The honey bee Apis mellifera Linnaeus (1758) provides many benefits to humans and ecosystems. This species is an important pollinator in natural environments, which may help to preserve and restore the biodiversity of wild plants. On the other hand, pollination in agro-ecosystems by managed honey bee colonies may enhance crop yield and quality, meeting the increasing food demand.
Beekeeping is also a high-valued and income-generating activity, which provides humans with honey as high-quality food as well as substances used as raw materials and in pharmaceuticals.
In addition, the honey bee and its products are valuable bioindicators and bioaccumulators of environmental pollution: they provide valuable information on the impact of human activities, enabling the implementation of measures to mitigate risks to human and ecosystem health.
The honey bee is also linked to many cultural ecosystem services and has a longstanding tradition in human culture, mysticism, and religion. Its popularity may be therefore used for educational purposes and to raise public awareness of important issues, such as the conservation of pollinator habitats and biodiversity.
- honey bee
- ecosystem services
- agro-ecosystems
- bee products
- provisioning services
- regulating services
- cultural services
- biodiversity
1. Regulating Services Provided by the Honey bee: The Conservation of Plant Biodiversity and Enhancement of Crop Production
1.1. Plant biodiversity conservation
Wild and domesticated bees are the most important pollinator group and the role played by bees as pollinators within natural and agro-ecosystems is becoming increasingly evident and recognised [4,8,9].
A. mellifera
A. mellifera itself, due to floral resource limitation and potential pest and pathogen transmission [10,11]. This is especially true in the case of massive introduction of non-native honey bees in natural, protected areas [10,12]; therefore, according to some researchers, the best option should be to avoid high-density beekeeping and to increase spacing among neighbouring apiaries to guarantee abundant floral resources for all pollinators [10,13]. In view of this, laws and regulations to ban “intensive
beekeeping” in natural ecosystems, while, in any case, favouring more sustainable approaches also through financial incentives for beekeepers should be promoted.
On the other hand, studies point out the global importance of honey bees as pollinators in natural habitats and the need to ensure their conservation to maintain the genetic diversity of local subspecies and their ecological function [11,14,15]. In natural habitats, honey bees appear to be the most frequent pollinators, averaging 13% of floral visits, with 5% of plant species being exclusively visited by
A. mellifera [15]. This confirms that honey bees may also aid in the maintenance of the biodiversity of native communities of flowering plants [14–16].
A. mellifera and biodiversity is the possibility of using honey bees to understand the diversity status of flowering plants. Pollen richness is often used as a means to estimate the floristic richness of an ecosystem [17] and honey bees may provide useful information for monitoring purposes through, for example, analysis of the pollen grains packed into the pollen basket, as well as the analysis of pollen-contaminating
bee products, especially honey. The use of molecular tools may offer further advantages in terms of quality and quantity of information compared to pollen identification through microscopic analysis. For example, DNA metabarcoding applied to honey reveals the presence of DNA from both pollen- and nectar-providing plants [18,19].
Of course, microscopic analysis and DNA metabarcoding applied to bee pollen and honey provide information on plant taxa on which honey bees forage, but, given that pollen composition in bee matrices is largely influenced by floristic local biodiversity and flowering phenology [18–23], these data may improve our understanding of the local biodiversity of flowering plants.
1.2. Crop pollination: quality and yield
1.2. Crop pollination: quality and yield
A. mellifera is easy to manage and transport, and the income the honey bee provides through the delivery of many products has made it the most valuable pollinator used to enhance agricultural production since ancient times. One of the best examples is almond pollination in California, where more than 70% of all honey bee colonies in the USA are moved to orchards for the promotion of pollination. To overcome the increasing demand for managed bees, growers are starting to breed almond varieties claimed as ‘pollinator-independent’ due to their presumed great capacity for self-pollination [33] [18]. However, researchers have
found that even in this case if bees are not present in the orchards, growers obtain a lower crop yield, as pollinators guarantee a 20% increase in kernel yield [33] [18]. In kiwi and avocado, while anemophilous and/or self-pollination alone ensures 12% and up to 17% of fruit set, respectively, when flowers are also exposed to honey bees, the yield increases to 80% and 90% (Figure 1a) [34,35][19][20]. In apples, one of the most important fruit crops in the world, insect pollination is necessary to obtain marketable fruits, i.e., large and symmetric [36][21]. Symmetry is a classical aesthetic principle, and consumers usually prefer to opt for beautiful fruits because they evoke naturalness and seem more appetising and healthier as compared to asymmetric fruits [37][22]. Therefore, honey bee colonies are usually placed into large commercial orchards to ensure fruit quality and quantity, even if honey bees do not seem to be the most efficient pollinators of apple flowers [38,39][23][24]. Similarly, supplementing raspberry and blueberry crops with beehives is necessary to obtain marketable berries [40,41][25][26]. Indeed, raspberry and blueberry fruits are characterised by an aggregation of drupelets, and under-pollinated flowers develop into crumbly, misshapen, or small berries that are avoided by consumers.
Honey bee supplementation is also important for ensuring yield stability over space and time [42]. If growers place small apiaries throughout a farm, this may enhance the spatial stability of bee visits, ensuring a homogeneous rate of yield quality and quantity [43]. The presence of healthy colonies also guarantees fruit quality and quantity across seasons in both apple and pear crops [44]. The strength of the bee colonies is also decisive for promoting crop production in the northern highbush blueberry, which is self-fertile, but higher fruit set and yields occur following visitation by honey bees from healthy colonies [45]. For watermelon crops, as native solitary bees are effective pollinators but do not allow optimal yield, supplementary pollination services through A. mellifera are suggested, even if in this case native managed stingless bees are preferable because they compete less with native pollinators [46]. In wild blueberry, both honey bee and bumblebee abundance increases fruit set and reduces spatial heterogeneity in crop production [47].
