The most common indications for splenectomy are a wide variety of diseases and conditions: raptured or enlarged spleen, blood disorders like idiopathic thrombocytopenic purpura and thalassemia, cancer (chronic lymphocytic leukemia, Hodgkin’s lymphoma, and non-Hodgkin’s lymphoma), infection, etc.
2. Autotransplantations of the Spleen
The orthotopic regeneration of the spleen is clinically unfeasible. At the same time, unattended splenectomies are fraught with delayed immunological complications
[1][5]. The problem can be solved by transplantation of splenic material to heterotopic locations.
The idea was born in connection with clinical cases of spontaneous splenic engraftment. In the aftermath of splenectomy, functionally active fragments of splenic lymphoid tissue may occasionally settle in the abdominal cavity, resembling accessory spleens. This phenomenon is known as ‘splenosis’
[2][3][30,31].
3. Splenic Influence on Repair Processes in the Liver
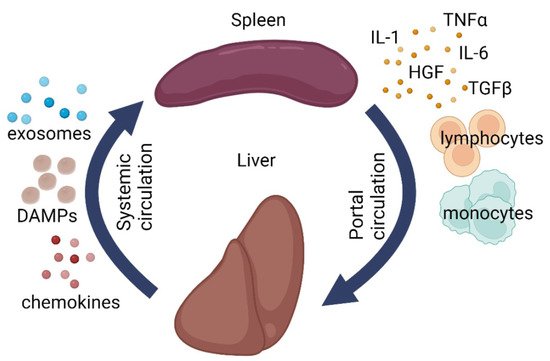
The immune system should be vigilant. Any minor failure in its functioning immediately affects the homeostasis, as exemplified by the post-splenectomy syndrome. Meanwhile, splenectomy is absolutely indicated in certain pathological conditions of the liver. The close relationship between the two organs is reflected by the concept of hepatosplenic axis (
Figure 1)
[4][5][77,78]. The apparent participation of the spleen in the regulation of hepatic repair can be further redefined as the role of immunity in regeneration. Pioneering research in this field was carried out by Anna G. Babaeva and her school
[6][7][8][79,80,81].
Figure 1. Schematic illustration of liver-splenic axis. DAMPs—damage-associated molecular patterns, IL1—interleukin 1, IL6—interleukin 6, TGF-β—transforming growth factor beta, TNFα—tumor necrosis factor alpha, HGF—hepatocyte growth factor.
The spleen and the liver are anatomically connected via portal circulation and have shared responsibilities (immune, barrier, metabolic, and hematopoietic). Clinical experience shows that liver diseases often disrupt the normal splenic architecture
[4][5][77,78]. At the same time, splenectomy has a positive effect on hepatic repair, though its mechanisms remain understudied
[9][10][82,83].
The impact of splenectomy on macrophage and lymphocyte populations of the liver in cirrhosis was emphasized in a number of studies. In a mouse model of concanavalin-induced hepatitis and cirrhosis, splenectomy promotes polarization of liver macrophages towards anti-inflammatory M2 phenotypes, which support the recovery
[11][88]. In mice with thioacetamide-induced cirrhosis, splenectomy reduces the degree of fibrotic lesions while enhancing hepatocyte proliferation and augmenting the numbers of Ly-6C(lo) monocytes and macrophages
[12][89]. On the other hand, splenectomy in rats with induced cirrhosis alleviates the damage, in particular, through increased production of TNFα by liver macrophages; at that, the macrophage numbers remain unaltered
[13][90]. It is noteworthy that in rats without liver damage, the liver reacts to splenectomy by enhanced proliferation of hepatocytes. Hepatic macrophages react as well: CD68+ cells increase in number, whereas the numbers of CD206+ cells decrease, with concomitant enhancement of
Il6, Il10, Tnfa, Hgf, and
Nos2 expression in the liver
[14][91].
The heavy-duty hepatosplenic circulation provides a permanent opportunity for the transportation of splenic monocytes/macrophages to the liver. On systemic scale, the spleen is viewed as a stock of monocytes to be released on demand for transportation and deployment at inflammatory foci. This is true for a number of murine models, including ischemic myocardial damage
[15][92], ischemic brain damage
[16][93], concussion spinal cord injury
[17][94], and muscular dystrophy in mice
[18][95]. However, the effect may as well be disease-specific; for instance, in a mouse model of lung carcinoma, the majority of monocytes arrive to the tumor directly from the bone marrow, bypassing the spleen
[19][96]. The arrival of monocytes/macrophages to the liver in the aftermath of hepatotoxic damage or resection has been demonstrated
[20][21][97,98], albeit without clear specification of their source. In
theour setting, intrasplenic injections of mesenchymal stromal cells (MSCs) labeled with a vital dye PKH26 led to appearance of PKH26-positive CD68+ cells (macrophages) in the liver 24 h after the injections
[22][99]. However, whether these are liver macrophages that have engulfed the labeled MSCs arriving from the spleen, rather than splenic macrophages that have engulfed MSCs on the spot before migrating to the liver, remains unknown.
