You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Juli Jing and Version 2 by Catherine Yang.
Meiosis is an essential cell-division process for ensuring genetic diversity across generations. Meiotic recombination ensures the accuracy of genetic interchange between homolous chromosomes and segregation of parental alleles. Programmed DNA double-strand breaks (DSBs), catalyzed by the evolutionarily conserved topoisomerase VIA (a subunit of the archaeal type II DNA topoisomerase)-like enzyme Spo11 and several other factors, is a distinctive feature of meiotic recombination initiation.
- meiosis
- double-strand break (DSB)
- homologous recombination
- chromosome
- hotspots
1. Introduction
In flowering plants, reproductive cells develop in the ‘sporophytic generation’ and then differentiate into the gamete-forming ‘gametophytic generation’ [1][2][1,2]. During the reproductive process, the production of gametes requires that the genetic complement be reduced by one-half [3][4][5][3,4,5]. This specialized nuclear division, called meiosis, includes one round of DNA replication followed by two successive rounds of cell division, thereby ensuring the ploidy of the zygotic genome [6][7][6,7]. In the first meiotic division (meiosis I), homologous chromosomes are brought in close to pair and undergo synapsis, promoting the reciprocal exchange of parental chromosome fragments and thereby increasing the genetic diversity of the progeny [7][8][9][7,8,9]. Meiotic recombination is initiated by the formation of programmed DNA double-strand breaks (DSBs) catalyzed by the evolutionarily conserved type II topoisomerase–like enzyme SPO11 and several accessary proteins [9][10][11][12][13][9,10,11,12,13]. After resection, two SPO11 molecules remain covalently bound to each 5′ end of the nicked DNA, which is then processed by the MRX complex (Mre11-Rad50-Xrs2) with the cooperation of Sae2 to release the SPO11-bound nicked DNA oligonucleotide [14][15][16][17][18][19][14,15,16,17,18,19]. Following the action of the 5′ to 3′ exonuclease Exo1, the DNA ends are further degraded to produce 3′ single-stranded DNA tails [9][20][21][22][9,20,21,22].
2. Defining Meiotic DSB Hotspots in Different Species
Meiotic DSBs are not randomly distributed along eukaryotic chromosomes; rather, they are concentrated within discrete regions described as DSB hotspots [23][92]. To generate a high-resolution physical map of the meiotic DSB landscape, several methodologies have been established over the past three decades. SPO11-oligo mapping is achieved by immunoprecipitating tagged-Spo11 bound with oligonucleotides, which subsequently go through end-labeling, purification and sequencing [24][98]. This method has been effectively applied in several yeast species, mouse and Arabidopsis (Table 12) [23][25][26][27][28][92,99,100,101,102]. SSDS takes advantage of an antibody specifically recognizing either one of two DNA recombinases, RAD51 or DMC1, and utilizes chromatin immunoprecipitation to enrich single-stranded DNA that has undergone single-end invasion. With the high-throughput sequencing of enriched single-stranded DNA, the genome-wide distribution of DSB hotpots was successfully obtained in maize, mouse and human [29][30][31][103,104,105] (Table 2).Table 12.
Meiotic DSB hotspots identified in different species by SPO11-oligo mapping or single-stranded DNA sequencing (SSDS).
