Bladder pathologies, very common in the aged population, have a considerable negative impact on quality of life. Novel targets are needed to design drugs and combinations to treat diseases such as overactive bladder and bladder cancers. A promising new target is the ubiquitous Rho GTPase Rac1, frequently dysregulated and overexpressed in bladder pathologies. Dysregulations of Rac signaling have been reported in atherosclerosis, neurodevelopmental disorders, rheumatic diseases, pulmonary hypertension and different types of cancers, including urothelial carcinoma. Protein Rac1 (RAS-related C3 botulinum toxin substrate (1) is considered a prime target to combat a variety of solid tumors and certain onco-hematological malignancies.
- bladder cancer
- Rho GTPase
- bladder dysfunction
- Rac inhibitors
- metastasis
- overactive bladder
- Rac1 protein
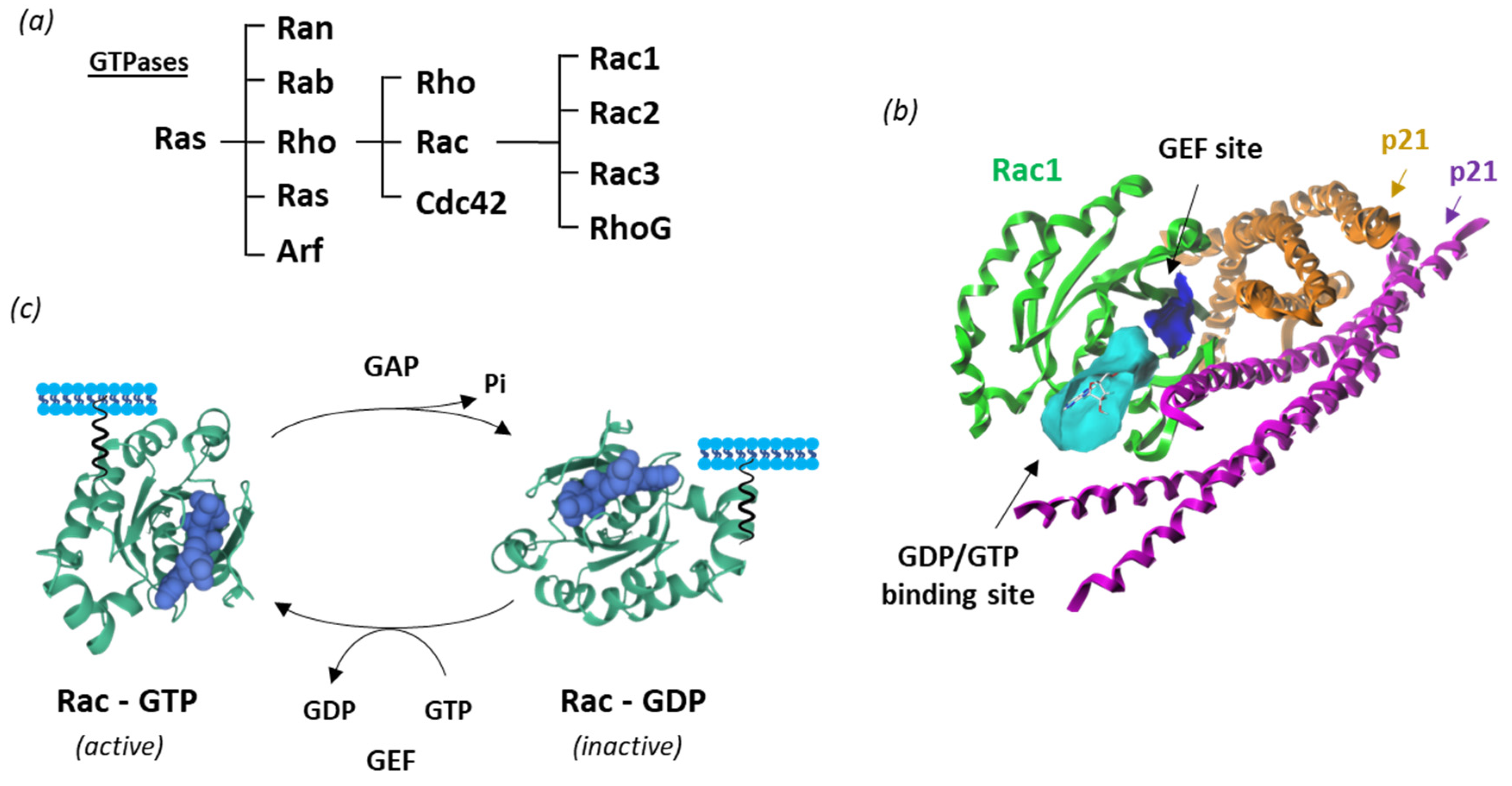
1. Rac1 Structure and Function
2. Rac1 in Non-Cancerous Bladder Pathologies
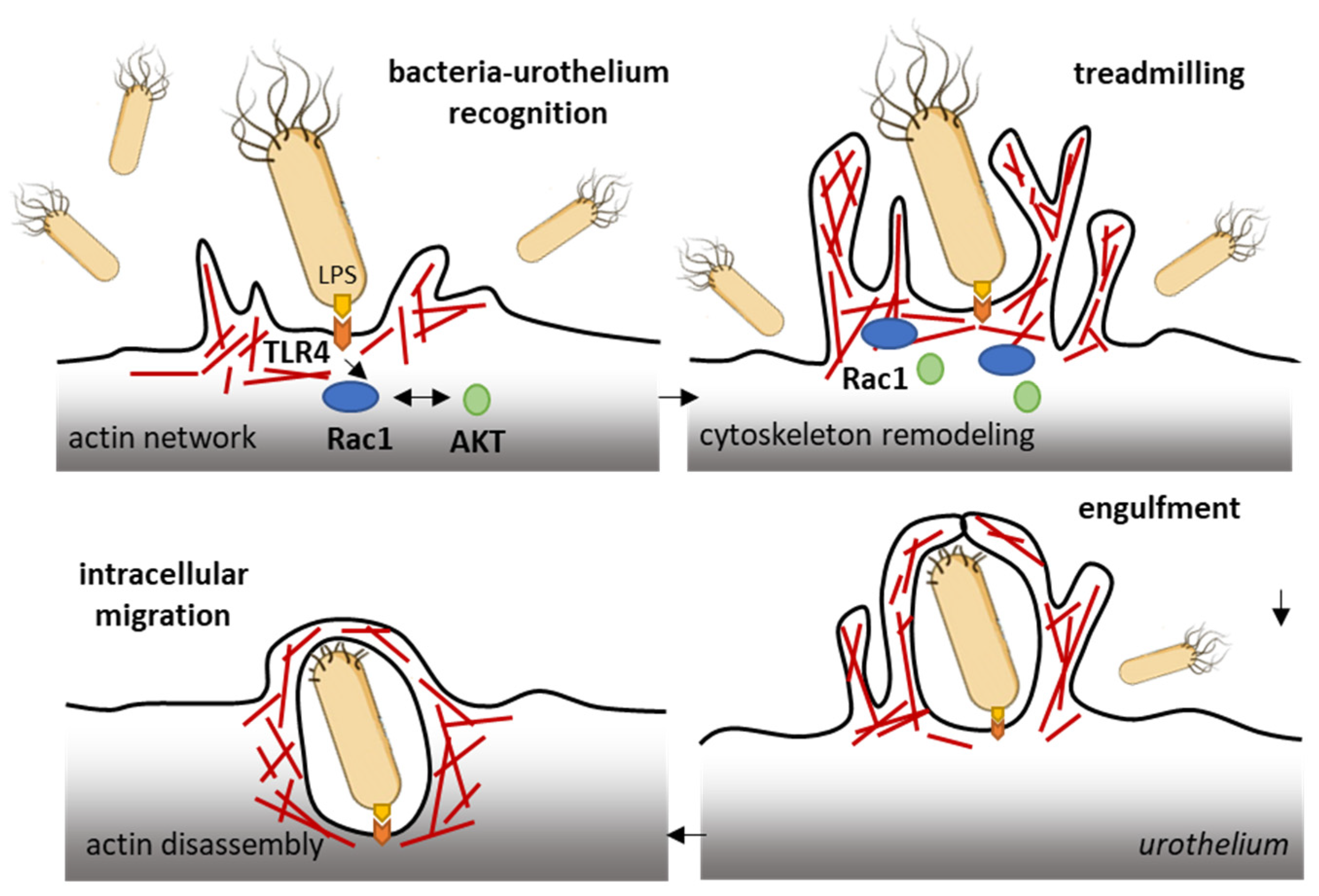
2.1. Rac1 and Bacterial Infections of the Bladder
2.2. Rac1 and Diabetes-Induced Bladder Dysfunctions
3. Rac1 in Bladder Cancer
3.1. Rac1 in Bladder Tumorigenesis