Migration of monocytes from the spleen to the liver is disputable; for splenic lymphocytes, this route has been confirmed. In a murine model of cirrhosis, the spleen becomes progressively depleted of CD4+ (helper) T lymphocytes, with a simultaneous increase in the content of Th2 lymphocytes (thought to augment fibrosis) in the liver. Under these circumstances splenectomy restores the Th1/Th2 balance and alleviates the fibrosis
[23][100]. Apart from the lymphocytes arriving from the spleen, the liver harbors several minor lymphocyte subpopulations including γδT cells
[24][101], NK cells
[25][26][102,103], and NKT cells
[27][104], which exert modulatory effects on liver repair
[28][105]. The impact of splenectomy on these subpopulations remains unknown.
Splenectomy (splenectomized status) is also beneficial for regeneration of the liver after massive resections. The majority of studies emphasize the elevated rates of hepatocyte proliferation in splenectomized animals, although its mechanistic causes have to be specified. The variants include (1) resolution of portal hypertension; (2) mitigation of the damage to sinusoidal endothelium; (3) alleviation of the inflammatory side effects by inhibiting the synthesis of pro-inflammatory cytokines, as well as the rates of macrophage and neutrophil infiltration; and (4) hepatocyte apoptosis inhibition
[4][29][77,106].
Splenectomy complementing 90% resections of the liver volume is accompanied by decreased expression of multiple acute phase markers in the liver remnant and increased expression of
heme oxygenase-1 gene, considered beneficial for the repair
[30][107]. Physical removal of the spleen abrogates the inflow of pro-inflammatory cytokines that cause hepatocyte damage, as well as the proliferation blocker TGFβ1
[31][32][33][108,109,110]. These conditions enhance the synthesis of HMOX1 in the liver, which inhibits the activity of TNFα with a net cytoprotective effect on hepatocytes; moreover, the synthesis of TGFβ1 and its receptor TGFβ RII decreases, while the synthesis of HGF and its receptor c-met increases
[31][32][34][35][108,109,111,112]. Other studies argue that the beneficial effect may be due to the withdrawal of IL10, which is a confirmed inhibitor of hepatocyte proliferation. IL10-deficient mice exhibit higher rates of hepatocyte proliferation in response to resections compared to ordinary animals. Partial hepatectomy promotes increased expression of IL10 not only in the liver, but also in the spleen; accordingly, splenectomy cuts off the inflow of IL10 via portal vein
[36][113]. A similar positive correlation between splenectomy and liver repair is observed in liver transplantation models. The benefits include a decrease in portal hypertension and alleviation of endothelial damage, apoptosis, and pro-inflammatory cytokine synthesis
[37][114].
On the other hand, some models question, and even disprove, the benefits of splenectomy for liver repair. For instance, Babaeva et al. observed the opposite, inhibitory effect of splenectomy on the compensatory growth of the liver after resections. The strength of the effect did not depend on the time lapse between the two interventions (splenectomy followed by liver resection);
[38][115]. At the same time, splenectomy promoted a significant increase in the volume of intact liver through increased hepatocyte proliferation
[38][115].
Contemporary studies on the role of immunity in regeneration involve model animals depleted of particular lymphocyte populations. The data obtained in such models are often controversial, which reflects the complexity of the regulatory mechanisms. In mice depleted of T cells, resections of 70% liver volume have lower rates of necrotic complications than in ordinary mice
[39][116]. Similar results were obtained in a model of lipopolysaccharide-induced hepatitis; animals depleted of T or B cells revealed lower degree of hepatotoxic damage and better survival
[40][117]. At the same time, the block of hepatocyte proliferation in rats depleted of T cells or NK cells is accompanied by the lack of proliferative response from hepatic oval cells, by contrast with the control animals, in which only the hepatocyte proliferation remained blocked
[41][118].
Other examples of lymphoid cell participation in repair processes include experiments with adoptive transfer of lymphocytes from actively regenerating organs to the orthotopic locations in non-operated syngeneic animals
[38][42][115,119]. Upon the transfer, lymphocytes retained their regeneration-supporting capacity to a degree depending on the organ, the phase of repair, and the type (population) of the lymphocytes. Transfers of helper T cells had the most pronounced effect
[38][42][115,119]. The nature of regeneration-activating signals in this case is obscure; possible transmitters are microRNA molecules contained in microvesicles and exosomes secreted by lymphocytes and other cell types
[43][120].
Thus, immune cells inside the liver, and some of those outside it, may influence repair processes within the organ or its remnant. The impact can be either activating or inhibiting; the latter is exerted by T killers, NK cells, and NKT cells. The stimulatory effect of splenectomy on the hepatic recovery after various types of damage, as well as the adoptive transfer of lymphocytes as the means for boosting liver repair, are subject to further investigation. Overall, these results indicate that the liver and spleen actively influence each other both at the level of cell migration and at the level of cytokine balance.