Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ines Bouaziz and Version 3 by Vivi Li.
Desalination brine is extremely concentrated saline water; it contains various salts, nutrients, heavy metals, organic contaminants, and microbial contaminants. Conventional disposal of desalination brine has negative impacts on natural and marine ecosystems that increase the levels of toxicity and salinity. These issues demand the development of brine management technologies that can lead to zero liquid discharge. Brine management can be productive by adopting economically feasible methodologies, which enables the recovery of valuable resources like freshwater, minerals, and energy.
- brine solution
- management
- sustainability
1. Introduction
Water scarcity has evolved into a global challenge with the population explosion and its demand for industrial and domestic applications. Research has been focused on the technological and material aspects to meet the growing water demand. With the exhaustion of freshwater sources and the abundance of seawater or brackish water, research has been focused on the production of clean water from these resources. Desalination has been an important advancement that has the potential to meet the water crisis, but with the advantages of this process there are disadvantages too. The large production of brine, i.e., the highly concentrated salt stream from the desalination plant, is a major concern as most of the desalination plants dispose of the brine into the original water source. The salt accumulation in brine increases the seawater salinity and consequently it increases the energy needed for desalination for a potable water supply. Brine also contains metals and chemicals (Table 1) that cause negative effects on marine ecosystems. The threats posed by brine discharge lead to socioeconomic and socio-political consequences such as energy demand, water stress, and negative health impacts. Therefore, the increasing pollution of the water resources has to be managed strategically to maintain the balance of the ecosystem.
However, there are socio-political and legal challenges that any management approach should address for the development and the proliferation of brine management. It is severely impacted by a variety of often neglected socio-political factors. These are major factors in the success or failure of many brine management projects around the world, and they are classified into four categories: strengths, weaknesses, opportunities, and dangers. There are links between brine management and society’s critical needs for political stability, better health, economic growth, and water security. For example, proper brine management can result in the commercialization of valuable resources like water, minerals, and energy, which will lower the overall cost and offer a business opportunity that definitely will ensure the economic, social, and environmental stability of countries.
This rentryview focuses on the advancement in brine management and proposes future strategies to overcome the crisis. Hybrid technologies that can be utilized to develop a circular solution of waste to energy or value-added products will also be discussed.
There are several review articles in this area that presented discussions on brine management and treatment-based and technology-based solutions. Bello et al. have recently given an overview of brine management, desalination technologies, life cycle assessment, and recovery methods [1], while Al-Absi et al. provided an update on the use of adsorption processes as a recovery option and discussed the various brine management strategies and technologies [2]. Mavukkandy et al. reviewed recent research and technological development on recovering water, minerals, and energy from desalination brine [3]. Soliman et al. have presented a comprehensive review of the current technologies of various desalination processes and the detailed energy consumption and water production costs of these technologies [4]. However, previous reports lack detailed analysis of future prospects to achieve sustainable brine management. Hence, the present rentryview focuses on the current brine disposal strategies, methods of treatment, hybrid methods for metal recovery, and zero liquid discharge (ZLD). More attention was given to analyzing futuristic developments of a sustainable hybrid strategy for brine management that could open gateways to remarkable water recovery and mineral recovery channels while attaining the near-ZLD approach.
| Parameters | Details |
|---|---|
| Physical characteristics | Salinity: above 55,000 mg/L of TDS; conductivity: 0.6 W/mK at 25 °C; temperature: ambient seawater; pH: 7–8. |
| Inorganic salts | Example: sodium chloride (NaCl), calcium chloride (CaCl2), and magnesium chloride (MgCl2) are the major constituents. |
| Metals caused by corrosion | Brine might have high levels of iron, chromium, nickel, and molybdenum if the facility uses low-quality stainless steel. |
| Nutrients | Ammonia, nitrate, and phosphorus. |
| Pretreatment chemicals | Antiscale additive (ethylenediaminetetraacetic acid: EDTA, sodium hexameta phosphate). Biofouling control additives such as chlorine (small quantities)—coagulants. |
| Halogenated organics | Trihalomethanes are common byproducts of chlorine addition (low content). |
| Cleaning chemicals | -Acidic solutions used to adjust the pH of the seawater. -Detergent such as EDTA, oxidants (sodium perborate) and biocides (formaldehyde) are used to clean the membrane. |
1.1. Brine Solution and Characteristics
Brine is a by-product or the end product of a desalination process that consists of various components. A list of typical physical and chemical characteristics of desalination brine is given in Table 1. Brine has a salinity above 55,000 mg/L of total dissolved solids (TDS) in the stream [5]. The chemical characteristics of brine discharge depend on various factors such as the quality of feed water and permeate water, type of desalination process, pre-treatment method, and cleaning procedures used. Each plant has a diverse concentration and components of contaminants in it. The presence of heavy metals, organic contaminants, strong acids/base, antiscalants, coagulants, and biocides add to the complexity of the brine solution.