3.2. Rac1 in Bladder Cancer Cells Proliferation and Tumor Progression
3.3. Rac1 in Epithelial-Mesenchymal Transition (EMT) of Bladder Cancer Cells
3.4. Rac1 in Bladder Cancer Metastasis
References
- Kulhanek, K.R.; Roose, J.P.; Rubio, I. Regulation of the Small GTPase Ras and Its Relevance to Human Disease. Methods Mol. Biol. 2021, 2262, 19–43.
- Marei, H.; Malliri, A. GEFs: Dual regulation of Rac1 signaling. Small GTPases 2017, 8, 90–99.
- Mosaddeghzadeh, N.; Ahmadian, M.R. The RHO Family GTPases: Mechanisms of Regulation and Signaling. Cells 2021, 10, 1831.
- Bianchi-Smiraglia, A.; Wolff, D.W.; Marston, D.J.; Deng, Z.; Han, Z.; Moparthy, S.; Wombacher, R.M.; Mussell, A.L.; Shen, S.; Chen, J.; et al. Regulation of local GTP availability controls RAC1 activity and cell invasion. Nat. Commun. 2021, 12, 6091, Correction in Nat. Commun. 2021, 12, 6482.
- Abdrabou, A.; Wang, Z. Post-Translational Modification and Subcellular Distribution of Rac1: An Update. Cells 2018, 7, 263.
- Lam, B.D.; Hordijk, P.L. The Rac1 hypervariable region in targeting and signaling: A tail of many stories. Small GTPases 2013, 4, 78–89.
- Payapilly, A.; Malliri, A. Compartmentalisation of RAC1 signalling. Curr. Opin. Cell Biol. 2018, 54, 50–56.