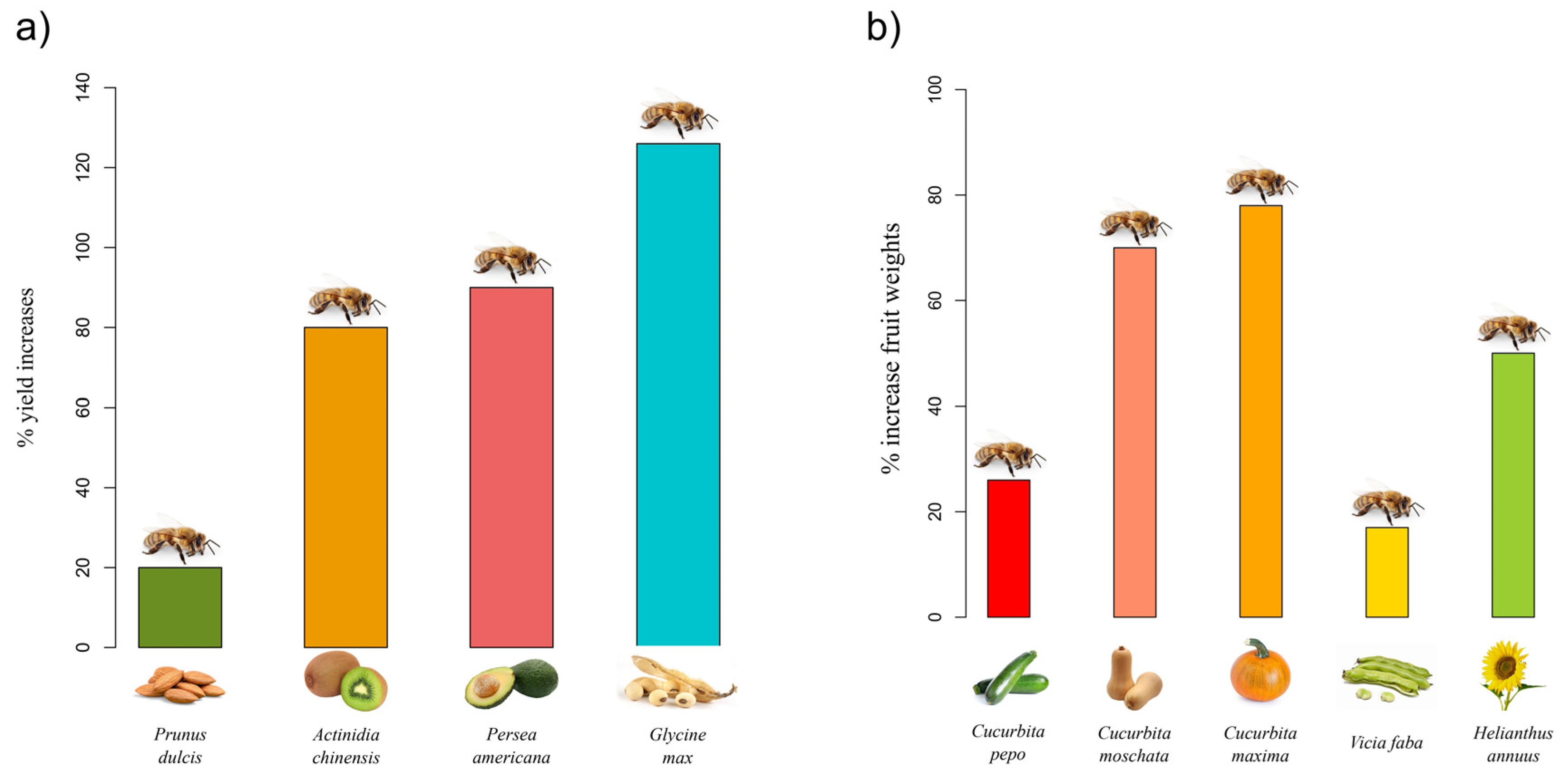
is also known to improve yields in horticultural, legume, oilseed, and feed crops. In a recent study by Garibaldi et al. [48], the authors highlighted the fact that soybean productivity can significantly increase through insect pollination. Among soybean pollinators, it has been demonstrated that the honey bee effectively increases crop yield, pod set, and seed set [48]. In Brazil, crop yield has increased by up to 126% [48]. Honey bee supplementation enhances fruit weights of Cucurbita pepo, C. moschata, and C. maxima by approximately 26%, 70%, and 78%, respectively [49]; in fava bean (Vicia faba), the yield increased by 17% [50], and in sunflower (Helianthus annuus), about 50% [51].
The effects of honey bees on fruit set and fruit/seed weight of biofuel crops, such as
Jatropha curcas and
Ricinus communis, have also been demonstrated [52,53]:
J. curcas, for example, fruit set can increase up to 70% [53].
2. Use of Bee Products as Raw Materials and Medicinal Resources
2.1. Wax as Raw Material: New Perspectives
2.2. Propolis
2.3. Royal Jelly
2.4. Venom and Apitherapy
3. Honey Bees and Bee Products to Safeguard Ecosystems from Pollution
-
Very limited purchase costs and maintenance—beekeeping is an easy and low-cost activity, which provides a potentially unlimited supply of bioindicators in many environments;
-
Self-sustaining biosensors for the pollutant collection;
-
Reliable samplers of pollutants, as the bees can fly for more than 3 km around a barycentre (the hive), exploring flowers, vegetation, water, and air for a maximum of three weeks.
