In the last decades, sensors based on screen-printed electrodes (SPEs) have gained increasing importance because of their advantageous characteristics, such as low-cost, disposability, ease of use and portability, which allow fast analysis in point-of-need scenarios.
The main characteristics of SPEs as electrochemical transducers for biosensors are described below.
- screen-printed electrode
- electroanalysis
- electrochemical sensor
- biosensor
1. Production and Design of Screen-Printed Electrodes
Figure 1
Figure 1
[9]
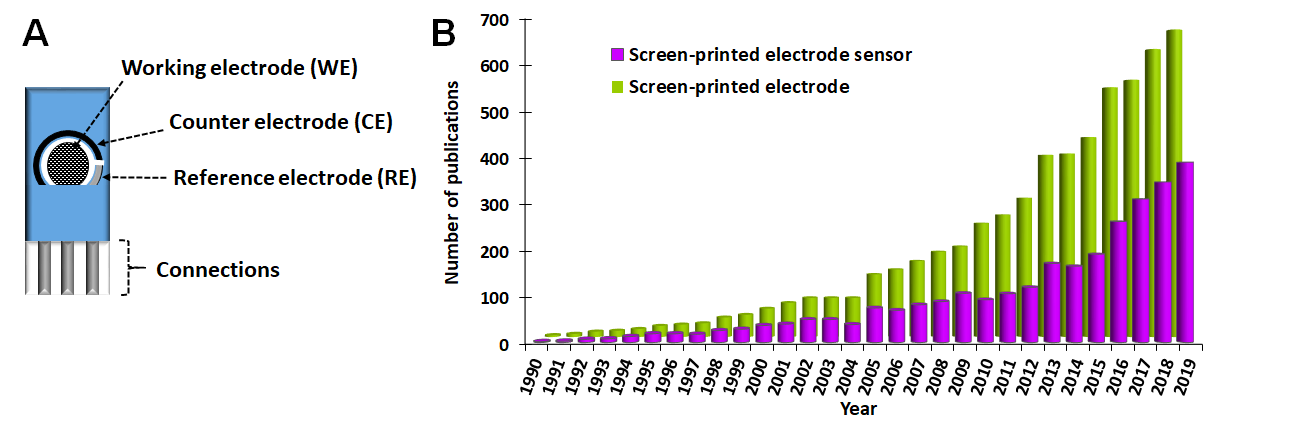
Figure 1.
A
B) Number of publications per year when searching “screen-printed electrode” and “screen-printed electrode sensor” in Scopus database for the last 30 years (1990–2019).
[13]
[13][14][15][16][17][18]. The RE is often made of silver or silver/silver chloride ink. This is considered a pseudo-reference or quasi-reference electrode since its potential is not as stable as that of an ideal reference electrode (e.g., conventional silver/silver chloride RE, which is the most common). Therefore, the applied potential is not as exact and reproducible as when an Ag/AgCl electrode is used. This can be problematic for electrochemical studies in which the control of the potential is essential; however, for sensing applications, this is rarely a problem. The CE is usually made of the same ink as the WE.
[1]
[2][7][12][22][23][24][25]. To choose the correct substrate, it is important to keep the final application in mind: for example, ceramics are easy to print on and are highly robust but are more expensive than paper. Paper is light and easy to transport but its flexibility and moisture tolerance is limited. Polymeric substrates, especially flexible ones, are interesting for wearable sensors; in these cases, the limitation is related to the bending endurance of the printed electrodes.
[7][13][26][27]. Thus, their high versatility together with their ease of use and portability make SPEs one of the main transducers for the development of electroanalytical devices.
2. (Bio)Sensors Based on SPEs
[12].
[5][3]. Immunosensors are highly specific and can be applied to a wide variety of analytes provided that an antibody that interacts with the analyte is available. Moreover, different strategies (e.g., the use of different labels or nanomaterials) can be used to improve their sensitivity. However, compared to enzymatic sensors, immunosensors are usually more labour intensive and less robust since several steps with long incubation times are required.
[32]
[30].
References
- Couto, R.A.S.; Lima, J.L.F.C.; Quinaz, M.B. Recent developments, characteristics and potential applications of screen-printed electrodes in pharmaceutical and biological analysis. Talanta 2016, 146, 801–814.
- Arduini, F.; Micheli, L.; Moscone, D.; Palleschi, G.; Piermarini, S.; Ricci, F.; Volpe, G. Electrochemical biosensors based on nanomodified screen-printed electrodes: Recent applications in clinical analysis. Trends Anal. Chem. 2016, 79, 114–126.
- Rama, E.C.; Costa-García, A. Screen-printed Electrochemical Immunosensors for the Detection of Cancer and Cardiovascular Biomarkers. Electroanalysis 2016, 28, 1700–1715.
- Smart, A.; Crew, A.; Pemberton, R.; Hughes, G.; Doran, O.; Hart, J.P. Screen-printed carbon based biosensors and their applications in agri-food safety. TrAC Trends Anal. Chem. 2020, 127, 115898.
- Vasilescu, A.; Nunes, G.; Hayat, A.; Latif, U.; Marty, J.L. Electrochemical affinity biosensors based on disposable screen-printed electrodes for detection of food allergens. Sensors 2016, 16, 1863.
- Mishra, R.K.; Nunes, G.S.; Souto, L.; Marty, J.L. Screen printed technology—An application towards biosensor development. In Encyclopedia of Interfacial Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 487–498.