| Species | Genome Size | Chromosome No. | Number of DSBs | DSB Hotspot No. | Most Common DSB Location |
Average Width (kb) | Predominantly DSB Formation Among Transposon |
Method | Hotspot Detection | References |
|---|---|---|---|---|---|---|---|---|---|---|
| S. cerevisiae (SK1) | 12.1 Mb | 16 | ~175 | 3604–4099 | Gene promoters | 0.248–0.264 | Ty retrotransposons | SPO11-oligos | Enrichment threshold | [23][27][32][33][92,101,110,111] |
| S. cerevisiae (YPS128) | 12.1 Mb | 16 | ~175 | 4177 | Gene promoters | 0.265 | n/a | SPO11-oligos | Enrichment threshold | [34][112] |
| S. cerevisiae (UWOPS03-461.4) | 12.1 Mb | 16 | ~175 | 3881 | Gene promoters | 0.256 | n/a | SPO11-oligos | Enrichment threshold | [34][112] |
| S. pombe | 13.8 Mb | 3 | ~60 | 603 | All chromosome regions | 1.4 | n/a | Rec12-oligos | Enrichment threshold | [35][113] |
| M. musculus (9R×13R) | 2.8 Gb | 20 | ~250 | 9874– 15,677 |
Intergenic | ~2.000–3.400 | LTR retrotransposons SINE |
SSDS | Peak calling | [29][30][103,104] |
| M. musculus (9R) | 2.8 Gb | 20 | ~250 | 14,869 | Intergenic | ~2.000 | n/a | SSDS | Peak calling | [30][104] |
| M. musculus (13R) | 2.8 Gb | 20 | ~250 | 15,481 | Intergenic | ~2.000 | n/a | SSDS | Peak calling | [30][104] |
| M. musculus (B6) |
2.8 Gb | 20 | ~250 | 18,313 | Intergenic | ~2.000 | n/a | SSDS | Peak calling | [30][104] |
| M. musculus (B6) |
2.8 Gb | 20 | ~250 | 13,960 | Intergenic | ~0.281 | n/a | SPO11-oligos | Enrichment threshold | [26][100] |
| Arabidopsis thaliana | 135 Mb | 5 | ~250–300 | 5914 | Gene promoters and terminators | 0.823 | Helitron /Pogo/Tc1/Mariner DNA transposons | SPO11-1-oligos | Peak calling | [25][99] |
| Zea mays | 2.4 Gb | 10 | ~500 | 3126 | All chromosome regions | 1.2 | Gypsy retrotransposons | SSDS | Peak calling | [31][105] |
3. Control of Meiotic DSB Formation by Protein Phosphorylation
3.1. Cyclin-Dependent Kinases (CDKs)
CDKs in conjunction with their cyclin partners represent an ancient molecular switch that promotes and regulates cell-cycle progression [4][40][41][4,114,115]. The fundamental theme of how CDKs mediate meiotic recombination initiation was mostly drawn from studies in yeast. In S. cerevisiae, the activation of Cdc28 by its two B-type cyclin partners, Clb5 and Clb6, stimulates the phosphorylation on Mer2 during pre-meiotic DNA replication (Figure 1A) [41][42][43][44][45][115,116,117,118,119]; subsequently, phosphorylated Mer2 recruits other Spo11-accessary proteins to initiate DSB formation (Figure 1B) [46][47][48][45,120,121]. Similarly, in S. pombe, the association between Cdc2 and any of the three cyclins, Crs1, Cig1 and Cig2, is crucial for DSB formation [40][114].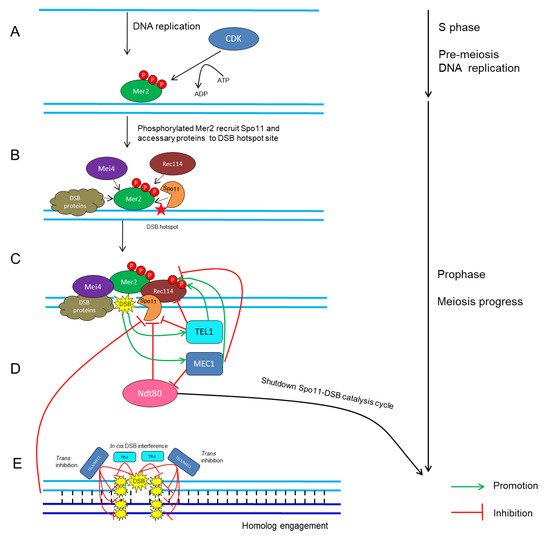
Figure 1. Schematic network of CDK- and ATM/ATR-mediated regulatory cycles of meiotic DSB timing and number in S. cerevisiae. (A) CDK phosphorylates Mer2 during pre-meiotic DNA replication [40][41][47][114,115,120]. (B) Phosphorylated Mer2 recruits Rec114, Mei4, Spo11 and other DSB proteins to DSB hotspot sites [46][47][48][45,120,121]. (C) DSB formation catalyzed by Spo11 and accessary proteins [4][49][4,66]. (D) Recurrent DSB formation activates TEL1/MEC1-dependent positive- and negative-feedback loops, which then restrains Spo11 activity and regulates the rate and number of DSB formation [50][51][52][53][136,140,147,156]. (E) Cis DSB interference mediated by TEL1 reduces the frequency of coincident DSB formation at the region adjacent to an already-formed DSB [54][55][143,149]. Trans inhibition mediated by TEL1 and MEC1 describes the ability of a DSB formation on one chromosome to suppress DSB formation on its homolog and sister chromatid at the same or adjacent regions [56][141].