-
No environmental impact.
-
Simultaneous collection of a wide range of pollutants during the foraging behaviour;
-
Collection of evidence for pollutants to enter the food chain (e.g., through honey or other edible bee products) and to expose pollinators to pollutant ingestion.
Pollution in Bees and Bee Products
- -
-
Agrochemicals
- -
-
Heavy metals
- -
-
Polycyclic aromatic hydrocarbons
- -
-
Radionuclides
- -
-
Dioxin, polychlorinated biphenyls,
- -
-
Particulate matter
4. Cultural Ecosystem Services and Bees
3.1. The Role of the Honey Bee in the Ecosystem of the Symbolic Tradition
4.1. The Role of the Honey Bee in the Ecosystem of the Symbolic Tradition
3.2. The Values of Pollinator Habitats
4.2. The Values of Pollinator Habitats


References
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326.
- Földesi, R.; Howlett, B.G.; Grass, I.; Batáry, P. Larger pollinators deposit more pollen on stigmas across multiple plant species—A meta-analysis. J. Appl. Ecol. 2021, 58, 699–707.
- Garibaldi, L.A.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham, S.A.; Kremen, C.; Carvalheiro, L.G.; Harder, L.D.; Afik, O.; et al. Wild Pollinators Enhance Fruit Set of Crops Regardless of Honey Bee Abundance. Science 2013, 339, 1608–1611.
- Iwasaki, J.M.; Hogendoorn, K. How protection of honey bees can help and hinder bee conservation. Curr. Opin. Insect Sci. 2021, 46, 112–118.
- Requier, F.; Garnery, L.; Kohl, P.L.; Njovu, H.K.; Pirk, C.W.W.; Crewe, R.M.; Steffan-Dewenter, I. The Conservation of Native Honey Bees Is Crucial. Trends Ecol. Evol. 2019, 34, 789–798.
- Hung, K.-L.J.; Kingston, J.M.; Lee, A.; Holway, D.A.; Kohn, J.R. Non-native honey bees disproportionately dominate the most abundant floral resources in a biodiversity hotspot. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182901.
- Henry, M.; Rodet, G. The apiary influence range: A new paradigm for managing the cohabitation of honey bees and wild bee communities. Acta Oecologica 2020, 105, 103555.
- Fontana, P.; Costa, C.; Di Prisco, G.; Ruzzier, E.; Annoscia, D.; Battisti, A.; Caoduro, G.; Carpana, E.; Contessi, A.; Dal Lago, A.; et al. Appeal for biodiversity protection of native honey bee subspecies of Apis mellifera in Italy (San Michele all’Adige declaration). Bull. Insectology 2018, 71, 257–271.
- Hung, K.-L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172140.
- Mallinger, R.E.; Gaines-Day, H.R.; Gratton, C. Do managed bees have negative effects on wild bees?: A systematic review of the literature. PLoS ONE 2017, 12, e0189268.
- Birks, H.J.B.; Felde, V.A.; Bjune, A.E.; Grytnes, J.-A.; Seppä, H.; Giesecke, T. Does pollen-assemblage richness reflect floristic richness? A review of recent developments and future challenges. Rev. Palaeobot. Palynol. 2016, 228, 1–25.
- Galimberti, A.; De Mattia, F.; Bruni, I.; Scaccabarozzi, D.; Sandionigi, A.; Barbuto, M.; Casiraghi, M.; Labra, M. A DNA Barcoding Approach to Characterize Pollen Collected by Honeybees. PLoS ONE 2014, 9, e109363.
- Prosser, S.W.J.; Hebert, P.D.N. Rapid identification of the botanical and entomological sources of honey using DNA metabarcoding. Food Chem. 2017, 214, 183–191.
- Utzeri, V.J.; Schiavo, G.; Ribani, A.; Tinarelli, S.; Bertolini, F.; Bovo, S.; Fontanesi, L. Entomological signatures in honey: An environmental DNA metabarcoding approach can disclose information on plant-sucking insects in agricultural and forest landscapes. Sci. Rep. 2018, 8, 9996.
- Bell, K.L.; de Vere, N.; Keller, A.; Richardson, R.T.; Gous, A.; Burgess, K.S.; Brosi, B.J. Pollen DNA barcoding: Current applications and future prospects. Genome 2016, 59, 629–640.
- Milla, L.; Sniderman, K.; Lines, R.; Mousavi-Derazmahalleh, M.; Encinas-Viso, F. Pollen DNA metabarcoding identifies regional provenance and high plant diversity in Australian honey. Ecol. Evol. 2021, 11, 8683–8698.
- Tremblay, É.D.; Duceppe, M.; Thurston, G.B.; Gagnon, M.; Côté, M.; Bilodeau, G.J. High-resolution biomonitoring of plant pathogens and plant species using metabarcoding of pollen pellet contents collected from a honey bee hive. Environ. DNA 2019, 1, 155–175.
- Sáez, A.; Aizen, M.A.; Medici, S.; Viel, M.; Villalobos, E.; Negri, P. Bees increase crop yield in an alleged pollinator-independent almond variety. Sci. Rep. 2020, 10, 3177.
- Gonzalez, M.V.; Coque, M.; Herrero, M. Influence of pollination systems on fruit set and fruit quality in kiwifruit (Actinidia deliciosa). Ann. Appl. Biol. 1998, 132, 349–355.
- Sagwe, R.N.; Peters, M.K.; Dubois, T.; Steffan-Dewenter, I.; Lattorff, H.M.G. Pollinator supplementation mitigates pollination deficits in smallholder avocado (Persea americana Mill.) production systems in Kenya. Basic Appl. Ecol. 2021, 56, 392–400.