- Maxwell, K.N.; Zhou, Y.; Hancock, J.F. Rac1 Nanoscale Organization on the Plasma Membrane Is Driven by Lipid Binding Specificity Encoded in the Membrane Anchor. Mol. Cell. Biol. 2018, 38, e00186-18.
- Killoran, R.C.; Smith, M.J. Conformational resolution of nucleotide cycling and effector interactions for multiple small GTPases determined in parallel. J. Biol. Chem. 2019, 294, 9937–9948.
- Sutton, S.S.; Magagnoli, J.; Cummings, T.; Hardin, J.W.; Love, B.L. Association between thiopurine exposure and depression in patients with inflammatory bowel disease and rheumatoid arthritis. J. Psychopharmacol. 2020, 34, 1163–1167.
- Sauzeau, V.; Beignet, J.; Vergoten, G.; Bailly, C. Overexpressed or hyperactivated Rac1 as a target to treat hepatocellular carcinoma. Pharmacol. Res. 2022, 179, 106220.
- Schnelzer, A.; Prechtel, D.; Knaus, U.; Dehne, K.; Gerhard, M.; Graeff, H.; Harbeck, N.; Schmitt, M.; Lengyel, E. Rac1 in human breast cancer: Overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene 2000, 19, 3013–3020.
- Casado-Medrano, V.; Baker, M.J.; Lopez-Haber, C.; Cooke, M.; Wang, S.; Caloca, M.J.; Kazanietz, M.G. The role of Rac in tumor susceptibility and disease progression: From biochemistry to the clinic. Biochem. Soc. Trans. 2018, 46, 1003–1012.
- Cannon, A.C.; Uribe-Alvarez, C.; Chernoff, J. RAC1 as a Therapeutic Target in Malignant Melanoma. Trends Cancer 2020, 6, 478–488.
- Uribe-Alvarez, C.; Guerrero-Rodríguez, S.L.; Rhodes, J.; Cannon, A.; Chernoff, J.; Araiza-Olivera, D. Targeting effector pathways in RAC1P29S-driven malignant melanoma. Small GTPases 2021, 12, 273–281.
- Acuner, S.E.; Sumbul, F.; Torun, H.; Haliloglu, T. Oncogenic mutations on Rac1 affect global intrinsic dynamics underlying GTP and PAK1 binding. Biophys. J. 2021, 120, 866–876.
- Colón-Bolea, P.; García-Gómez, R.; Casar, B. RAC1 Activation as a Potential Therapeutic Option in Metastatic Cutaneous Melanoma. Biomolecules 2021, 11, 1554.
- Hodge, R.G.; Schaefer, A.; Howard, S.V.; Der, C.J. RAS and RHO family GTPase mutations in cancer: Twin sons of different mothers? Crit. Rev. Biochem. Mol. Biol. 2020, 55, 386–407.
- Melzer, C.; Hass, R.; Lehnert, H.; Ungefroren, H. RAC1B: A Rho GTPase with Versatile Functions in Malignant Transformation and Tumor Progression. Cells 2019, 8, 21.
- Ungefroren, H.; Wellner, U.F.; Keck, T.; Lehnert, H.; Marquardt, J.U. The Small GTPase RAC1B: A Potent Negative Regulator of-and Useful Tool to Study-TGFbeta Signaling. Cancers 2020, 12, 3475.
- Martinez, J.J.; Hultgren, S.J. Requirement of Rho-family GTPases in the invasion of Type 1-piliated uropathogenic Escherichia coli. Cell. Microbiol. 2002, 4, 19–28.
- Montalbetti, N.; Dalghi, M.G.; Bastacky, S.I.; Clayton, D.R.; Ruiz, W.G.; Apodaca, G.; Carattino, M.D. Bladder infection with uropathogenic Escherichia coli increases the excitability of afferent neurons. Am. J. Physiol. Renal. Physiol. 2022, 322, F1–F13.
- Duncan, M.J.; Li, G.; Shin, J.S.; Carson, J.L.; Abraham, S.N. Bacterial penetration of bladder epithelium through lipid rafts. J. Biol. Chem. 2004, 279, 18944–18951.
- Song, J.; Bishop, B.L.; Li, G.; Duncan, M.J.; Abraham, S.N. TLR4-initiated and cAMP-mediated abrogation of bacterial invasion of the bladder. Cell Host Microbe 2007, 1, 287–298.
- Yin, Q.; Jiang, D.; Li, L.; Yang, Y.; Wu, P.; Luo, Y.; Yang, R.; Li, D. LPS Promotes Vascular Smooth Muscle Cells Proliferation through the TLR4/Rac1/Akt Signalling Pathway. Cell. Physiol. Biochem. 2017, 44, 2189–2200.
- Davis, J.M.; Rasmussen, S.B.; O’Brien, A.D. Cytotoxic necrotizing factor type 1 production by uropathogenic Escherichia coli modulates polymorphonuclear leukocyte function. Infect. Immun. 2005, 73, 5301–5310.
- Shen, X.F.; Teng, Y.; Sha, K.H.; Wang, X.Y.; Yang, X.L.; Guo, X.J.; Ren, L.B.; Wang, X.Y.; Li, J.; Huang, N. Dietary flavonoid luteolin attenuates uropathogenic Escherichia. Coli invasion of the urinary bladder. Biofactors 2016, 42, 674–685.