1.2. Conventional Methodologies for Disposal of Brine
The conventional strategies involved in the disposal of brine from desalination processes can vary depending on the geographical location, quality, and volume of the brine. Some conventional disposal methods include surface water discharge, deep well injection, land application, evaporation ponds, and conventional crystallizers. There are several factors that influence which option of disposal method can be adopted such as quantity and quality of the brine, geographical location of discharge point, availability and authorization of dump sites, and operational and transportation costs. All these are critical factors to be addressed when a desalination plant needs to be installed. It has been reported that almost 5% to 33% of the total cost will be spent on the disposal processes for the brine. In addition, revenue that can be made from the brine, such as minerals recovery, waste-to-value-added products like fertilizers, etc., are alternatives for a more cost-effective model. The conventional brine disposal strategies have been tabulated in the Table 2.
Table 2.
Conventional brine disposal strategies and its environmental impacts.
| Disposal Methods | Requirements Prior Disposal | Cost in US$ 0.00/m3 | Environmental Impact |
|---|---|---|---|
| Surface water discharge | Compatibility with the receiving water body, i.e., dilution to maintain salinity. | 0.05–0.30 | Pollution of the marine ecosystem by altering the salinity and pH. |
| Sewer discharge | Basic pretreatment is essential like pH neutralization to maintain the TDS concentration lower than 3000 mg/L. | 0.32–0.66 | Potential environmental hazards due to brine’s high TDS content. |
| Deep-well injection |
Wells of depth 500–1500 m is a requisite and should be able to receive brine for 25–30 years. Other parameters are pond size, lining material and monitoring of the injection site. | 0.54–2.65 | Pollution of nearby water aquifers and ground water contamination. Unsuitable for countries with high seismic activity. |
| Evaporation ponds |
Availability of solar energy, land and favorable climatic conditions affect the evaporation rate. | 3.28–10.04 | Improper lining or damage can cause percolation into the water aquifer underneath the pond and deteriorate the water quality. |
| Land application |
The concentration of nutrients in the brine needs to be well within the limits when used for irrigation purposes. Other factors include dilution of concentrated discharge, availability of irrigation land, salinity tolerance interval and follow the groundwater quality regulations. There should not be any pathogenic organisms in the stream. | 0.74–1.95 | High-salinity tolerant plants can only be irrigated with a TDS higher than 2000 g/L. Ground percolation and surface water runoff can increase the aquifer salinity thereby causing a negative impact on ground water aquifer. |
| Conventional crystallizers | A process used at the last stage of brine disposal. It can be a combination of RO, electrodialysis or evaporation process to obtain zero liquid discharge. | 3–27 | Recovery and reuse of waste metal is the objective so that it can reduce environmental impact and generate revenue from brine. |
1.3. Environmental Impact of Brine
The improper disposal methods of brine can cause several environmental hazards that bring negative impacts to the air and water quality. The toxicity imposed by brine disposal can vary depending on the potential hazardous substances it contains, such as toxic metals (mercury (Hg), cobalt (Co), copper (Cu), iron (Fe), zinc (Zn), and nickel (Ni)), pesticides, and acids, which cause irrevocable changes to the environment. The direct disposal of brine into the ecosystem has also caused severe imbalance to aquatic life by fluctuating the pH, salinity, temperature, eutrophication, etc. There are several reports wherein the direct influence of heavy metals has impacted the flora and fauna [6][7][6,7]. The methods for brine disposal vary depending on the geographical location of the desalination plant. The plants that are located in the coastal line usually dispose of the brine back into the seawater, thus affecting its salinity and the marine ecosystem as mentioned earlier, whereas the land-based plants result in the contamination of the groundwater resources and surrounding environment. There are several reports that have highlighted the environmental impacts of brine disposal from desalination plants at specific geological locations [5][7][5,7].
