Scarring and regeneration are two physiologically opposite endpoints to skin injuries, with mammals, including humans, typically healing wounds with fibrotic scars. We aim to provide an updated review on fibroblast heterogeneity as determinants of the scarring–regeneration continuum. We discuss fibroblast-centric mechanisms that dictate scarring–regeneration continua with a focus on intercellular and cell–matrix adhesion. Improved understanding of fibroblast lineage-specific mechanisms and how they determine scar severity will ultimately allow for the development of antiscarring therapies and the promotion of tissue regeneration.
- scarring
- fibrosis
- fibroblasts
- regeneration
- adhesion
1. Introduction
The most common response to injury in mammalian tissues and organs is scarring and fibrosis[1]. The more favorable outcome would be complete regeneration, such as occurs in invertebrates and lower vertebrates[2], with new tissue retaining the same structural, aesthetic, and functional attributes as the original uninjured tissue. Mammalian skin exhibits a remarkable diversity of scar severities that depend on the injury type, anatomic location, age, gender, and species, and this kaleidoscope of responses includes cases, albeit rare in mammals, of scarless regeneration. In the back-skin, scarless skin regeneration is conserved in early mammalian embryos, but it is lost in humans before the third trimester (week 24 of human gestation) and in rodents around embryonic day 16.5–18.5[3].
2. Dermal Fibroblasts Drive Diverse Skin Wound Responses
A multitude of therapies have been introduced to treat and/or prevent skin scars, but the efficacy of commercially available therapies remains limited[4], largely due to lack of a comprehensive understanding of the underlying cellular and molecular mechanisms that lead to scar formation. Scarring is most noticeable in the skin, but it affects almost all adult mammalian and human tissues and organs[5].
In this review, we summarize recent findings on the skin’s response to injury, with a focus on “decision-making” mechanisms of fibroblasts in injured skin that dictate the skin’s wound response to scar or regenerate.
Skin wound repair is a multifaceted process that aims to accomplish two major tasks: restoring the barrier functions of the skin so as to prevent further blood loss and infection, and restoring the physiological and mechanical properties. Scarring and regeneration can be viewed as extreme restoration of one versus the other: patching wounds with dense plugs of ECM makes for quick sealing of wounds, whereas regeneration takes longer but culminates in functional restoration. Healed wounds are therefore a compromise of the bifurcation of fibrosis and regeneration, and skin repair is carried out on a spectrum from overhealing, as in hypertrophic scars, keloids, and scleroderma, to regeneration, as in fetal wound healing, oral mucosal repair, and WIHN. Understanding of the mechanism underlying the balance of scarring and regeneration is the key for future manipulation towards scarless regeneration in a fully controlled manner.
The new studies reviewed here shed light on the cellular and molecular mechanisms leading to scarring and regeneration upon skin injury. The key signaling pathways are summarized in Figure 1. We believe that these pathways are not isolated, but rather interconnected. Hence, the triggers of the fibrotic or regenerative process likely require cross-talk of multiple pathways. Among them, the signal cascades involving cell–matrix adhesion and cell–cell adhesion between fibroblasts emerge as important candidates for future research on scarring mechanisms.
Despite fast progress in the field in recent years, the governing mechanism as an integrated network is far from being understood. New state-of-the-art techniques such as lineage tracing, intravital microscopy, and single-cell transcriptional and epigenetic profiling, coupled with ECM composition and migration analysis, are expected to continually illuminate the underlying mechanisms of skin scarring, and may pave the way to future promising pro-regenerative approaches for skin injuries.
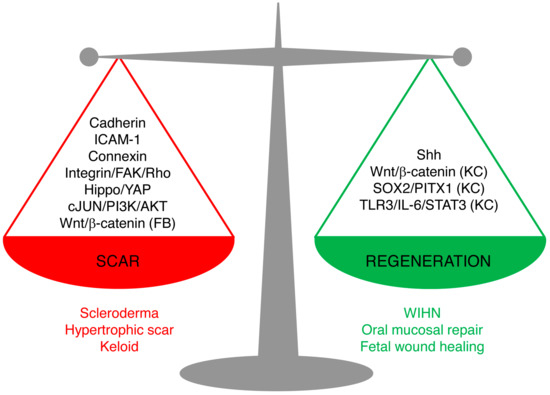
Figure 1. Signaling pathways leading to scarring or regeneration. The outcome of skin wound healing is a balance of signaling pathways leading to scarring or regeneration. Upregulation of Cadherins, ICAM-1, or Connexins or overactivation of Hippo/YAP, integrin/FAK/Rho GTPase, c-JUN/PI3K/AKT, or Wnt/β-catenin signaling in fibroblasts leads to pathological scarring, such as scleroderma, hypertrophic scar, and keloids. Activation of Sonic Hedgehog (Shh) signaling in keratinocytes and wound fibroblasts, or activation of Wnt/β-catenin, SOX2/PITX1, or TLR3/IL-6/STAT3 in keratinocytes (but not in fibroblasts) promotes regeneration, as seen in wound-induced hair follicle regeneration, oral mucosa repair, and fetal scarless healing. (FB), only in fibroblasts; (KC), only in keratinocytes.
References
- Geoffrey C. Gurtner; Sabine Werner; Yann Barrandon; Michael T. Longaker; Wound repair and regeneration. Nature 2008, 453, 314-321, 10.1038/nature07039.
- A Bayat; D A McGrouther; M W J Ferguson; Skin scarring. BMJ 2003, 326, 88-92, 10.1136/bmj.326.7380.88.
- Michael T. Longaker; David J. Whitby; N. Scott Adzick; Timothy M. Crombleholme; Jacob C. Langer; Brian W. Duncan; Scott M. Bradley; Robert Stern; Mark W.J. Ferguson; Michael R. Harrison; et al. Studies in fetal wound healing VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. Journal of Pediatric Surgery 1990, 25, 63-69, 10.1016/s0022-3468(05)80165-4.
- Ruth Ellen Jones; Deshka S. Foster; Michael S. Hu; Michael T. Longaker; Wound healing and fibrosis: current stem cell therapies. Transfusion 2019, 59, 884-892, 10.1111/trf.14836.
- Jagannath Padmanabhan; Zeshaan N. Maan; Sun Hyung Kwon; Revanth Kosaraju; Clark A. Bonham; Geoffrey C. Gurtner; In Vivo Models for the Study of Fibrosis. Advances in Wound Care 2019, 8, 645-654, 10.1089/wound.2018.0909.