- Alaridah, N.; Lutay, N.; Tenland, E.; Rönnholm, A.; Hallgren, O.; Puthia, M.; Westergren-Thorsson, G.; Godaly, G. Mycobacteria Manipulate G-Protein-Coupled Receptors to Increase Mucosal Rac1 Expression in the Lungs. J. Innate Immun. 2017, 9, 318–329.
- Redelman-Sidi, G.; Iyer, G.; Solit, D.B.; Glickman, M.S. Oncogenic activation of Pak1-dependent pathway of macropinocytosis determines BCG entry into bladder cancer cells. Cancer Res. 2013, 73, 1156–1167.
- Wu, Y.; Li, C.; Riehle, A.; Pollmeier, B.; Gulbins, E.; Grassmé, H. Mycobacterial Infection is Promoted by Neutral Sphingomyelinase 2 Regulating a Signaling Cascade Leading to Activation of β1-Integrin. Cell. Physiol. Biochem. 2018, 51, 1815–1829.
- Palugan, L.; Cerea, M.; Cirilli, M.; Moutaharrik, S.; Maroni, A.; Zema, L.; Melocchi, A.; Uboldi, M.; Filippin, I.; Foppoli, A.; et al. Intravesical drug delivery approaches for improved therapy of urinary bladder diseases. Int. J. Pharm. X 2021, 3, 100100.
- Josephs-Spaulding, J.; Krogh, T.J.; Rettig, H.C.; Lyng, M.; Chkonia, M.; Waschina, S.; Graspeuntner, S.; Rupp, J.; Møller-Jensen, J.; Kaleta, C. Recurrent Urinary Tract Infections: Unraveling the Complicated Environment of Uncomplicated rUTIs. Front. Cell. Infect. Microbiol. 2021, 11, 562525.
- Bolgeo, T.; Maconi, A.; Bertolotti, M.; Roveta, A.; Betti, M.; Gatti, D.; Boccafoschi, C. Physiopathology of the diabetic bladder. Arch. Ital. Urol. Androl. 2020, 92, 314–317.
- Kirschner-Hermanns, R.; Daneshgari, F.; Vahabi, B.; Birder, L.; Oelke, M.; Chacko, S. Does diabetes mellitus-induced bladder remodeling affect lower urinary tract function? ICI-RS 2011. Neurourol. Urodyn. 2012, 31, 359–364.
- Lu, Y.; Tao, J. Diabetes Mellitus and Obesity as Risk Factors for Bladder Cancer Prognosis: A Systematic Review and Meta-Analysis. Front. Endocrinol. (Lausanne) 2021, 12, 699732.
- Yuan, Z.; Tang, Z.; He, C.; Tang, W. Diabetic cystopathy: A review. J. Diabetes. 2015, 7, 442–447, Erratum in J. Diabetes. 2016, 8, 170.
- Ying, C.; Zhou, Z.; Dai, J.; Wang, M.; Xiang, J.; Sun, D.; Zhou, X. Activation of the NLRP3 inflammasome by RAC1 mediates a new mechanism in diabetic nephropathy. Inflamm. Res. 2022, 71, 191–204.
- Poladia, D.P.; Bauer, J.A. Oxidant driven signaling pathways during diabetes: Role of Rac1 and modulation of protein kinase activity in mouse urinary bladder. Biochimie 2004, 86, 543–551.
- Evcim, A.S.; Micili, S.C.; Karaman, M.; Erbil, G.; Guneli, E.; Gidener, S.; Gumustekin, M. The Role of Rac1 on Carbachol-induced Contractile Activity in Detrusor Smooth Muscle from Streptozotocin-induced Diabetic Rats. Basic Clin. Pharmacol. Toxicol. 2015, 116, 476–484.
- Wu, T.; Chen, L.; Wei, T.; Wang, Y.; Xu, F.; Wang, K. Effect of cyclic hydrodynamic pressure-induced proliferation of human bladder smooth muscle through Ras-related C3 botulinum toxin substrate 1, mitogen-activated protein kinase kinase 1/2 and extracellular regulated protein kinases 1/2. Int. J. Urol. 2012, 19, 867–874.
- Rahman, A.; Davis, B.; Lövdahl, C.; Hanumaiah, V.T.; Feil, R.; Brakebusch, C.; Arner, A. The small GTPase Rac1 is required for smooth muscle contraction. J. Physiol. 2014, 592, 915–926.
- Wang, R.; Yu, Q.; Wang, X.; Li, B.; Ciotkowska, A.; Rutz, B.; Wang, Y.; Stief, C.G.; Hennenberg, M. Rac1 silencing, NSC23766 and EHT1864 reduce growth and actin organization of bladder smooth muscle cells. Life Sci. 2020, 261, 118468.
- Erdogan, B.R.; Liu, G.; Arioglu-Inan, E.; Michel, M.C. Established and emerging treatments for diabetes-associated lower urinary tract dysfunction. Naunyn-Schmiedebergs Arch. Pharmacol. 2022. Online ahead of print.
- Volanis, D.; Zaravinos, A.; Kadiyska, T.; Delakas, D.; Zoumpourlis, V.; Spandidos, D.A. Expression profile of Rho kinases in urinary bladder cancer. J. BUON 2011, 16, 511–521.