Generally, the treatment of brine depends on the composition, such as the removal of all organic matter initially and further removal of salts and other elements. Proper treatment and conversion of brine to value-added substances for industrial and irrigation purposes can be a good brine management strategy [8]. As formerly mentioned, brine has a salinity at least 1.6–2.1 times higher than seawater and at elevated temperature up to 50 °C, which is extremely high compared to the surrounding temperature, thus the potential of affecting the marine flora and fauna. The most devastating effect it can cause is the ‘lethal osmotic shock’ to the fishes, plankton, algae, and seagrass, causing irrevocable damage to their cells, leading to extinction [9][10][11][9,10,11]. Water bodies with abundant marine life such as closed or semi-closed shallow places should not be disposal sites as it greatly affects the marine life because of the change in salinity and lowered dissolved oxygen levels. The seasonal and cumulative effects of brine discharges from desalination plants along the Israeli coast was studied using benthic foraminifera, a known sensitive marine bio-indicator [12]. Another study reported fish survival for three months in raw and calcium-reduced concentrate discharge from the desalination plant [13]. However, a very recent short-term study for six years at two mega-size seawater desalination plants on the Mediterranean coast of Israel has reported that brine discharge has no significant impact on seawater quality. The study presented that it did not impact the oxygen saturation, turbidity, pH, nutrients (except for total organic phosphorus (TOP)), chlorophyll-a, and metal concentrations [5]. An environmental risk assessment is a prerequisite to assess the environmental impacts associated with desalination plants. It studies and processes a proper location for installation of a desalination plant with mitigation strategies and waste disposal methods and their impact on marine and coastal environments [14]. Marine monitoring and assessment should continue for as long as the plants are operational and critically reviewed. The regulations should be re-evaluated periodically for frequency, sampling stations, and parameters measured, and updated when necessary.
2. Conventional Technologies for Brine Treatment
Proper brine management must be designed to fulfill the criteria of a brine recycling loop. Subjecting desalination brine to chemical/electro chemical coagulation, chemical oxidation, chemical precipitations, and biological assimilation are the traditional ways of brine treatment for decontamination/resource recovery before the most modern technology replaced the conventional techniques. Among conventional methods, chemical precipitation is mainly used only for inorganic removal, whereas other methods are adopted for organic impurities. For example, electro-/chemical coagulation and chemical precipitations are closely related techniques that involve the summoning of smaller impurities into larger debris to help them settle at the bottom, on top, or on a targeted site. Coagulation manipulates electrostatic charge neutralization on organic impurities, especially non-settleable solids, upon absorption against suitably added chemical agents called coagulants or flocculants (e.g., metal oxides). The types and dosages of coagulants depend upon the nature, concentration, and composition of the brine. Frequently used coagulants are Al3+, Fe3+-based salts, polymerized inorganic metal salts, etc. Polymers like polyamine or polydiallyldimethylammonium chloride containing large numbers of charges may also be found useful as effective coagulants [15][16][15,16]. However, state-of-the-art advancements made in this field could achieve only a maximum of 58% dissolved organic content (DOC), so far, with a high dosage of coagulant (8.95 mM Fe3+) [16]. This is because most classes of brines consist of high concentrations of salts containing organic impurities with all ranges of molecular weight (MW), whereas coagulation is effective only in the case of high-MW organics removal. It has been observed that over-dosage of these salts (especially Fe-based or old alum-based salts) in a treatment process may lead to machinery impairment, mandating additional maintenance. Because of this very reason, coagulation/flocculation is not extensively used for brine treatment. Another concern of using a metal-based coagulant is its adverse effect on the ecosystem and human health. Thus, a flawless coagulation technology has a long route ahead to attain an acceptable competence. Mohamed et al. found that Al3+ and Fe3+ ions impregnated onto activated silica, i.e., hydrolyzed poly aluminum ferric chloride plus silicate (PAlFeCl + Si), is a good alternative to conventional coagulants offering removal of 89% COD [17]. In an attempt to reduce the environmental impact, synthetic derivatives of many natural coagulants have also been developed by exploiting a number of