- Li, M.; Li, Y.T.; Li, D.W.; Long, Y.T. Recent developments and applications of screen-printed electrodes in environmental assays-A review. Anal. Chim. Acta 2012, 734, 31–44.
- Hayat, A.; Marty, J.L. Disposable screen printed electrochemical sensors: Tools for environmental monitoring. Sensors 2014, 14, 10432–10453.
- Cano-Raya, C.; Denchev, Z.Z.; Cruz, S.F.; Viana, J.C. Chemistry of solid metal-based inks and pastes for printed electronics–A review. Appl. Mater. Today 2019, 15, 416–430.
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Electrochemical (bio)sensors: Promising tools for green analytical chemistry. Curr. Opin. Green Sustain. Chem. 2019, 19, 1–7.
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal. Chem. 2013, 50, 78–84.
- Sanati, A.; Jalali, M.; Raeissi, K.; Karimzadeh, F.; Kharaziha, M.; Mahshid, S.S.; Mahshid, S. A review on recent advancements in electrochemical biosensing using carbonaceous nanomaterials. Microchim. Acta 2019, 186, 773.
- Metrohm DropSens. Available online: (accessed on 29 September 2020).
- Micrux Technologies. Available online: (accessed on 29 September 2020).
- Pine Research. Available online: (accessed on 29 September 2020).
- Gwent Group. Available online: (accessed on 29 September 2020).
- PalmSens. Available online: (accessed on 29 September 2020).
- Rusens. Available online: (accessed on 29 September 2020).
- Putzbach, W.; Ronkainen, N.J. Immobilization techniques in the fabrication of nanomaterial-based electrochemical biosensors: A review. Sensors (Basel) 2013, 13, 4811–4840.
- Antuña-Jiménez, D.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Screen-printed electrodes modified with metal nanoparticles for small molecule sensing. Biosensors 2020, 10, 9.
- Duffy, G.F.; Moore, E.J. Electrochemical Immunosensors for Food Analysis: A Review of Recent Developments. Anal. Lett. 2017, 50, 1–32.
- Windmiller, J.R.; Bandodkar, A.J.; Parkhomovsky, S.; Wang, J. Stamp transfer electrodes for electrochemical sensing on non-planar and oversized surfaces. Analyst 2012, 137, 1570–1575.
- Mishra, R.K.; Hubble, L.J.; Martín, A.; Kumar, R.; Barfidokht, A.; Kim, J.; Musameh, M.M.; Kyratzis, I.L.; Wang, J. Wearable flexible and stretchable glove biosensor for on-site detection of organophosphorus chemical threats. ACS Sens. 2017, 2, 553–561.
- Desmet, C.; Marquette, C.A.; Blum, L.J.; Doumèche, B. Paper electrodes for bioelectrochemistry: Biosensors and biofuel cells. Biosens. Bioelectron. 2016, 76, 145–163.
- Moro, G.; Bottari, F.; Van Loon, J.; Du Bois, E.; De Wael, K.; Moretto, L.M. Disposable electrodes from waste materials and renewable sources for (bio)electroanalytical applications. Biosens. Bioelectron. 2019, 146.
- Neves, M.M.P.S.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Screen-Printed Electrochemical 96-Well Plate: A High-Throughput Platform for Multiple Analytical Applications. Electroanalysis 2014, 26, 2764–2772.
- Piermarini, S.; Micheli, L.; Ammida, N.H.S.; Palleschi, G.; Moscone, D. Electrochemical immunosensor array using a 96-well screen-printed microplate for aflatoxin B1 detection. Biosens. Bioelectron. 2007, 22, 1434–1440.
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification1International Union of Pure and Applied Chemistry: Physical Chemistry Division, Commission I.7 (Biophysical Chemistry); Analytical Chemistry Division, Commission V.5 (Electroanalytical). Biosens. Bioelectron. 2001, 16, 121–131.
- Sharma, H.; Mutharasan, R. Review of biosensors for foodborne pathogens and toxins. Sens. Actuators B Chem. 2013, 183, 535–549.
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763.
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv. 2012, 30, 489–511.
- Crapnell, R.D.; Hudson, A.; Foster, C.W.; Eersels, K.; van Grinsven, B.; Cleij, T.J.; Banks, C.E.; Peeters, M. Recent advances in electrosynthesized molecularly imprinted polymer sensing platforms for bioanalyte detection. Sensors (Switzerland) 2019, 19, 1204.
- Tudorache, M.; Bala, C. Biosensors based on screen-printing technology, and their applications in environmental and food analysis. Anal. Bioanal. Chem. 2007, 388, 565–578.
- Ricci, F.; Adornetto, G.; Palleschi, G. A review of experimental aspects of electrochemical immunosensors. Electrochim. Acta 2012, 84, 74–83.
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214.
- Wang, Y.; Duncan, T.V. Nanoscale sensors for assuring the safety of food products. Curr. Opin. Biotechnol. 2017, 44, 74–86.
- Silva, N.F.D.; Neves, M.M.P.S.; Magalhães, J.M.C.S.; Freire, C.; Delerue-Matos, C. Emerging electrochemical biosensing approaches for detection of Listeria monocytogenes in food samples: An overview. Trends Food Sci. Technol. 2020, 99, 621–633.
- Silva, N.F.D.; Magalhães, J.M.C.S.; Freire, C.; Delerue-Matos, C. Electrochemical biosensors for Salmonella: State of the art and challenges in food safety assessment. Biosens. Bioelectron. 2018, 99, 667–682.
