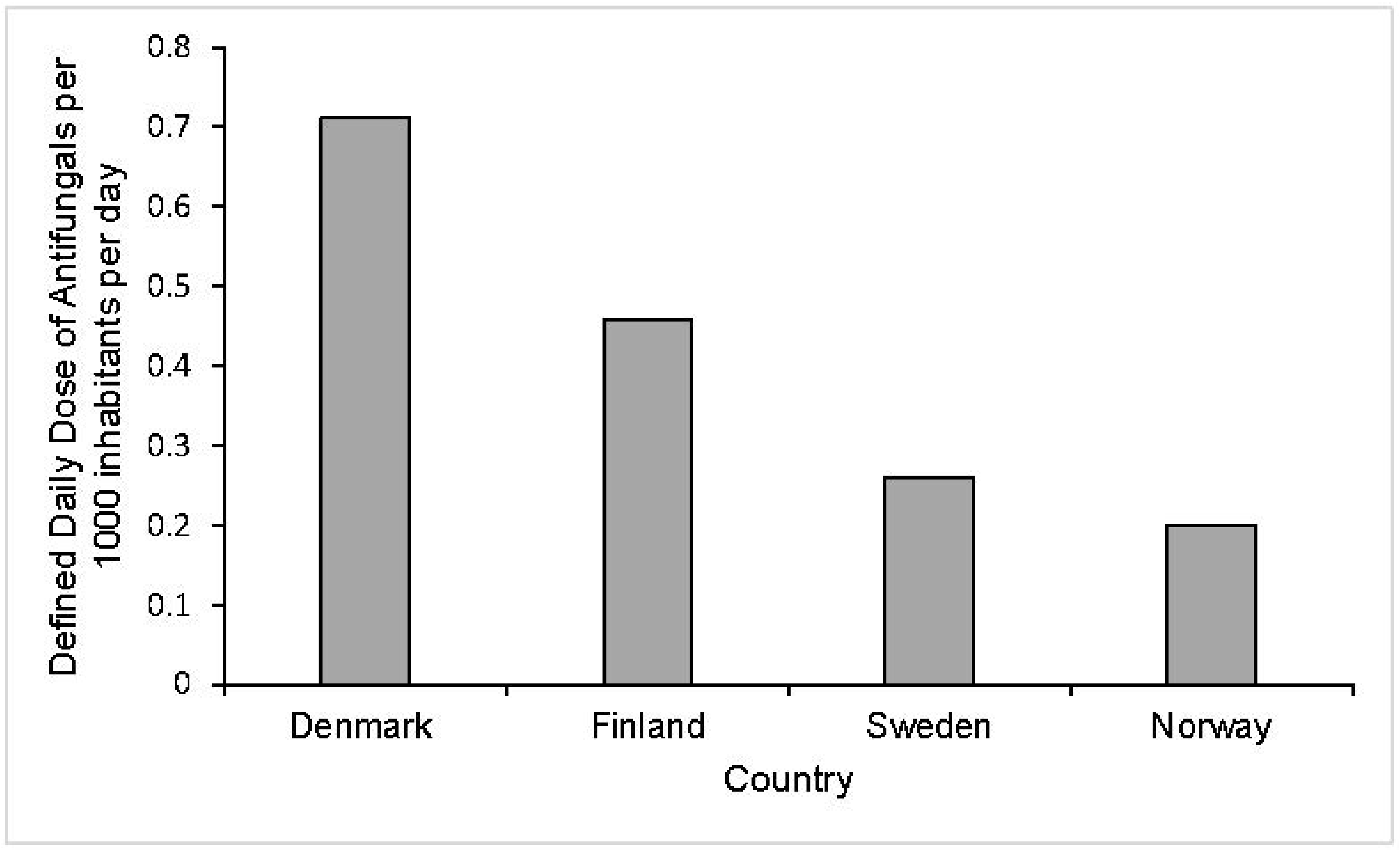
2.2.2. Europe
2.2.3. South America
2.2.4. Asia
3. Diagnostics
3.1. Culture-Based Diagnostics
3.2. CHROMagar for Species Identification
3.3. Disease Management and Patient Care Impacts
References
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 11, 18026.