biopolymers, viz., lignin, tannin, starch, etc. [18]. In electrocoagulation, an electrochemical reactor deployed with stainless steel and aluminum as electrodes is being used. The elevated electrical conductivity of high saline water is highly suitable to be treated by this method with added the advantage of less electricity consumption. However, the electrodes must be regularly maintained or replaced for consistent performance as the dissolution of metals from electrodes cause the coagulation/flocculation of charged impurity metal ions like Ca, Sr, or non-metal like SiO2, to affect the overall performance [19]. A major affliction that ever retards the working performance of any bulk brine treatment plant is the frequent deposition of scale-forming substances. Thus, the presence of scale precursor ions, viz. Ca2+, Ba2+, Mg2+, Si, Sr2+, etc., are invariably responsible for inefficient water recovery since they tend to form deposits on machinery parts because of their lower solubility limits. An ancient method to resolve this problem is to remove such ions with the aid of chemical agents such as lime-soda, ash, etc., so-called precipitants or softening agents. Lime softening is a widely employed robust technology for eliminating high scale-forming ions. Several studies have long focused on using Ca2+ to remove silica with other metals like Ba or Mg as their hydroxides [20][21][20,21]. Recently Boo et al. introduced a thermomorphic hydrophilicity base-induced precipitation strategy for the removal of scalants driven by basic conditions by thermoresponisive amines. The use of diisopropylamine managed to remove ~80% hardness of ultra-high-saline brine with recovery of amines for reuse in warm conditions [22]. Apart from chemical softening methods, there exists another pretreatment approach, known as seeded slurry precipitation, suitable for low-saline brine particularly rich with calcium sulfate [21][23][21,23]. The mechanism herein involves the growth of scalants onto the seed crystals. In this procedure, a slurry made out of seed crystals is introduced into the brine. The seed crystals serve as nucleating centers for the deposition of scalants like silica and calcium sulfate. In a more convenient approach called a pellet reactor, the same methodology was applied but in a heterogenous manner with an added advantage of formation of dry sludge [24]. A typical bed fluidized bed reactor contain packed calcium carbon crystals as seeding platforms for preventing super-saturation of scale-forming salts [21]. The above discussed conventional methods may not be well-applicable for the treatment of brine with high salinity, containing organic pollutants such as hormones, pharmaceuticals, personal care products, and soluble microbial products. A special caution must be taken for the removal of such organic contaminants present in trace amounts [25]. A most common way of treating recalcitrant organic contaminants is by converting them into viable smaller fragments. Different combinations of advanced oxidation processes by means of O3 (ozonization), UV-H2O2/O3 [26], UVA-TiO2 [27], electro oxidation [28], non-thermal plasma [29][30][29,30], photo-Fenton oxidation [31], etc., have been documented by a number of groups. Almost all these schemes work on the principle of free radical formation by photolysis. However, it is crucial to pretreat the brine prior to this stage because the presence of groups like sulfates in the medium most likely deactivate hydroxyl free radicals for further oxidation. Despite a well-inculcated track record of oxidation methodologies, very few groups have so far really focused on the scaling-up and assessment of hazardous consequences associated with the generated low-molecular-weight fragments/byproducts [32]. A relatively less and inefficiently explored, but far older, technique for removal of sulfate or ammonia is through bioprocessing, wherein useful microbes assimilate them by converting them into remediable forms, but only a low-saline brine could be treated by this method since higher-saline brine contains large varieties of heavy metals that can inhibit/reduce microbial growth. Most of the studies in this area concentrate on the reduction of nitrate to N2 and sulfate to sulfides. A typical example is the conversion of nitrate content into ammonia and then N2 by denitrifying bacteria [33]. Many groups have come up with woodchip bioreactors, known as a convenient treatment method for nitrate removal [34][35][36][37][38][34,35,36,37,38]. However, these techniques require the addition or attachment of electron-donating groups such as ethanol or acetate in an attempt to enhance the conversion efficiency. Unfortunately, this procedure causes the increase in DOC, which has to further alleviate serious environmental impacts. Another limitation of this method is its inconsistent performance. This is caused by the high initial DOC content that is due to bacterial multiplication resulting in better performance in the first weeks, as revealed by Díaz-García et al. This trend could be minimized by performing an alternate drying–rewetting cycle while using wood chip reactors [39].3. Brine Management and Zero Liquid Discharge