- Pardo, A.; Borges, P.A.V. Worldwide importance of insect pollination in apple orchards: A review. Agric. Ecosyst. Environ. 2020, 293, 106839.
- Hagen, L. Pretty Healthy Food: How and When Aesthetics Enhance Perceived Healthiness. J. Mark. 2021, 85, 129–145.
- Pilati, L.; Fontana, P.; Angeli, G. Commercial Pollination of Apple Orchards: Val di Non Case Study. In Modern Beekeeping—Bases for Sustainable Production; IntechOpen: London, UK, 2020.
- Delaplane, K.; Mayer, D. Crop Pollination by Bees; CABI: London, UK, 2000.
- Hall, M.A.; Jones, J.; Rocchetti, M.; Wright, D.; Rader, R. Bee Visitation and Fruit Quality in Berries Under Protected Cropping Vary Along the Length of Polytunnels. J. Econ. Entomol. 2020, 113, 1337–1346.
- Andrikopoulos, C.J.; Cane, J.H. Comparative Pollination Efficacies of Five Bee Species on Raspberry. J. Econ. Entomol. 2018, 111, 2513–2519.
- Hünicken, P.L.; Morales, C.L.; Aizen, M.A.; Anderson, G.K.S.; García, N.; Garibaldi, L.A. Insect pollination enhances yield stability in two pollinator-dependent crops. Agric. Ecosyst. Environ. 2021, 320, 107573.
- Cunningham, S.A.; Fournier, A.; Neave, M.J.; Le Feuvre, D. Improving spatial arrangement of honeybee colonies to avoid pollination shortfall and depressed fruit set. J. Appl. Ecol. 2016, 53, 350–359.
- Geslin, B.; Aizen, M.A.; Garcia, N.; Pereira, A.-J.; Vaissière, B.E.; Garibaldi, L.A. The impact of honey bee colony quality on crop yield and farmers’ profit in apples and pears. Agric. Ecosyst. Environ. 2017, 248, 153–161.
- Grant, K.J.; DeVetter, L.; Melathopoulos, A. Honey bee (Apis mellifera) colony strength and its effects on pollination and yield in highbush blueberries (Vaccinium corymbosum). PeerJ 2021, 9, e11634.
- Layek, U.; Kundu, A.; Bisui, S.; Karmakar, P. Impact of managed stingless bee and western honey bee colonies on native pollinators and yield of watermelon: A comparative study. Ann. Agric. Sci. 2021, 66, 38–45.
- Bushmann, S.L.; Drummond, F.A. Analysis of Pollination Services Provided by Wild and Managed Bees (Apoidea) in Wild Blueberry (Vaccinium angustifolium Aiton) Production in Maine, USA, with a Literature Review. Agronomy 2020, 10, 1413.
- Garibaldi, L.A.; Schulte, L.A.; Nabaes Jodar, D.N.; Gomez Carella, D.S.; Kremen, C. Time to Integrate Pollinator Science into Soybean Production. Trends Ecol. Evol. 2021, 36, 573–575.
- Walters, S.A.; Taylor, B.H. Effects of Honey Bee Pollination on Pumpkin Fruit and Seed Yield. HortScience 2006, 41, 370–373.
- Cunningham, S.A.; Le Feuvre, D. Significant yield benefits from honeybee pollination of faba bean (Vicia faba) assessed at field scale. Field Crops Res. 2013, 149, 269–275.
- Abbasi, K.H.; Jamal, M.; Ahmad, S.; Ghramh, H.A.; Khanum, S.; Khan, K.A.; Ullah, M.A.; Aljedani, D.M.; Zulfiqar, B. Standardization of managed honey bee (Apis mellifera) hives for pollination of Sunflower (Helianthus annuus) crop. J. King Saud Univ.—Sci. 2021, 33, 101608.
- Rizzardo, R.A.G.; Milfont, M.O.; da Silva, E.M.S.; Freitas, B.M. Apis mellifera pollination improves agronomic productivity of anemophilous castor bean (Ricinus communis). An. Acad. Bras. Ciências 2012, 84, 1137–1145.
- Romero, M.J.; Quezada-Euán, J.J.G. Pollinators in biofuel agricultural systems: The diversity and performance of bees (Hymenoptera: apoidea) on Jatropha curcas in Mexico. Apidologie 2013, 44, 419–429.
- Chauzat, M.-P.; Cauquil, L.; Roy, L.; Franco, S.; Hendrikx, P.; Ribière-Chabert, M. Demographics of the European Apicultural Industry. PLoS ONE 2013, 8, e79018.
- Svečnjak, L.; Chesson, L.A.; Gallina, A.; Maia, M.; Martinello, M.; Mutinelli, F.; Muz, M.N.; Nunes, F.M.; Saucy, F.; Tipple, B.J.; et al. Standard methods for Apis mellifera beeswax research. J. Apic. Res. 2019, 58, 1–108.
- Fratini, F.; Cilia, G.; Turchi, B.; Felicioli, A. Beeswax: A minireview of its antimicrobial activity and its application in medicine. Asian Pac. J. Trop. Med. 2016, 9, 839–843.
- Luo, X.; Dong, Y.; Gu, C.; Zhang, X.; Ma, H. Processing Technologies for Bee Products: An Overview of Recent Developments and Perspectives. Front. Nutr. 2021, 8, 727181.
- Sano, K.; Arrighi, S.; Stani, C.; Aureli, D.; Boschin, F.; Fiore, I.; Spagnolo, V.; Ricci, S.; Crezzini, J.; Boscato, P.; et al. The earliest evidence for mechanically delivered projectile weapons in Europe. Nat. Ecol. Evol. 2019, 3, 1409–1414.