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163.
- Fischer, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats posed by the fungal kingdom to humans, wildlife and agriculture. mBio 2020, 11, e00449-20.
- Yapar, N. Epidemiology and risk factors for invasive candidiasis. Ther. Clin. Risk Manag. 2014, 10, 95–105.
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and multi-national prevalence of fungal diseases—Estimate precision. J. Fungi 2017, 3, 57.
- Toda, M.; Williams, S.R.; Berkow, E.L.; Farley, M.M.; Harrison, L.H.; Bonner, L.; Marceaux, K.M.; Hollick, R.; Zhang, A.Y.; Schaffner, W.; et al. Population-based active surveillance for culture confirmed candidemia—Four sites, United States, 2012–2016. MMWR Surveill. Summ. 2019, 68, 1–15.
- Saville, S.P.; Lazzell, A.L.; Monteagudo, C.; Lopez-Ribot, J. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell 2003, 2, 1053–1060.
- Pfaller, M.A.; Moet, G.J.; Messer, S.A.; Jones, R.N.; Castanheira, M. Candida bloodstream infections: Comparison of species distributions and antifungal resistance patterns in community-onset and nosocomial isolates in the SENTRY Antimicrobial Surveillance Program, 2008–2009. Antimicrob. Agents Chemother. 2011, 55, 561–566.
- Clancy, C.J.; Nguyen, M.H. Finding the “missing 50%” of invasive candidiasis: How nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin. Infect. Dis. 2013, 56, 1284–1292.
- Clancy, C.J.; Nguyen, M.H. Diagnosing invasive candidiasis. J. Clin. Microbiol. 2018, 56, e01909-17.
- Garey, K.W.; Rege, M.; Pai, M.P.; Mingo, D.E.; Suda, K.J.; Turpin, R.S.; Bearden, D.T. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: A multi-institutional study. Clin. Infect. Dis. 2006, 43, 25–31.
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical practice guideline for the management of candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50.
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID Guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin. Microbiol. Infect. 2012, 7, 19–37.
- Bassetti, M.; Righi, E.; Montravers, P.; Cornely, O.A. What has changed in the treatment of invasive candidiasis? A look at the past 10 years and ahead. J. Antimicrob. Chemother. 2018, 73, i14–i25.
- Ostrosky-Zeichner, L.; Casadevall, A.; Galgiani, J.N.; Odds, F.C.; Rex, J.H. An insight into the antifungal pipeline: Selected new molecules and beyond. Nat. Rev. Drug Discov. 2010, 9, 719–727.
- Hazen, K.C. New and emerging yeast pathogens. Clin. Microbiol. Rev. 1995, 8, 462–478.
- Pfaller, M.A.; Diekema, D.J. Rare and emerging opportunistic fungal pathogens: Concern for resistance beyond Candida albicans and Aspergillus fumigatus. J. Clin. Microbiol. 2004, 42, 4419–4431.
- Cleaveland, A.A.; Harrison, L.H.; Farley, M.M.; Hollick, R.; Stein, B.; Chiller, T.M.; Lockhart, S.R.; Park, B.J. Declining incidence of candidemia and the shifting epidemiology of Candida resistance in two US metropolitan areas, 2008—2013: Results from population-based surveillance. PLoS ONE 2015, 10, e0120452.
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of healthcare associated infections. N. Engl. J. Med. 2014, 370, 1198–1208.
- Risum, M.; Astvad, K.; Johansen, H.K.; Schonheyder, H.C.; Rosenvinge, F.; Knudsen, J.D.; Hare, R.; Datcu, R.; Roder, B.L.; Antsupova, V.S.; et al. Update 2016–2018 of the nationwide Danish Fungaemia Surveillance Study: Epidemiologic changes in a 15-year perspective. J. Fungi 2021, 7, 491.