It being said that one side of a coin shows cases of the removal of toxic components/elements from brine, the flip side of the coin shows that a large volume of potentially reusable water is abandoned in the form of liquid waste. The recovery the water content thus provides a solution to compensate for water scarcity, and also alleviate the major concern with liquid waste disposal. A cutting-edge technology for brine management and resource recovery is the ZLD scheme. Since the invention of this concept, traced back to the 1970s in the U. S. (put forward for regulating the salinity of the Colorado River, U.S.), ZLD has witnessed tremendous advancement, especially in the last decade [40]. It is a strategic engineering approach for waste management ensuring the complete elimination/recovery of liquid, as well as minerals, from the feed wastewater, leaving solid waste to be disposed of. On the other hand, liquid portions of brine and valuable salts are effectively recovered and reused, enabling them to enter into a circular cycle, entitling effective net zero liquid discharge to the environment. The foremost drive for this innovation is the quest for the maximum recovery/reuse of water in dry lands and the easy and convenient disposal of the solid waste. The major outcome of ZLD, i.e., solid waste, prevents the entering of liquefied contaminants into the main flow stream, making it easier to treat them. Thus, ZLD on one hand averts the effluent-drain-water discharge and associated threats of aquatic environmental pollution; on the other hand, it demands greater overhead because of the involvement of energy-intensive sophisticated technologies. A rough estimation of the global market of ZLD requires funding of a minimum of $100–200 million per annum [40]. This made the execution of this technology limited to economic First World countries such as those in North America and Europe (not 100% execution), while it is prompt to implement in developing countries such as China and India [40]. In First World countries, factories are investing in recovering/recycling of water, implementing ZLD even without regulatory push to achieve better sustainability. Though ZLD negotiates a better balance between waste management and the environment, economization of ZLD technology is often hit by cheaper near-ZLD/close-to-ZLD technology by possible on-site removal/recovery of liquid/water from the effluents at the production site. There are cases wherein near-ZLD technologies are largely put forward to compromise the economical constrains associated with the ZLD technique, often involving incomplete removal of liquid waste/water recovery. They simply achieve lower volumes of brine [40]. Thus, recent studies apparently focus on bridging the gap between economic constraints and the efficacy of the overall ZLD system. Operations like forward osmosis (FO), electrodialysis (ED), and membrane distillation (MD) are majorly performed in conjunction with reverse osmosis (RO) for treating RO brine concentrates to achieve ZLD, since these methods can treat brine of high salinity (>200,000 mg/L) [40]. An ideal ZLD process is designed for the maximum recovery of resources. Regardless, purified water is the first and foremost incentive of any brine treatment process. Forward osmosis, electrodialysis, membrane distillation, and hybrid processes are the major approaches adopted for freshwater recovery, and they are discussed in the proceeding sections. This step is followed by mineral recovery techniques in the subsequent stages. It should be noted that a careful screening of technologies must be made, rendering the concentration and composition of RO brine.4. Brine Management: Resource Recovery Technologies
4.1. Freshwater Recovery Technologies
4.1.1. Forward Osmosis
Forward osmosis, as the name implies, is an osmotic pressure-driven membrane process, unlike RO (which uses hydraulic pressure), it uses the osmotic pressure gradient across the membrane to separate the feed water and allow it to permeate. In brine treatment, this method is majorly adopted for water recovery. In principle, as shown in Figure 1, to attain an osmotic pressure gradient, a high-saline solution called a draw solution will be used. During the process, water from feed water (low saline) will pass through the semipermeable membrane to the draw solution (DS), which is highly saline, to achieve the osmotic equilibrium. As the process continues, there will a diluted draw solution and concentrated feed. The freshwater and draw solution can be separated via a regeneration process using RO/evaporation/mechanical methods. The remaining concentrated DS can be reused further. The obtained concentrated brine feed can be subjected to crystallizers/evaporators for minerals recovery.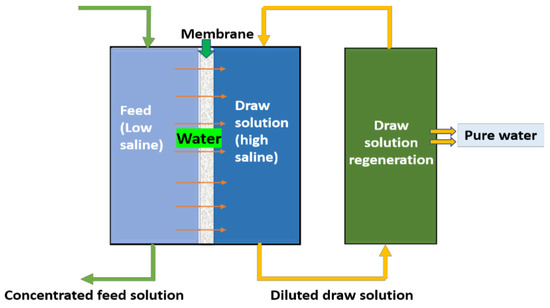
Figure 1.