- La Nasa, J.; Nardella, F.; Andrei, L.; Giani, M.; Degano, I.; Colombini, M.P.; Ribechini, E. Profiling of high molecular weight esters by flow injection analysis-high resolution mass spectrometry for the characterization of raw and archaeological beeswax and resinous substances. Talanta 2020, 212, 120800.
- Stacey, R.J. The composition of some Roman medicines: Evidence for Pliny’s Punic wax? Anal. Bioanal. Chem. 2011, 401, 1749–1759.
- Kramberger, B.; Berthold, C.; Spiteri, C. Fifth millennium BC miniature ceramic bottles from the south-eastern Prealps and Central Balkans: A multi-disciplinary approach to study their content and function. J. Archaeol. Sci. Rep. 2021, 38, 102993.
- Hao, P.; Lou, X.; Tang, L.; Wang, F.; Chong, Z.; Guo, L. Solvent-free fabrication of slippery coatings from edible raw materials for reducing yogurt adhesion. Prog. Org. Coat. 2022, 162, 106590.
- Sarkisyan, V.; Sobolev, R.; Frolova, Y.; Malinkin, A.; Makarenko, M.; Kochetkova, A. Beeswax Fractions Used as Potential Oil Gelling Agents. J. Am. Oil Chem. Soc. 2021, 98, 281–296.
- Damodaran, T. Propolis. In Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2021; pp. 795–812.
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical Aspects of Propolis Research in Modern Times. Evid.-Based Complement. Altern. Med. 2013, 2013, 964149.
- Ghisalberti, E.L. Propolis: A Review. Bee World 1979, 60, 59–84.
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363.
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703.
- Marcucci, M.C. Propolis: Chemical composition, biological properties and therapeutic activity. Apidologie 1995, 26, 83–99.
- de Castro, S.L. Propolis: Biological and Pharmacological Activities. Therapeutic Uses of This Bee-product. Annu. Rev. Biomed. Sci. 2001, 3, 49–83.
- Boudourova-Krasteva, G.; Bankova, V.; Sforcin, J.M.; Nikolova, N.; Popov, S. Phenolics from Brazilian Propolis. Z. Nat. C 1997, 52, 676–679.
- Bankova, V.; Boudourova-Krasteva, G.; Popov, S.; Sforcin, J.M.; Funari, S.R.C. Seasonal Variations in Essential Oil from Brazilian Propolis. J. Essent. Oil Res. 1998, 10, 693–696.
- Bankova, V.; Boudourova-Krasteva, G.; Popov, S.; Sforcin, J.M.; Cunha Funari, S.R. Seasonal variations of the chemical composition of Brazilian propolis. Apidologie 1998, 29, 361–367.
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxidative Med. Cell. Longev. 2017, 2017, 1259510.
- Orsi, R.O.; Sforcin, J.M.; Funari, S.R.C.; Bankova, V. Effects of Brazilian and Bulgarian propolis on bactericidal activity of macrophages against Salmonella Typhimurium. Int. Immunopharmacol. 2005, 5, 359–368.
- Orsi, R.D.O.; Sforcin, J.M.; Funari, S.R.C.; Fernandes Junior, A.; Bankova, V. Synergistic effect of propolis and antibiotics on the Salmonella Typhi. Braz. J. Microbiol. 2006, 37, 108–112.
- Scazzocchio, F.; D’Auria, F.D.; Alessandrini, D.; Pantanella, F. Multifactorial aspects of antimicrobial activity of propolis. Microbiol. Res. 2006, 161, 327–333.
- Nandre, V.S.; Bagade, A.V.; Kasote, D.M.; Lee, J.H.J.; Kodam, K.M.; Kulkarni, M.V.; Ahmad, A. Antibacterial activity of Indian propolis and its lead compounds against multi-drug resistant clinical isolates. J. Herb. Med. 2021, 29, 100479.
- Gekker, G.; Hu, S.; Spivak, M.; Lokensgard, J.R.; Peterson, P.K. Anti-HIV-1 activity of propolis in CD4+ lymphocyte and microglial cell cultures. J. Ethnopharmacol. 2005, 102, 158–163.
- Liao, N.; Sun, L.; Wang, D.; Chen, L.; Wang, J.; Qi, X.; Zhang, H.; Tang, M.; Wu, G.; Chen, J.; et al. Antiviral properties of propolis ethanol extract against norovirus and its application in fresh juices. LWT 2021, 152, 112169.
- Sforcin, J.M.; Fernandes Júnior, A.; Lopes, C.A.M.; Funari, S.R.C.; Bankova, V. Seasonal effect of brazilian propolis on Candida albicans and Candida tropicalis. J. Venom. Anim. Toxins 2001, 7, 139–144.
- Ibrahim, M.E.E.-D.; Alqurashi, R.M. Anti-fungal and antioxidant properties of propolis (bee glue) extracts. Int. J. Food Microbiol. 2022, 361, 109463.
- Salomao, K.; Dantas, A.P.; Borba, C.M.; Campos, L.C.; Machado, D.G.; Aquino Neto, F.R.; Castro, S.L. Chemical composition and microbicidal activity of extracts from Brazilian and Bulgarian propolis. Lett. Appl. Microbiol. 2004, 38, 87–92.
- Freitas, S.F.; Shinohara, L.; Sforcin, J.M.; Guimarães, S. In vitro effects of propolis on Giardia duodenalis trophozoites. Phytomedicine 2006, 13, 170–175.