- Banerjee, S.N.; Emori, T.G.; Culver, D.H.; Gaynes, R.P.; Jarvis, W.R.; Horan, T.; Edwards, J.R.; Tolson, J.; Henderson, T.; Martone, W.J. Secular trends in nosocomial primary bloodstream infections in the United States, 1980–1989. Am. J. Med. 1991, 91, S86–S89.
- Lass-Flörl, C. The changing face of epidemiology of invasive fungal disease in Europe. Mycoses 2009, 52, 197–205.
- Pfaller, M.A.; Castanheira, M.; Messer, S.A.; Moet, G.J.; Jones, R.N. Echinocandin and triazole antifungal susceptibility profiles for Candida spp., Cryptococcus neoformans, and Aspergillus fumigatus: Application of new CLSI clinical breakpoints and epidemiologic cut-off values to characterise resistance in the SENTRY Antimicrobial Surveillance Program (2009). Diagn. Microbiol. Infect. Dis. 2011, 69, 45–50.
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44.
- Pfeiffer, C.D.; Samsa, G.P.; Schell, W.A.; Reller, L.B.; Perfect, J.R.; Alexander, B.D. Quantification of Candida CFU in initial positive blood cultures. J. Clin. Microbiol. 2011, 49, 2878–2883.
- Sandven, P.; Bevanger, L.; Digranes, A.; Haukland, H.H.; Mannsaker, T.; Gaustad, P. Candidemia in Norway (1991–2003): Results from a nationwide study. J. Clin. Microbiol. 2006, 44, 1977–1981.
- Horvath, L.L.; George, B.J.; Murray, C.K.; Harrison, L.S.; Hospenthal, D.R. Direct comparison of the BACTEC 9240 and BacT/Alert 3D automated blood culture systems for Candida growth detection. J. Clin. Microbiol. 2004, 42, 115–118.
- Horvath, L.L.; Hospenthal, D.R.; Murray, C.K.; Dooley, D.P. Detection of simulated candidemia by the BACTEC 9240 system with plus aerobic/F and anaerobic/F blood culture bottles. J. Clin. Microbiol. 2003, 41, 4714–4717.
- Abi-Said, D.; Anaissie, E.; Uzun, O.; Raad, I.; Pinzcowski, H.; Vartivarian, S. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin. Infect. Dis. 1997, 24, 1122–1128.
- McCarty, T.P.; Pappas, P.G. Invasive candidiasis. Infect. Dis. Clin. North Am. 2016, 30, 103–124.
- Alexander, B.D.; Schell, W.A.; Miller, J.L.; Long, G.D.; Perfect, J.R. Candida glabrata fungemia in transplant patient receiving voriconazole after fluconazole. Transplantation 2005, 80, 868–871.
- Baran, J.; Muckatira, B.; Khatib, R. Candidemia before and during the fluconazole era: Prevalence, type of species and approach to treatment in a tertiary care community hospital. Scand. J. Infect. Dis. 2001, 33, 137–139.
- Lin, M.Y.; Carmeli, Y.; Zumsteg, J.; Flores, E.L.; Tolentino, J.; Sreeramoju, P.; Weber, S.G. Prior antimicrobial therapy and risk for hospital-acquired Candida glabrata and Candida krusei fungemia: A case-case-control study. Antimicrob. Agents Chemother. 2005, 49, 4555–4560.
- Blumberg, H.M.; Jarvis, W.R.; Soucie, J.M.; Edwards, J.E.; Patterson, J.E.; Pfaller, M.A.; Rangel-Frausto, M.S.; Rinaldi, M.G.; Saiman, L.; Wiblin, R.T.; et al. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: The NEMIS prospective multicentre study. The National Epidemiology of Mycosis Survey. Clin. Infect. Dis. 2001, 33, 177–186.
- White, M.H. The contribution of fluconazole to the changing epidemiology of invasive candidal infections. Clin. Infect. Dis. 1997, 24, 1129–1130.