Schematic diagram for forward osmosis.
Table 3.
Summary of water recovery studies recently reported using FO process.
| Source of Brine | Draw Solution and FO Membrane | Water Recovery and Salinity Level | Ref. |
|---|---|---|---|
| High-saline water | NH3/CO2 as DS and polyamide FO thin film composite membrane | 64% water recovery with 300 mg/L TDS | [43] |
| Reverse osmosis brine | NaCl as DS and flat-sheet cellulose triacetate membrane | 90% water recovery | [44] |
| NaCl-based synthetic brine | Industrial-grade fertilizer ammonium sulfate as DS and commercial FO membrane | 12.7% water recovery | [45] |
| RO brine | 3 M MgCl2 as DS; cellulose-based polymers with an embedded polyester mesh | 50% water recovery | [46] |
| Synthetic brine | Fructose as DS; hydrophilic cotton-derived cellulose-ester plastics embedded on top of a microfiltration membrane | 56.8% recovery with 5 M Fructose; 61.4% recovery with 6 M Fructose | [47] |
| Brine from multi-effect distillation systems | 3 mol/L NaCl as DS; cellulose triacetate membrane and polyamide thin film composite membranes | Brine volume reduced to 54.9% | [48] |
| Four source of high-saline wastewater | Sodium alginate sulfate as DS | - | [49] |
| RO concentrate produced from coal chemical industry | DS: NaCl; membrane: active rejection layer made of cellulose triacetate (CTA) as well as a polyester support layer | 72.1% (4.6g/L TDS), 84.3%, 90.9% and 92.5% (17.4 g/L TDS) water recovery using 1 M, 2 M, 3 M and 4 M DS | [50] |
| Anaerobic palm oil mill effluent | DS: 3 reagent-grade fertilizers (i.e., (NH4)2SO4, monoammonium phosphate (MAP) and KCl) and three commercial grade chemical fertilizers (i.e., (NH4)2SO4-f, monoammonium phosphate-f and muriate of potash; membrane: cellulose triacetate | Highest recovery with MAP, 5.9% for a 4 h operation | [51] |
4.1.2. Electrodialysis Technologies
In electrodialysis, an alternating series of cation and anion selective semipermeable membranes (ion exchange membranes—IEM) are placed in between cathode and anode; clean water is produced by the electrochemical separation of ions, i.e., ions in solution are separated by the influence of electric potential. A schematic diagram illustrating the principle of electrodialysis process is shown in Figure 2. The brine solution is passed through into the cells in the ED system; the voltage gradient makes the movement of anions and cations through the selective membranes to anode and cathode, respectively. The cation-exchange membranes (CEM) allow the cations to block the anions; similarly, anions get passed through anion-exchange membranes (AEM) and cations are blocked. This leads to the complete separation of ions in brine, and ends up in ion enrichment at one side and freshwater recovery in another side. All cations such as Na+, K+, Mg2+, and Ca2+ and all anions such as chlorides, sulfates, and nitrates are found to be separated effectively from brine using ED technology.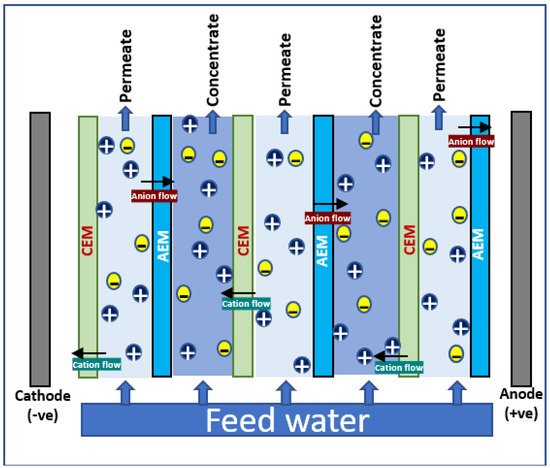
Figure 2.