- Paula, L.A.; Cândido, A.C.B.B.; Santos, M.F.C.; Caffrey, C.R.; Bastos, J.K.; Ambrósio, S.R.; Magalhães, L.G. Antiparasitic Properties of Propolis Extracts and Their Compounds. Chem. Biodivers. 2021, 18, e2100310.
- de Groot, A.C. Propolis. Dermatitis 2013, 24, 263–282.
- da Silva Barboza, A.; Aitken-Saavedra, J.P.; Ferreira, M.L.; Fábio Aranha, A.M.; Lund, R.G. Are propolis extracts potential pharmacological agents in human oral health?—A scoping review and technology prospecting. J. Ethnopharmacol. 2021, 271, 113846.
- Banskota, A.H.; Tezuka, Y.; Kadota, S. Recent progress in pharmacological research of propolis. Phytother. Res. 2001, 15, 561–571.
- Gheflati, A.; Dehnavi, Z.; Ghannadzadeh Yazdi, A.; Khorasanchi, Z.; Raeisi-Dehkordi, H.; Ranjbar, G. The effects of propolis supplementation on metabolic parameters: A systematic review and meta-analysis of randomized controlled clinical trials. Avicenna J. Phytomed. 2021, 11, 551–565.
- Souto, E.B.; Silva, G.F.; Dias-Ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part I—Clinical Trials Legislation and Good Manufacturing Practices (GMP) of Nanotherapeutics in the EU. Pharmaceutics 2020, 12, 146.
- Souto, E.B.; Silva, G.F.; Dias-Ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part II—Production Scales and Clinically Compliant Production Methods. Nanomaterials 2020, 10, 455.
- Botteon, C.E.A.; Silva, L.B.; Ccana-Ccapatinta, G.V.; Silva, T.S.; Ambrosio, S.R.; Veneziani, R.C.S.; Bastos, J.K.; Marcato, P.D. Biosynthesis and characterization of gold nanoparticles using Brazilian red propolis and evaluation of its antimicrobial and anticancer activities. Sci. Rep. 2021, 11, 1974.
- Kamakura, M. Royalactin induces queen differentiation in honeybees. Nature 2011, 473, 478–483.
- Melliou, E.; Chinou, I. Chemistry and Bioactivities of Royal Jelly. J. Agric. Food. Chem. 2005, 53, 261–290.
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. Int. J. Mol. Sci. 2020, 21, 382.
- Uversky, V.N.; Albar, A.H.; Khan, R.H.; Redwan, E.M. Multifunctionality and intrinsic disorder of royal jelly proteome. Proteomics 2021, 21, 2000237.
- Melliou, E.; Chinou, I. Chemistry and Bioactivity of Royal Jelly from Greece. J. Agric. Food Chem. 2005, 53, 8987–8992.
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Foods 2012, 4, 39–52.
- Guo, J.; Wang, Z.; Chen, Y.; Cao, J.; Tian, W.; Ma, B.; Dong, Y. Active components and biological functions of royal jelly. J. Funct. Foods 2021, 82, 104514.
- Baracchi, D.; Francese, S.; Turillazzi, S. Beyond the antipredatory defence: Honey bee venom function as a component of social immunity. Toxicon 2011, 58, 550–557.
- Scaccabarozzi, D.; Dods, K.; Le, T.T.; Gummer, J.P.A.; Lussu, M.; Milne, L.; Campbell, T.; Wafujian, B.P.; Priddis, C. Factors driving the compositional diversity of Apis mellifera bee venom from a Corymbia calophylla (marri) ecosystem, Southwestern Australia. PLoS ONE 2021, 16, e0253838.
- Hider, R.C. Honeybee venom: A rich source of pharmacologically active peptides. Endeavour 1988, 12, 60–65.
- de Lima, P.R.; Brochetto-Braga, M.R. Hymenoptera venom review focusing on Apis mellifera. J. Venom. Anim. Toxins Incl. Trop. Dis. 2003, 9, 149–162.
- Chen, J.; Guan, S.M.; Sun, W.; Fu, H. Melittin, the Major Pain-Producing Substance of Bee Venom. Neurosci. Bull. 2016, 32, 265–272.
- Silva, J.; Monge-Fuentes, V.; Gomes, F.; Lopes, K.; dos Anjos, L.; Campos, G.; Arenas, C.; Biolchi, A.; Gonçalves, J.; Galante, P.; et al. Pharmacological alternatives for the treatment of neurodegenerative disorders: Wasp and bee venoms and their components as new neuroactive tools. Toxins 2015, 7, 3179–3209.
- Son, D.; Lee, J.; Lee, Y.; Song, H.; Lee, C.; Hong, J. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol. Ther. 2007, 115, 246–270.
- Park, J.H.; Jeong, Y.-J.; Park, K.-K.; Cho, H.-J.; Chung, I.-K.; Min, K.-S.; Kim, M.; Lee, K.-G.; Yeo, J.-H.; Park, K.-K.; et al. Melittin suppresses PMA-induced tumor cell invasion by inhibiting NF-κB and AP-1-dependent MMP-9 expression. Mol. Cells 2010, 29, 209–215.
- Zhang, S.; Liu, Y.; Ye, Y.; Wang, X.R.; Lin, L.T.; Xiao, L.Y.; Zhou, P.; Shi, G.X.; Liu, C.Z. Bee venom therapy: Potential mechanisms and therapeutic applications. Toxicon 2018, 148, 64–73.
- Khalil, W.K.B.; Assaf, N.; ElShebiney, S.A.; Salem, N.A. Neuroprotective effects of bee venom acupuncture therapy against rotenone-induced oxidative stress and apoptosis. Neurochem. Int. 2015, 80, 79–86.