- Oeser, C.; Lamagni, T.; Heath, P.T.; Sharland, M.; Ladhani, S. The epidemiology of neonatal and pediatric candidemia in England and Wales, 2000–2009. Pediatr. Infect. Dis. J. 2013, 32, 23–26.
- Oeser, C.; Vergnano, S.; Naidoo, R.; Anthony, M.; Chang, J.; Chow, P.; Clarke, P.; Embleton, N.; Kennea, N.; Pattnayak, S.; et al. Neonatal invasive fungal infection in England 2004–2010. Clin. Microbiol. Infect. 2014, 20, 936–941.
- Levy, I.; Rubin, L.G.; Vasishtha, S.; Tucci, V.; Sood, S.K. Emergence of Candida parapsilosis as the predominant species causing candidemia in children. Clin. Infect. Dis. 1998, 26, 1086–1088.
- Lupetti, A.; Tavanti, A.; Davini, P.; Ghelardi, E.; Corsini, V.; Merusi, I.; Boldrini, A.; Campa, M.; Senesi, S. Horizontal transmission of Candida parapsilosis candidemia in a neonatal intensive care unit. J. Clin. Microbiol. 2002, 40, 2363–2369.
- Branchini, M.L.; Pfaller, M.A.; Rhine-Chalberg, J.; Frempong, T.; Isenberg, H.D. Genotypic variation and slime production among blood and catheter isolates of Candida parapsilosis. J. Clin. Microbiol. 1994, 32, 452–456.
- Clark, T.A.; Slavinski, S.A.; Morgan, J.; Lott, T.; Arthington-Skaggs, B.A.; Brandt, M.E.; Webb, R.M.; Currier, M.; Flowers, R.H.; Fridkin, S.K.; et al. Epidemiologic and molecular characterization of an outbreak of Candida parapsilosis bloodstream infections in a community hospital. J. Clin. Microbiol. 2004, 42, 4468–4472.
- Fridkin, S.K. The changing face of fungal infections in healthcare settings. Clin. Infect. Dis. 2005, 41, 1455–1460.
- Almirante, B.; Rodriguez, D.; Cuenca-Estrella, M.; Almela, M.; Sanchez, F.; Ayats, J.; Alonso-Tarres, C.; Rodriquez-Tudela, J.L.; Pahissa, A. Epidemiology, risk factors, and prognosis of Candida parapsilosis bloodstream infections: Case-control population-based surveillance study of patients in Barcelona, Spain, from 2002–2003. J. Clin. Microbiol 2006, 44, 1681–1685.
- Kontoyiannis, D.P.; Vaziri, I.; Hanna, H.A.; Boktour, M.; Thornby, J.; Hachem, R.; Bodey, G.P.; Raad, I.I. Risk factors for Candida tropicalis fungemia in patients with cancer. Clin. Infect. Dis. 2001, 33, 1676–1681.
- Marr, K.A.; Seidel, K.; White, T.C.; Bowden, R.A. Candidemia in allogenic blood and marrow transplant recipients: Evolution of risk factors after the adoption of prophylactic fluconazole. J. Infect. Dis. 2000, 181, 309–316.
- Castanheira, M.; Messer, S.A.; Rhomberg, P.R.; Pfaller, M.A. Antifungal susceptibility patterns of a global collection of fungal isolates: Results of the SENTRY Antifungal Surveillance Program (2013). Diagn. Microbiol. Infect. Dis. 2016, 85, 200–204.
- Merz, W.G.; Karp, J.E.; Schron, D.; Saral, R. Increased incidence of fungemia caused by Candida krusei. J. Clin. Microbiol. 1986, 24, 581–584.
- Teoh, F.; Pavelka, N. How chemotherapy increases the risk of systemic candidiasis in cancer patients: Current paradigm and future directions. Pathogens 2016, 5, 6.