- Brait, M.; Munari, E.; LeBron, C.; Noordhuis, M.G.; Begum, S.; Michailidi, C.; Gonzalez-Roibon, N.; Maldonado, L.; Sen, T.; Guerrero-Preston, R.; et al. Genome-wide methylation profiling and the PI3K-AKT pathway analysis associated with smoking in urothelial cell carcinoma. Cell Cycle. 2013, 12, 1058–1070.
- Huang, K.; Chen, G.; Luo, J.; Zhang, Y.; Xu, G. Clinicopathological and cellular signature of PAK1 in human bladder cancer. Tumour Biol. 2015, 36, 2359–2368.
- Huang, K.; Chen, G.; Li, Y.; Liu, J.K.; Wang, Z.Y.; Zhou, G.C. . Zhonghua Yi Xue Za Zhi 2016, 96, 3227–3231.
- Chandrashekar, D.S.; Chakravarthi, B.V.S.K.; Robinson, A.D.; Anderson, J.C.; Agarwal, S.; Balasubramanya, S.A.H.; Eich, M.L.; Bajpai, A.K.; Davuluri, S.; Guru, M.S.; et al. Therapeutically actionable PAK4 is amplified, overexpressed, and involved in bladder cancer progression. Oncogene 2020, 39, 4077–4091.
- Kuroda, K.; Asano, T.; Horiguchi, A.; Ito, K. Effect of increased expression of both ras-related C3 botulinum toxin substrsate 1 and p21-activated kinase 1 in patients with N0M0 upper urinary tract urothelial carcinoma and cancer-free surgical margins. Jpn. J. Clin. Oncol. 2020, 50, 465–472.
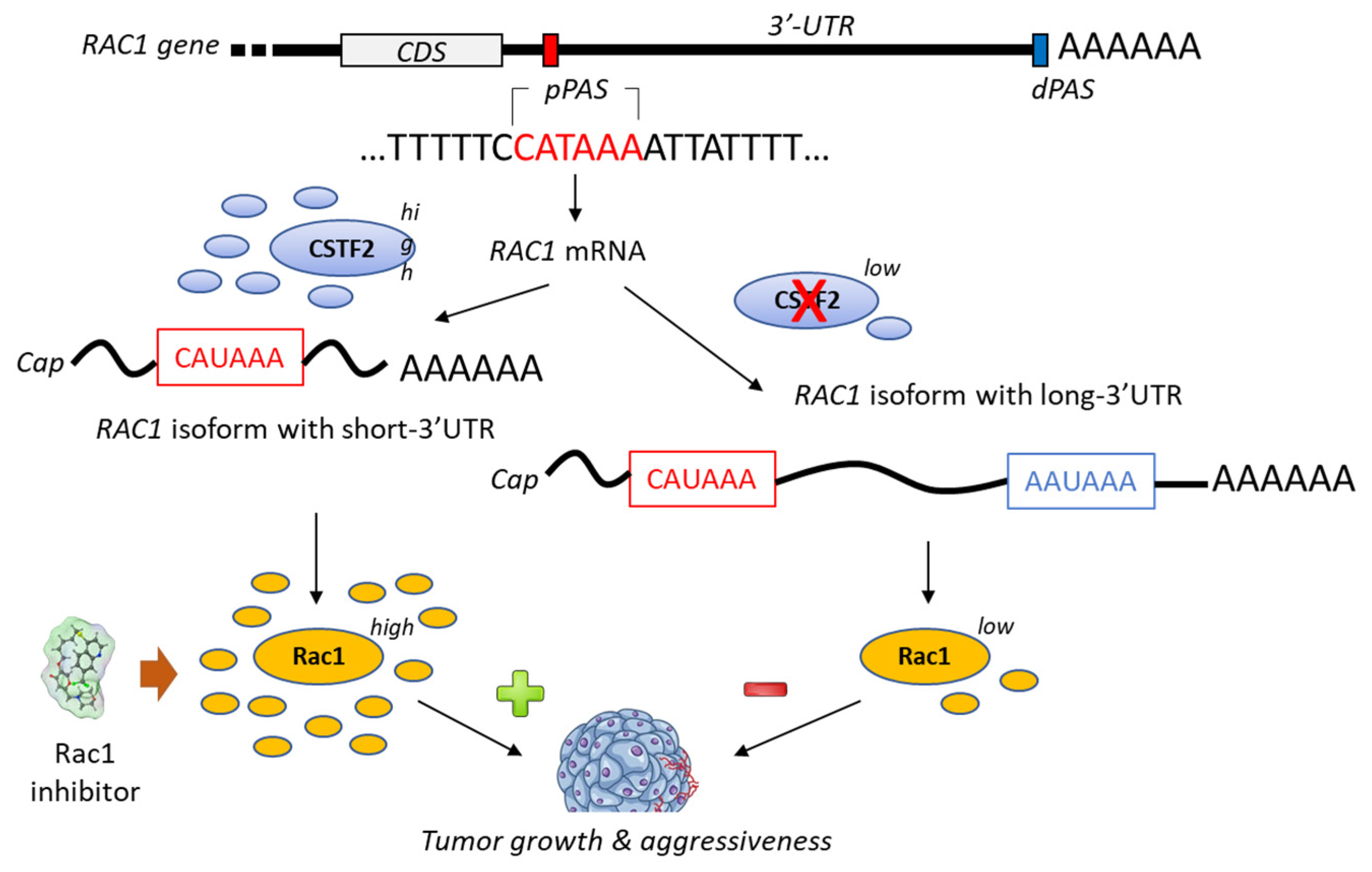
- Chen, X.; Zhang, J.X.; Luo, J.H.; Wu, S.; Yuan, G.J.; Ma, N.F.; Feng, Y.; Cai, M.Y.; Chen, R.X.; Lu, J.; et al. CSTF2-Induced Shortening of the RAC1 3’UTR Promotes the Pathogenesis of Urothelial Carcinoma of the Bladder. Cancer Res. 2018, 78, 5848–5862.