Schematic diagram for electrodialysis.
Table 4.
Summarized reports of ED systems utilized for water recovery from various sources of brine.
| Source of Brine and Salinity Level | IEMs and Conditions of ED Technologies | Water Recovery Rate | Ref. | ||||
|---|---|---|---|---|---|---|---|
| RO concentrate discharged from RO plant | Series of ion exchange membranes such as FAS-PET-130, FKS-PET-130, Neosepta-CMX, Neosepta-AMX, LabAM-NR, LabCM-NR were used | 67.78% | Bolivian salt-lake brine | Adsorption by column packed with iron-doped lithium manganese oxides, Li1.33FexMn1.67−xO4 (x = 0.15, 0.30, and 0.40) | [56] | ||
| [ | 66 | ] | [115] | RO brine concentrate | RO-ED integrated system | 95% | [59 |
| Li | ] | ||||||
| - | Adsorption using dihydrate lithium acetate (C | 2H3LiO2·2H2O) | [67][116] | Brackish water RO concentrate | Lab-scale EDR system with three cell pairs of AEM and CEM | 85% | |
| Li | Salt-lake brine | Electrochemical adsorption or capacitive deionization using oxygen vacancy-rich CoP/Co3O4-graphene aerogel (GA/CoP/Co3O | [60] | ||||
| 4 | ) as bifunctional anode and cathode | [ | 68][117] | Synthetic brine | Electrodialyzer with 25 cell pairs of monovalent selective AEM and CEM | 70% | [58] |
| Li | Salt-lake brine | Adsorption by column packed with layered lithium-aluminum hydroxides | [69][118] | Brackish Water RO brine | Bipolar membrane electrodialysis (BMED) | Acid (0.7 mol/L) and base (0.6 mol/L) recovery | [61] |
| Seawater reverse osmosis brine | Monovalent selective electrodialysis (S-ED) | 55% | [62] |
4.2. Mineral’s Recovery Technologies
A major route by which researchwers can make the brine treatment economical is by the recovery of profitable minerals. FO, membrane-based technologies, or other advanced brine treatment techniques include sophisticated amenities, which on the other hand can only be balanced by the profit envisaged by mining brine for commercially relevant products. Mineral recovery ideas have gained momentum based on this very fundamental aspect [63][112]. From the economic point of view, metal recovery from seawater desalination or geothermal brine leaves less environment footprint when compared to traditional mining and purification processes. However, its extraction is a matter of controversy. Only limited mineral recovery technologies are so far commercialized and implemented in industrial scale for real-time applications. Despite finding it to be highly dependable with potential for yielding high throughput, even the most modern technologies are far from the ideal necessities of global market. However, before heading to the recovery, it is important to know the global demand. For example, rubidium (Rb) is known for being a high-priced element but stands for its low requirements, whereas Li has high market demand and therefore is more viable for extraction. Other economically viable metals are magnesium (Mg), cesium (Cs), uranium (U), etc. [63][64][65][112,113,114]. Moreover, while opting a suitable recovery technology, other factors such as fast reaction kinetics, ability to withstand quite high temperature (60–200 °C), and pressure 15–25 bar must also be considered. This is because most brine-management plants are working on high flow rates and temperatures. Similar to water-recovery technologies, electrodialysis and membrane distillation have also been adopted for metal recovery from brine solution (see Table 59). The other commonly used methodologies for mineral recovery are adsorption, crystallization, precipitation, and hybrid processes. The proceeding sections discuss major effective practices reported recently for the recovery of industrially relevant metals/minerals from the high-concentrate brine. Table 59 summarizes the recent studies on metal/minerals recovery from various concentrated brines.