- Kaur, H.; Singh, S.; Rudramurthy, S.M.; Ghosh, A.K.; Jayashree, M.; Narayana, Y.; Ray, P.; Chakrabarti, A. Candidemia in a tertiary care centre of developing country: Monitoring possible change in spectrum of agents and antifungal susceptibility. Indian J. Med. Microbiol. 2020, 38, 110–116.
- Zeng, Z.; Ding, Y.; Tian, G.; Yang, K.; Deng, J.; Li, G.; Liu, J. A seven-year surveillance study of the epidemiology, antifungal susceptibility, risk factors and mortality of candidemia among paediatric and adult inpatients in a tertiary teaching hospital in China. Antimicrob. Resist. Infect. Control 2020, 9, 133.
- Zeng, Z.; Tiang, G.; Ding, Y.; Yang, K.; Liu, J.; Deng, J. Surveillance study of the prevalence, species distribution, antifungal susceptibility, risk factors and mortality of invasive candidiasis in a tertiary teaching hospital in Southwest China. BMC Infect. Dis. 2019, 19, 939.
- Xiao, Z.; Wang, Q.; Zhu, F.; An, Y. Epidemiology, species distribution, antifungal susceptibility and mortality risk factors of candidemia among critically ill patients: A retrospective study from 2011–2017 in a teaching hospital in China. Antimicrob. Resist. Infect. Control 2019, 8, 89.
- Boonslip, S.; Homkaew, A.; Phumisantiphong, U.; Nutalai, D.; Wongsuk, T. Species distribution, antifungal susceptibility and molecular epidemiology of candida species causing candidemia in a tertiary care hospital in Bangkok, Thailand. J. Fungi 2021, 7, 577.
- Yamin, D.; Husin, A.; Harun, A. Distribution of candidemia in a Malaysian tertiary care hospital revealed predominance of Candida parapsilosis. Trop. Biomed. 2020, 37, 903–910.
- Hesstvedt, L.; Arendrup, M.C.; Poikonen, E.; Klingpor, L.; Friman, V.; Nordoy, I. Differences in epidemiology of candidemia in the Nordic countries—What is to blame? Mycoses 2017, 60, 11–19.
- Puig-Asensio, M.; Padilla, B.; Garnacho-Montero, J.; Zaragoza, O.; Aguado, J.M.; Zaragoza, R.; Montejo, M.; Munoz, P.; Ruiz-Camps, I.; Cuenca-Estrella, M.; et al. Epidemiology and predictive factors for early and late mortality in Candida bloodstream infections: A population-based surveillance in Spain. Clin. Microbiol. Infect. 2014, 20, O245–O254.
- Barchiesi, F.; Orsetti, E.; Gesuita, R.; Skrami, E.; Manso, E. Epidemiology, clinical characteristics, and outcome of candidemia in a tertiary referral center in Italy from 2010–2014. Infection 2016, 44, 205–213.
- Nucci, M.; Queiroz-Telles, F.; Alvarado-Matute, T.; Tiraboschi, I.N.; Cortes, J.; Zurita, J.; Guzman-Blanco, M.; Santolaya, M.E.; Thompson, L.; Sifuentes-Osornio, J.; et al. Epidemiology of candidemia in Latin America: A laboratory-based survey. PLoS ONE 2013, 8, e59373.
- Kao, A.S.; Brandt, M.E.; Pruitt, W.R.; Conn, L.A.; Perkins, B.A.; Stephens, D.S.; Baughman, W.S.; Reingold, A.L.; Rothrock, G.A.; Pfaller, M.A.; et al. The epidemiology of candidemia in two United States cities: Results of a population based active surveillance. Clin. Infect. Dis. 1999, 29, 1164–1170.
- Cleaveland, A.A.; Farley, M.M.; Harrison, L.H.; Stein, B.; Hollick, R.; Lockhart, S.R.; Magill, S.S.; Derado, G.; Park, B.J.; Chiller, T.M. Changes in incidence and antifungal drug resistance in candidemia: Results from population-based laboratory surveillance in Atlanta and Baltimore, 2008-2011. Clin. Infect. Dis. 2012, 55, 1352–1361.