- Hu, X.; Xiang, L.; He, D.; Zhu, R.; Fang, J.; Wang, Z.; Cao, K. The long noncoding RNA KTN1-AS1 promotes bladder cancer tumorigenesis via KTN1 cis-activation and the consequent initiation of Rho GTPase-mediated signaling. Clin. Sci. 2021, 135, 555–574.
- De Conti, A.; Tryndyak, V.; Heidor, R.; Jimenez, L.; Moreno, F.S.; Beland, F.A.; Rusyn, I.; Pogribny, I.P. Butyrate-containing structured lipids inhibit RAC1 and epithelial-to-mesenchymal transition markers: A chemopreventive mechanism against hepatocarcinogenesis. J. Nutr. Biochem. 2020, 86, 108496.
- Yue, X.; Wu, F.; Li, Y.; Liu, J.; Boateng, M.; Mandava, K.; Zhang, C.; Feng, Z.; Gao, J.; Hu, W. Gain of function mutant p53 protein activates AKT through the Rac1 signaling to promote tumorigenesis. Cell Cycle 2020, 19, 1338–1351.
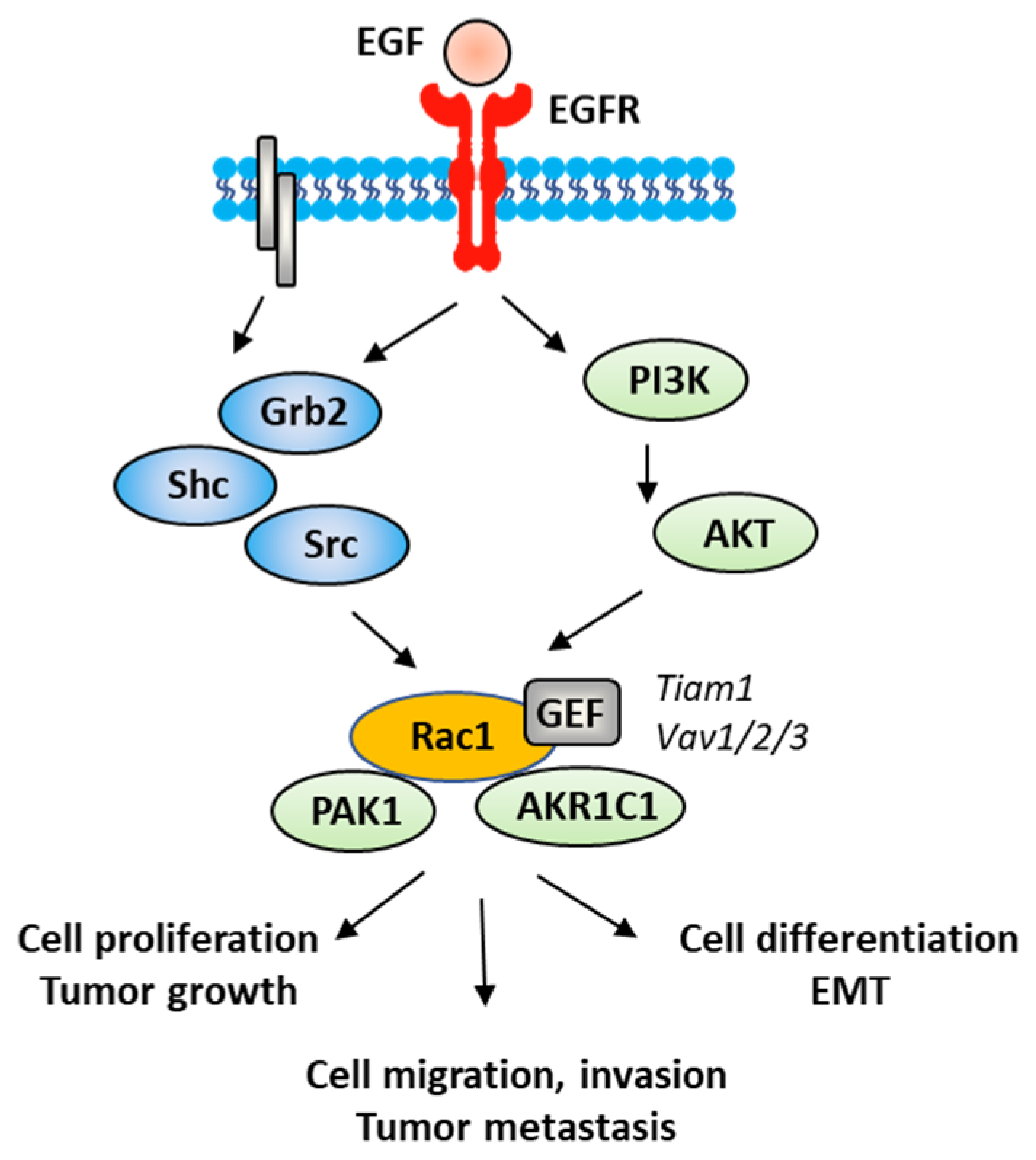
- Yin, H.; Zhang, C.; Wei, Z.; He, W.; Xu, N.; Xu, Y.; Li, T.; Ren, K.; Kuang, Y.; Zhu, X.; et al. EGF-induced nuclear translocation of SHCBP1 promotes bladder cancer progression through inhibiting RACGAP1-mediated RAC1 inactivation. Cell Death Dis. 2022, 13, 39.