- Chung, E.S.; Kim, H.; Lee, G.; Park, S.; Kim, H.; Bae, H. Neuro-protective effects of bee venom by suppression of neuroinflammatory responses in a mouse model of Parkinson’s disease: Role of regulatory T cells. Brain Behav. Immun. 2012, 26, 1322–1330.
- Mirshafiey, A. Venom therapy in multiple sclerosis. Neuropharmacology 2007, 53, 353–361.
- Bromenshenk, J.J.; Carlson, S.R.; Simpson, J.C.; Thomas, J.M. Pollution Monitoring of Puget Sound with Honey Bees. Science 1985, 227, 632–634.
- Markert, B.A.; Breure, A.M.; Zechmeister, H.G. Bioindicators and Biomonitors; Elsevier: Amsterdam, The Netherlands, 2003.
- Parmar, T.K.; Rawtani, D.; Agrawal, Y.K. Bioindicators: The natural indicator of environmental pollution. Front. Life Sci. 2016, 9, 110–118.
- Lambert, O.; Piroux, M.; Puyo, S.; Thorin, C.; Larhantec, M.; Delbac, F.; Pouliquen, H. Bees, honey and pollen as sentinels for lead environmental contamination. Environ. Pollut. 2012, 170, 254–259.
- Bargańska, Ż.; Ślebioda, M.; Namieśnik, J. Honey bees and their products: Bioindicators of environmental contamination. Crit. Rev. Environ. Sci. Technol. 2016, 46, 235–248.
- Porrini, C.; Sabatini, A.G.; Girotti, S.; Ghini, S.; Medrzycki, P.; Grillenzoni, F.; Bortolotti, L.; Gattavecchia, E.; Celli, G. Honey bees and bee products as monitors of the environmental contamination. Apiacta 2003, 38, 63–70.
- Girotti, S.; Ghini, S.; Maiolini, E.; Bolelli, L.; Ferri, E.N. Trace analysis of pollutants by use of honeybees, immunoassays, and chemiluminescence detection. Anal. Bioanal. Chem. 2013, 405, 555–571.
- Horn, U.; Helbig, M.; Molzahn, D.; Hentschel, E.J. Transfer von 226 Ra in den Honig und die mögliche Nutzung der Honigbiene (Apis meilifera) als Bioindikator im radioaktiv belasteten Uranabbaugebiet der Wismut. Apidologie 1996, 27, 211–217.
- Amorena, M.; Visciano, P.; Giacomelli, A.; Marinelli, E.; Sabatini, A.G.; Medrzycki, P.; Oddo, L.P.; De Pace, F.M.; Belligoli, P.; Di Serafino, G.; et al. Monitoring of levels of polycyclic aromatic hydrocarbons in bees caught from beekeeping: Remark 1. Vet. Res. Commun. 2009, 33, 165–167.
- Mohr, S.; García-Bermejo, Á.; Herrero, L.; Gómara, B.; Costabeber, I.H.; González, M.J. Levels of brominated flame retardants (BFRs) in honey samples from different geographic regions. Sci. Total Environ. 2014, 472, 741–745.
- Edo, C.; Fernández-Alba, A.R.; Vejsnæs, F.; van der Steen, J.J.M.; Fernández-Piñas, F.; Rosal, R. Honeybees as active samplers for microplastics. Sci. Total Environ. 2021, 767, 144481.
- Dively, G.P.; Kamel, A. Insecticide Residues in Pollen and Nectar of a Cucurbit Crop and Their Potential Exposure to Pollinators. J. Agric. Food Chem. 2012, 60, 4449–4456.
- Martinello, M.; Manzinello, C.; Dainese, N.; Giuliato, I.; Gallina, A.; Mutinelli, F. The Honey Bee: An Active Biosampler of Environmental Pollution and a Possible Warning Biomarker for Human Health. Appl. Sci. 2021, 11, 6481.
- Perugini, M.; Manera, M.; Grotta, L.; Abete, M.C.; Tarasco, R.; Amorena, M. Heavy Metal (Hg, Cr, Cd, and Pb) Contamination in Urban Areas and Wildlife Reserves: Honeybees as Bioindicators. Biol. Trace Elem. Res. 2011, 140, 170–176.
- Couvillon, M.J.; Ratnieks, F.L.W. Environmental consultancy: Dancing bee bioindicators to evaluate landscape “health”. Front. Ecol. Evol. 2015, 3, 44.
- Negri, I.; Mavris, C.; Di Prisco, G.; Caprio, E.; Pellecchia, M. Honey bees (Apis mellifera, L.) as active samplers of airborne particulate matter. PLoS ONE 2015, 10, e0132491.
- Pellecchia, M.; Negri, I. Particulate matter collection by honey bees (Apis mellifera, L.) near to a cement factory in Italy. PeerJ 2018, 2018, e5322.
- Papa, G.; Capitani, G.; Capri, E.; Pellecchia, M.; Negri, I. Vehicle-derived ultrafine particulate contaminating bees and bee products. Sci. Total Environ. 2020, 750, 141700.
- Capitani, G.; Papa, G.; Pellecchia, M.; Negri, I. Disentangling multiple PM emission sources in the Po Valley (Italy) using honey bees. Heliyon 2021, 7, e06194.
- Zaric, N.M.; Deljanin, I.; Ilijević, K.; Stanisavljević, L.; Ristić, M.; Gržetić, I. Assessment of spatial and temporal variations in trace element concentrations using honeybees (Apis mellifera) as bioindicators. PeerJ 2018, 6, e5197.