- Hajjeh, R.A.; Sofair, A.N.; Harrison, L.H.; Lyon, G.M.; Arthington-Skaggs, B.A.; Mirza, S.A.; Phelan, M.; Morgan, J.; Lee-Yang, W.; Ciblak, M.A.; et al. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J. Clin. Microbiol. 2004, 42, 1519–1527.
- Perlin, D.S. Echinocandin resistance in Candida. Clin. Infect. Dis. 2015, 61, S612–S617.
- Marchetti, O.; Bille, J.; Fluckiger, U.; Eggimann, P.; Ruef, C.; Garbino, J.; Calandra, T.; Glauser, M.; Tauber, M.G.; Pittet, D. Epidemiology of candidemia in Swiss tertiary care hospitals: Secular trends, 1991–2000. Clin. Infect. Dis. 2004, 38, 311–320.
- Adam, K.; Osthoff, M.; Lamoth, F.; Conen, A.; Erard, V.; Boggian, K.; Schreiber, P.W.; Zimmerli, S.; Bochud, P.; Neofytos, D.; et al. Trends of the epidemiology of candidemia in Switzerland: A 15-year FUNGINOS survey. Open Forum Infect. Dis. 2021, 8, ofab471.
- Asmundsdottir, L.R.; Erlendsdottir, H.; Gottfredsson, M. Nationwide study of candidemia, antifungal use, and antifungal drug resistance in Iceland, 2000 to 2011. J. Clin. Microbiol 2013, 51, 841–848.
- Poikonen, E.; Lyytikäinen, O.; Anttila, V.J.; Koivula, I.; Lumio, J.; Kotilainen, P.; Syrjälä, H.; Ruutu, P. Secular trend in candidemia and the use of fluconazole in Finland, 2004–2007. BMC Infect. Dis. 2010, 10, 312.
- Asmundsdottir, L.R.; Erlendsdottir, H.; Gottfredsson, M. Increasing incidence of candidemia: Results from a 20-year nationwide study in Iceland. J. Clin. Microbiol. 2002, 40, 3482–3492.
- Poikonen, E.; Lyytikäinen, O.; Anttila, V.J.; Ruutu, P. Candidemia in Finland, 1995–1999. Emerg. Infect. Dis. 2003, 9, 985–990.
- Ericsson, J.; Chryssanthou, E.; Klingspor, L.; Johansson, A.G.; Ljungman, P.; Svensson, E.; Sjölin, J. Candidemia in Sweden: A nationwide prospective observational study. Clin. Microbiol. Infect. 2013, 19, E218–E221.
- Arendrup, M.C.; Bruun, B.; Christensen, J.J.; Fuursted, K.; Johansen, H.K.; Kjaeldgaard, P.; Knudsen, J.D.; Kristensen, L.; Moller, J.; Nielsen, L.; et al. National surveillance of fungemia in Denmark (2004–2009). J. Clin. Microbiol. 2010, 49, 325–334.
- Arendrup, M.C.; Dzajic, E.; Jensen, R.H.; Johansen, H.K.; Kjaeldgaard, P.; Knudsen, J.D.; Kristensen, L.; Leitz, C.; Lemming, L.E.; Nielsen, L.; et al. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: Data from a nationwide fungaemia surveillance programme. Clin. Microbiol. Infect. 2013, 19, E343–E353.
- Hesstvedt, L.; Gaustad, P.; Andersen, C.T.; Haarr, E.; Hannula, R.; Haukland, H.H.; Hermansen, N.-O.; Larssen, K.W.; Mylvaganam, H.; Ranheim, T.E.; et al. Twenty-two years of candidemia surveillance: Results from a Norwegian national study. Clin. Microbiol. Infect. 2015, 21, 938–945.
- Chalmers, C.; Gaur, S.; Chew, J.; Wright, T.; Kumar, A.; Mathur, S.; Wan, W.Y.; Gould, I.M.; Leanord, A.; Bal, A.M. Epidemiology and management of candidemia—A retrospective, multicentre study in five hospitals in the UK. Mycoses 2011, 54, e795–e800.