- Rose, M.; Maurer, A.; Wirtz, J.; Bleilevens, A.; Waldmann, T.; Wenz, M.; Eyll, M.; Geelvink, M.; Gereitzig, M.; Rüchel, N.; et al. EGFR activity addiction facilitates anti-ERBB based combination treatment of squamous bladder cancer. Oncogene 2020, 39, 6856–6870, Correction in Oncogene 2021, 40, 1390.
- Railkar, R.; Krane, L.S.; Li, Q.Q.; Sanford, T.; Siddiqui, M.R.; Haines, D.; Vourganti, S.; Brancato, S.J.; Choyke, P.L.; Kobayashi, H.; et al. Epidermal Growth Factor Receptor (EGFR)-targeted Photoimmunotherapy (PIT) for the Treatment of EGFR-expressing Bladder Cancer. Mol. Cancer Ther. 2017, 16, 2201–2214.
- Nagaya, T.; Okuyama, S.; Ogata, F.; Maruoka, Y.; Knapp, D.W.; Karagiannis, S.N.; Fazekas-Singer, J.; Choyke, P.L.; LeBlanc, A.K.; Jensen-Jarolim, E.; et al. Near infrared photoimmunotherapy targeting bladder cancer with a canine anti-epidermal growth factor receptor (EGFR) antibody. Oncotarget 2018, 9, 19026–19038.
- Lee, S.W.; Commisso, C. Rac1 and EGFR cooperate to activate Pak in response to nutrient stress. Biochem. Biophys. Res. Commun. 2020, 533, 437–441.
- Yao, D.; Li, C.; Rajoka, M.S.R.; He, Z.; Huang, J.; Wang, J.; Zhang, J. P21-Activated Kinase 1: Emerging biological functions and potential therapeutic targets in Cancer. Theranostics 2020, 10, 9741–9766.
- Beier, I.; Düsing, R.; Vetter, H.; Schmitz, U. Epidermal growth factor stimulates Rac1 and p21-activated kinase in vascular smooth muscle cells. Atherosclerosis 2008, 196, 92–97.
- Li, W.Q.; Zhao, W.C.; Xin, J.; Niu, T.L.; Chao, Y.F.; Zhou, P.; Zheng, M.H.; Xu, B. MicroRNA-142-3p suppresses cell proliferation and migration in bladder cancer via Rac1. J. Biol. Regul. Homeost. Agents 2020, 34. Online ahead of print.
- Ashrafizadeh, M.; Hushmandi, K.; Hashemi, M.; Akbari, M.E.; Kubatka, P.; Raei, M.; Koklesova, L.; Shahinozzaman, M.; Mohammadinejad, R.; Najafi, M.; et al. Role of microRNA/Epithelial-to-Mesenchymal Transition Axis in the Metastasis of Bladder Cancer. Biomolecules. 2020, 10, 1159.
- Crosas-Molist, E.; Samain, R.; Kohlhammer, L.; Orgaz, J.L.; George, S.L.; Maiques, O.; Barcelo, J.; Sanz-Moreno, V. Rho GTPase signaling in cancer progression and dissemination. Physiol. Rev. 2022, 102, 455–510.
- Kang, J.; Zhong, Y.; Tian, W.; Li, J.; Li, X.; Zhai, L.; Hou, H.; Li, D. A novel anthraquinone-quinazoline hybrid 7B blocks breast cancer metastasis and EMT via targeting EGFR and Rac1. Int. J. Oncol. 2021, 58, 19.
- Li, X.; Jiang, F.; Wang, X.; Gu, X. SPAG9 regulates HEF1 expression and drives EMT in bladder transitional cell carcinoma via rac1 signaling pathway. Am. J. Cancer Res. 2018, 8, 2467–2480.
- Matsumoto, R.; Tsuda, M.; Yoshida, K.; Tanino, M.; Kimura, T.; Nishihara, H.; Abe, T.; Shinohara, N.; Nonomura, K.; Tanaka, S. Aldo-keto reductase 1C1 induced by interleukin-1beta mediates the invasive potential and drug resistance of metastatic bladder cancer cells. Sci. Rep. 2016, 6, 34625.
- Xia, L.; Lin, J.; Su, J.; Oyang, L.; Wang, H.; Tan, S.; Tang, Y.; Chen, X.; Liu, W.; Luo, X.; et al. Diallyl disulfide inhibits colon cancer metastasis by suppressing Rac1-mediated epithelial-mesenchymal transition. Onco Targets Ther. 2019, 12, 5713–5728.
- Tan, S.; Yi, P.; Wang, H.; Xia, L.; Han, Y.; Wang, H.; Zeng, B.; Tang, L.; Pan, Q.; Tian, Y.; et al. RAC1 Involves in the Radioresistance by Mediating Epithelial-Mesenchymal Transition in Lung Cancer. Front. Oncol. 2020, 10, 649, Erratum in Front. Oncol. 2020, 10, 1106.