Table 59.
Summary of metals/minerals recovered using several technologies based on recent reports.
| Minerals/Components Recovered | Brine Source | Mineral Recovery Method | Ref. |
|---|---|---|---|
| Lithium (Li) | |||
| Li | |||
| Salt-lake brine | Adsorption by Mn-based cylindrical | granular adsorbent EP/HMO (Epoxyresin/H4Mn5O12) |
[70][119] |
| Li | Seawater brine | Adsorption by lithium-ion sieves (LIS) embedded in a cross-linked hydroxyethyl cellulose (HEC) | [71][120] |
| Li | East Taigener Salt-lake brine | granulated lithium adsorbents made of puckered layer double hydroxide NH4Al3(SO4)2(OH)6 | [72][121] |
| Li | Seawater brine | Adsorption by spinel-type manganese oxide (Li1.33Mn1.67O4) ion sieve | [73][122] |
| Li | Salt-lake brine | Adsorption on porous titanium-based Lithium-ion sieves (LIS) nanofibers | [68][117] |
| Li | Salt brine eluate | solar evaporation crystallization | [74][123] |
| Li | Salt-lake brine | Constant-current electrodialysis (ED) | [75][124] |
| Li | Salt-lake brine | Selective-electrodialysis (S-ED) for recovering Li from Mg2+/Li+ ratio brines | [76][125] |
| Li | Lake brines | Sandwiched liquid-membrane electrodialysis used by combining liquid-membrane extraction and electrodialysis |
[77][126] |
| Lithium hydroxide (LiOH) | Lithium-rich salt-lake brine | mass transfer based on three-chamber bipolar membrane electrodialysis based | [78][127] |
| Li | Salt-lake brine | GO-composite-based pervaporation membrane and crystallizer | [79][128] |
| Magnesium (Mg) | Brackish water mimicked RO brine | Electrochemical nitrate removal with simultaneous magnesium recovery | [80][129] |
| Rubidium (Rb) | SWRO brine | integrated submerged MD-adsorption by granular KCuFC | [81][82][130,131] |
| Salt-lake brine | Adsorption by biomass-derived adsorbents (BCA@STS, CBCA@STS) modified with sodium titanium silicate (STS) | [83][132] | |
| Cesium (Cs) | Salt-lake brine | Adsorption by titanosilicate modified BCA and CBCA (carbonized biomass carbonaceous aerogel) | [83][132] |
| Calcium (Ca) | RO brine, seawater and stored urine | Eutectic freeze crystallization (EFC) | [84][133] |
| Boron (B) | Salt-lake brine | Electrochemical adsorption or capacitive deionization using oxygen vacancy-rich CoP/Co3O4-graphene aerogel (GA/CoP/Co3O4) as bifunctional anode and cathode |
[68][117] |
| Neodymium (Nd), Gadolinium (Gd), Holmium (Ho) |
Acid mine drainage or geothermal fluid | Adsorption using ligand-functionalized silica particles | [85][134] |
| Sodium chloride (NaCl) | Seawater brine | quartz glass fibrous filter membrane enabled solar crystallizer coupled with a salt crystallization inhibitor |
[86][135] |
| Magnesium sulphate (MgSO4) | Seawater brine | Precipitation using Slaked dolomite | [87][136] |
| Magnesium hydroxide Mg(OH)2 | RO brine | Precipitation using NaOH | [88][137] |
| Lithium phosphate (Li3PO4) | Salt-lake brine | Precipitation using facet engineered Li3PO4 seed | [89][138] |
| MgSO4 | Seawater brine | Precipitation using paper sludge ash, sulfuric acid, and ethanol | [90][139] |