- Vannini, M.; Emery, S.; Lieutier-Colas, F.; Legueult, K.; Mondain, V.; Retur, N.; Gastaud, L.; Pomares, C.; Hasseine, L. Epidemiology of candidemia in NICE area, France: A five-year study of antifungal susceptibility and mortality. J. Mycol. Med. 2022, 32, 101210.
- Schroeder, M.; Weber, T.; Denker, T.; Winterland, S.; Wichmann, D.; Rohde, H.; Ozga, A.-K.; Fischer, M.; Kluge, S. Epidemiology, clinical characteristics, and outcome of candidemia in critically ill patients in Germany: A single-centre retrospective 10-year analysis. Ann. Intensive Care 2020, 10, 142.
- Bassetti, M.; Ansaldi, F.; Nicolini, L.; Malfatto, E.; Molinari, M.P.; Mussap, M.; Rebesco, B.; Pallavicini, F.B.; Icardi, G.; Viscoli, C. Incidence of candidemia and relationship with fluconazole use in an intensive care unit. J. Antimicrob. Chemother. 2009, 64, 625–629.
- Rodriguez, L.; Bustamante, B.; Huaroto, L.; Agurto, C.; Illescas, R.; Ramirez, R.; Diaz, A.; Hidalgo, J. A multi-centric study of Candida bloodstream infection in Lima-Callao, Peru: Species distribution, antifungal resistance and clinical outcomes. PLoS ONE 2017, 12, e0175172.
- Rodrigues, D.K.B.; Bonfietti, L.X.; Garcia, R.A.; Araujo, M.R.; Rodrigues, J.S.; Gimenes, V.M.F.; Melhem, M.S.C. Antifungal susceptibility profile of Candida clinical isolates from 22 hospitals of Sao Paulo state, Brazil. Braz. J. Med. Biol. Res. 2021, 54, e10928.
- Garcia-Effron, G.; Katiyar, S.K.; Park, S.; Edlind, T.D.; Perlin, D.S. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 2008, 52, 2305–2312.
- Douglas, C.M.; D’Ippolito, J.A.; Shei, G.J.; Meinz, M.; Onishi, J.; Marrinan, J.A.; Li, W.; Abruzzo, G.K.; Flattery, A.; Bartizal, K.; et al. Identification of the FKS1 gene of candida albicans as the essential target of 1,3-beta-D-glucan synthase inhibitors. Antimicrob. Agents Chemother. 1997, 41, 2471–2479.
- Jones, J.M. Laboratory diagnosis of invasive candidiasis. Clin. Microbiol. Rev. 1990, 3, 32–45.
- Ellepola, A.; Morrison, C. Laboratory diagnosis of invasive candidiasis. J. Microbiol. 2005, 43, 65–84.
- Leon, C.; Ruiz-Santana, S.; Saavedra, P.; Castro, C.; Loza, A.; Zakariya, I.; Ubeda, A.; Parra, M.; Macias, D.; Tomas, J.I.; et al. Contribution of Candida biomarkers and DNA detection for the diagnosis of invasive candidiasis in ICU patients with severe abdominal conditions. Crit. Care 2016, 20, 149.
- Clancy, C.J.; Nguyen, M.H. Rapid diagnosis of invasive candidiasis: Ready for prime-time? Curr. Opin, Infect. Dis. 2019, 32, 546–552.
- Clancy, C.J.; Nguyen, M.H. Non-culture diagnostics for invasive candidiasis: Promise and unintended consequences. J. Fungi 2018, 4, 27.
- Clancy, C.J.; Shields, R.K.; Nguyen, M.H. Invasive candidiasis in various patient populations: Incorporating non-culture diagnostic tests into rational management strategies. J Fungi 2016, 2, 10.