- Kim, S.; Jang, Y.W.; Ku, Y.A.; Shin, Y.; Rahman, M.M.; Chung, M.H.; Kim, Y.H.; Kim, D.H. Investigating the Anti-Inflammatory Effects of RCI001 for Treating Ocular Surface Diseases: Insight Into the Mechanism of Action. Front. Immunol. 2022, 13, 850287.
- Liu, H.; Wang, W.; Shen, W.; Wang, L.; Zuo, Y. ARHGAP24 ameliorates inflammatory response through inactivating Rac1/Akt/NF-κB pathway in acute pneumonia model of rat. Ann. Transl. Med. 2020, 8, 1289.
- Shin, B.; Kupferman, J.; Schmidt, E.; Polleux, F.; Delany, A.M.; Lee, S.K. Rac1 Inhibition Via Srgap2 Restrains Inflammatory Osteoclastogenesis and Limits the Clastokine, SLIT3. J. Bone Miner. Res. 2020, 35, 789–800.
- Alves, A.; Diel, L.; Ramos, G.; Pinto, A.; Bernardi, L.; Yates, J.; Lamers, M. Tumor microenvironment and Oral Squamous Cell Carcinoma: A crosstalk between the inflammatory state and tumor cell migration. Oral Oncol. 2021, 112, 105038.
- Ibrahim, J.N.; Jounblat, R.; Jalkh, N.; Abou Ghoch, J.; Al Hageh, C.; Chouery, E.; Mégarbané, A.; Lecron, J.C.; Medlej-Hashim, M. RAC1 expression and role in IL-1β production and oxidative stress generation in familial Mediterranean fever (FMF) patients. Eur. Cytokine Netw. 2018, 29, 127–135.
- Shi, H.; Li, S.; Geng, Y.; Fan, H.; Zhang, R.; Zhang, Y.; Pan, J.; Song, G.; Ge, L.; Xie, T.; et al. Euphorbia factor L3 ameliorates rheumatoid arthritis by suppressing the inflammatory response by targeting Rac family small GTPase 1. Bioengineered 2022, 13, 10984–10997.
- Ciarlantini, M.S.; Barquero, A.; Bayo, J.; Wetzler, D.; Dodes Traian, M.M.; Bucci, H.A.; Fiore, E.J.; Gandolfi Donadío, L.; Defelipe, L.; Turjanski, A.; et al. Development of an Improved Guanidine-Based Rac1 Inhibitor with in vivo Activity against Non-Small Cell Lung Cancer. Chem. Med. Chem. 2021, 16, 1011–1021.
- Li, Y.; Zhao, M.; Guo, C.; Chu, H.; Li, W.; Chen, X.; Wang, X.; Li, Y.; Jia, Y.; Koussatidjoa, S.; et al. Intracellular mature IL-37 suppresses tumor metastasis via inhibiting Rac1 activation. Oncogene 2018, 37, 1095–1106.
- Pereira, J.F.S.; Bessa, C.; Matos, P.; Jordan, P. Pro-Inflammatory Cytokines Trigger the Overexpression of Tumour-Related Splice Variant RAC1B in Polarized Colorectal Cells. Cancers 2022, 14, 1393.
- Zinn, R.; Otterbein, H.; Lehnert, H.; Ungefroren, H. RAC1B: A Guardian of the Epithelial Phenotype and Protector against Epithelial-Mesenchymal Transition. Cells 2019, 8, 1569.
- De, P.; Aske, J.C.; Dey, N. RAC1 Takes the Lead in Solid Tumors. Cells 2019, 8, 382.
- Kotelevets, L.; Chastre, E. Rac1 Signaling: From Intestinal Homeostasis to Colorectal Cancer Metastasis. Cancers 2020, 12, 665.
- Liu, L.; Cui, J.; Zhao, Y.; Liu, X.; Chen, L.; Xia, Y.; Wang, Y.; Chen, S.; Sun, S.; Shi, B.; et al. KDM6A-ARHGDIB axis blocks metastasis of bladder cancer by inhibiting Rac1. Mol. Cancer 2021, 20, 77.
- Kamai, T.; Shirataki, H.; Nakanishi, K.; Furuya, N.; Kambara, T.; Abe, H.; Oyama, T.; Yoshida, K. Increased Rac1 activity and Pak1 overexpression are associated with lymphovascular invasion and lymph node metastasis of upper urinary tract cancer. BMC Cancer 2010, 10, 164.
- Liang, J.; Oyang, L.; Rao, S.; Han, Y.; Luo, X.; Yi, P.; Lin, J.; Xia, L.; Hu, J.; Tan, S.; et al. Rac1, A Potential Target for Tumor Therapy. Front. Oncol. 2021, 11, 674426.
- Zeng, R.J.; Zheng, C.W.; Chen, W.X.; Xu, L.Y.; Li, E.M. Rho GTPases in cancer radiotherapy and metastasis. Cancer Metastasis Rev. 2020, 39, 1245–1262.
- Abd El-Salam, M.A.; Smith, C.E.P.; Pan, C.X. Insights on recent innovations in bladder cancer immunotherapy. Cancer Cytopathol. 2022. Online ahead of print.