- Leita, L.; Muhlbachova, G.; Cesco, S.; Barbattini, R.; Mondini, C. Investigation of the use of honey bees and honey bee products to assess heavy metals contamination. Environ. Monit. Assess. 1996, 43, 1–9.
- González-Alcaraz, M.N.; Malheiro, C.; Cardoso, D.N.; Prodana, M.; Morgado, R.G.; van Gestel, C.A.M.; Loureiro, S. Bioaccumulation and Toxicity of Organic Chemicals in Terrestrial Invertebrates; Springer Science and Business Media Deutschland GmbH: Berlin Germany, 2020; pp. 149–189.
- de Oliveira, R.C.; do Nascimento Queiroz, S.C.; da Luz, C.F.; Porto, R.S.; Rath, S. Bee pollen as a bioindicator of environmental pesticide contamination. Chemosphere 2016, 163, 525–534.
- Zioga, E.; Kelly, R.; White, B.; Stout, J.C. Plant protection product residues in plant pollen and nectar: A review of current knowledge. Environ. Res. 2020, 189, 109873.
- Mahé, C.; Jumarie, C.; Boily, M. The countryside or the city: Which environment is better for the honeybee? Environ. Res. 2021, 195, 110784.
- Roman, A.; Majewska, B.; Pleban, E. Comparative study of selected toxic elements in propolis and honey. J. Apic. Sci. 2011, 55, 97–105.
- Goretti, E.; Pallottini, M.; Rossi, R.; La Porta, G.; Gardi, T.; Cenci Goga, B.T.; Elia, A.C.; Galletti, M.; Moroni, B.; Petroselli, C.; et al. Heavy metal bioaccumulation in honey bee matrix, an indicator to assess the contamination level in terrestrial environments. Environ. Pollut. 2020, 256, 113388.
- Reitmayer, C.M.; Ryalls, J.M.W.; Farthing, E.; Jackson, C.W.; Girling, R.D.; Newman, T.A. Acute exposure to diesel exhaust induces central nervous system stress and altered learning and memory in honey bees. Sci. Rep. 2019, 9, 5793.
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; Island Press, Ed.; Island Press/Center for Resource Economics: Washington, DC, USA, 2005; ISBN 9781597260404.
- Topal, E.; Adamchuk, L.; Negri, I.; Kösoğlu, M.; Papa, G.; Dârjan, M.S.; Cornea-Cipcigan, M.; Mărgăoan, R. Traces of Honeybees, Api-Tourism and Beekeeping: From Past to Present. Sustainability 2021, 13, 11659.
- Aryal, S.; Ghosh, S.; Jung, C. Ecosystem Services of Honey Bees; Regulating, Provisioning and Cultural Functions. J. Apic. 2020, 35, 119–128.
- Crane, E. The rock art of honey hunters. Bee World 2005, 86, 11–13.
- Carozza, G. La parola è più dolce del miele. Le api e il miele nella Bibbia e nella tradizione cristiana; Edizioni Messaggero Padova: Padova, Italy, 2019.
- Chevalier, J.; Gheerbrant, A. Dictionnaire des Symboles. Mythes, Rêves, Coutumes, Gestes, Formes, Figures, Couleurs, Nombres; Robert Laffont: Paris, France, 1969.
- Charbonneau-Lassay, L. The Bestiary of Chris; original ed.; Parabola Books: New York, NY, USA, 1991.
- Wratten, S.D.; Gillespie, M.; Decourtye, A.; Mader, E.; Desneux, N. Pollinator habitat enhancement: Benefits to other ecosystem services. Agric. Ecosyst. Environ. 2012, 159, 112–122.
- Fleury, P.; Seres, C.; Dobremez, L.; Nettier, B.; Pauthenet, Y. “Flowering Meadows”, a result-oriented agri-environmental measure: Technical and value changes in favour of biodiversity. Land Use Policy 2015, 46, 103–114.
- Bonnet, X.; Brischoux, F.; Pearson, D.; Rivalan, P. Beach rock as a keystone habitat for amphibious sea snakes. Environ. Conserv. 2009, 36, 62.
- Hitchman, S.M.; Mather, M.E.; Smith, J.M.; Fencl, J.S. Identifying keystone habitats with a mosaic approach can improve biodiversity conservation in disturbed ecosystems. Glob. Change Biol. 2018, 24, 308–321.
- Sikorska, D.; Ciężkowski, W.; Babańczyk, P.; Chormański, J.; Sikorski, P. Intended wilderness as a Nature-based Solution: Status, identification and management of urban spontaneous vegetation in cities. Urban For. Urban Green. 2021, 62, 127155.
- Kowarik, I. Urban wilderness: Supply, demand, and access. Urban For. Urban Green. 2018, 29, 336–347.
- Hutcheson, W.; Hoagland, P.; Jin, D. Valuing environmental education as a cultural ecosystem service at Hudson River Park. Ecosyst. Serv. 2018, 31, 387–394.
- Mocior, E.; Kruse, M. Educational values and services of ecosystems and landscapes—An overview. Ecol. Indic. 2016, 60, 137–151.
- van Dijk-Wesselius, J.E.; van den Berg, A.E.; Maas, J.; Hovinga, D. Green Schoolyards as Outdoor Learning Environments: Barriers and Solutions as Experienced by Primary School Teachers. Front. Psychol. 2020, 10, 2919.
- S2S—Erasmus Plus project From seed to spoon 2019-1-IT02-KA201-062392 Catching insect diversity with a sweep net 2020.
- Riolo, F. The social and environmental value of public urban food forests: The case study of the Picasso Food Forest in Parma, Italy. Urban For. Urban Green. 2019, 45, 126225.