- Nguyen, M.H.; Wissel, M.C.; Shields, R.K.; Salomoni, M.A.; Hao, B.; Press, E.G.; Shields, R.M.; Cheng, S.; Mitsani, D.; Vadnerkar, A.; et al. Performance of Candida real-time polymerase chain reaction, β-D-glucan assay, and blood cultures in the diagnosis of invasive candidiasis. Clin. Infect. Dis. 2012, 54, 1240–1248.
- Cuenca-Estrella, M.; Verweij, P.E.; Arendrup, M.C.; Arikan-Akdagli, S.; Bille, J.; Donnelly, J.P.; Jensen, H.E.; Lass-Flörl, C.; Richardson, M.D.; Akova, M.; et al. ESCMID guideline for the diagnosis of Candida diseases 2012: Diagnostic procedures. Clin. Microbiol. Infect. 2012, 18, 9–18.
- Ness, M.J.; Vaughan, W.P.; Woods, G.L. Candida antigen latex test for detection of invasive candidiasis in immunocompromised patients. J. Infect. Dis. 1989, 159, 495–502.
- Telenti, A.; Steckelberg, J.M.; Stockman, L.; Edson, R.S.; Roberts, G.D. Quantitative blood cultures in candidemia. Mayo Clin. Proc. 1991, 66, 1120–1123.
- Beyda, N.D.; Amadio, J.; Rodriguez, J.R.; Malinowski, K.; Garey, K.W.; Wanger, A.; Ostrosky-Zeichner, L. In vitro evaluation of BacT/Alert FA blood culture bottles and T2 Candida assay for detection of Candida in the presence of antifungals. J. Clin. Microbiol. 2018, 56, e00471-18.
- Odds, F.C. Sabouraud(’s) agar. J. Med. Vet. Mycol. 1991, 29, 355–359.
- Odds, F.C.; Bernaerts, R. CHROMagar Candida, a new differential isolation medium for presumptive isolation of clinically important Candida species. J. Clin. Microbiol. 1994, 32, 1923–1929.
- Horvath, L.L.; Hospenthal, D.R.; Murray, C.K.; Dooley, D.P. Direct isolation of Candida spp. from blood cultures on the chromogenic medium CHROMagar Candida. J. Clin. Microbiol. 2003, 41, 2629–2632.
- Bernal, S.; Mazuelos, E.M.; Garcia, M.; Aller, A.I.; Martinez, M.A.; Gutierrez, M.J. Evaluation of CHROMagar Candida medium for the isolation and presumptive identification of species of Candida of clinical importance. Diagn. Microbiol. Infect. Dis. 1996, 24, 201–204.
- Hospenthal, D.R.; Murray, C.K.; Beckius, M.L.; Green, J.A.; Dooley, D.P. Persistence of pigment production by yeast isolates grown on CHROMagar Candida medium. J. Clin. Microbiol. 2002, 40, 4768–4770.
- Powell, H.L.; Sand, C.A.; Rennie, R.P. Evaluation of CHROMagar Candida for presumptive identification of clinically important Candida species. Diagn. Microbiol. Infect. Dis. 1998, 32, 201–204.
- Pfaller, M.A.; Houston, A.; Coffmann, S. Application of CHROMagar Candida for rapid screening of clinical specimens for Candida albicans, Candida tropicalis, Candida krusei, and Candida (Torulopsis) glabrata. J. Clin. Microbiol. 1996, 34, 58–61.
- Hospenthal, D.R.; Beckius, M.L.; Floyd, K.L.; Horvath, L.L.; Murray, C.K. Presumptive identification of Candida species other than C. albicans, C. krusei, and C. tropicalis with the chromogenic medium CHROMagar Candida. Ann. Clin. Microbiol. Antimicrob. 2006, 5, 1.
- Clancy, C.J.; Nguyen, M.H. Undiagnosed invasive candidiasis: Incorporating non-culture diagnostics into rational prophylactic and pre-emptive antifungal strategies. Expert Rev. Anti Infect. Ther. 2014, 12, 731–734.
- Matsui, D. Current issues in pediatric medication adherence. Paediatr. Drugs 2007, 9, 283–288.
