Intima-media thickness (IMT) measurement is a non-invasive method of arterial wall assessment. An increased IMT is a common manifestation of atherosclerosis associated with endothelial dysfunction. In the course of pregnancy, various maternal organs, including the endothelium, are prepared for their new role. However, several pre-gestational conditions involving endothelial dysfunction, such as diabetes, chronic hypertension, and obesity, may impair the adaptation to pregnancy, whereas vascular changes may also affect fetal development, thus, influencing the fetal IMT. In fact, data indicate that following the delivery, the endothelial dysfunction persists and influences the future health of the mother and the newborn.
- intima-media thickness
- vascular programming
- preeclampsia
- growth restriction
1. Introduction
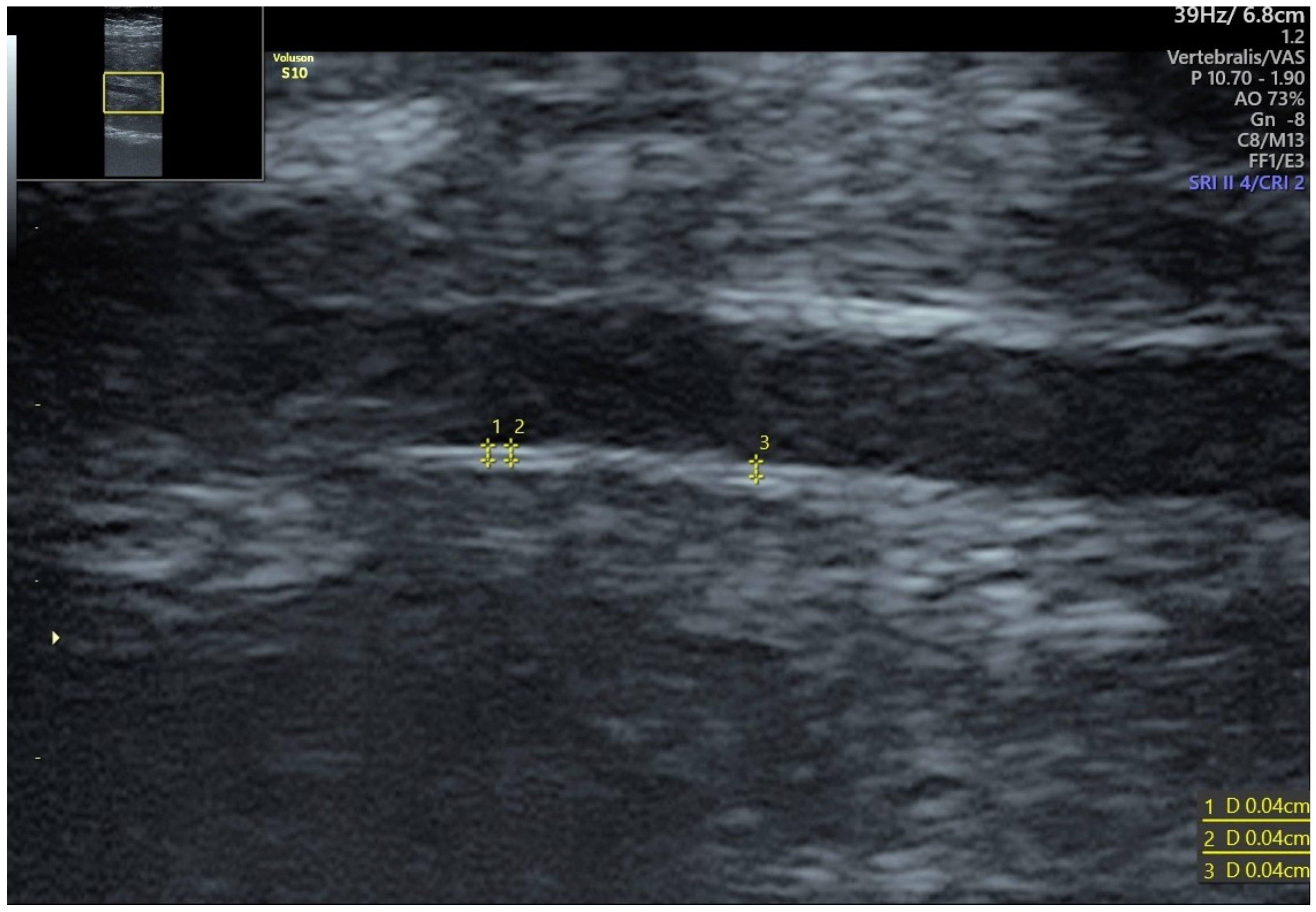
2. Effectiveness of IMT Measurements
Carotid intima-media thickness (cIMT) is a strong predictor of vascular events, slightly more associated with stroke than with myocardial infarction [4][3]. Additionally, increased carotid IMT is a marker of atherosclerosis, and it is used for the risk assessment of vascular events. However, adding carotid IMT measurement to traditional cardiovascular risk prediction models does not statistically improve these models’ performance [5][4]. Similar observations were found in pediatric populations where patients with diabetes mellitus, hypertension, or chronic renal failure presented a statistically increased cIMT, hence confirming early vascular damage and the increased cardiovascular risk for this patient group in adulthood [6][5].
An elevated carotid IMT is a common manifestation of generalized atherosclerosis involving endothelial damage [7][6]. The endothelium, as a part of tunica intima, participates in maintaining thrombolytic and fibrinolytic balance. However, several factors may impair endothelial function. Obesity, hypertension, hyperglycemia, and increased blood pressure contribute to the endothelial damage, leading to a local inflammatory reaction [8][7]. Inflammation, in turn, prevents endothelium from its anticoagulative role, resulting in atherosclerosis and ultimately in vascular events. Furthermore, atherosclerosis is a leading vascular complication in diabetes. Patients suffering from diabetes, particularly type 2 diabetes, need to maintain regular physical activity in order to reduce atherosclerosis and the risk of vascular events. It is worth bearing in mind that hyperglycemia-induced oxidative stress in diabetic patients also impairs both the endothelium and vascular smooth muscle. Hence, vascular smooth muscle changes contribute to the development of hypertension and cardiovascular disease in diabetic patients [10][8].3. IMT in Pregnancy
Physiological changes in the maternal body affect vascular wall and the intima-media thickness. Maternal metabolism in early pregnancy becomes anabolic-oriented, and insulin levels increase as well as an increased insulin sensitivity are observed. Consequently, fat tissue deposits are formed, and the accumulation of fatty acids and cholesterol in maternal serum is noted [11,12][9][10]. In the course of pregnancy, endothelium adapts by increasing the endothelium-dependent vasodilatation, although this adaptation is less expressed during complicated pregnancies [16][11]. Diabetes mellitus, hypertension, and dyslipidemia are associated with an increased cIMT, suggesting that this parameter might be involved in the pathogenesis of the metabolic syndrome. In addition, similar subclinical vascular features are present during pregnancy in some cases. Preeclampsia (PE) is one of the most common multisystem pregnancy complications. It is a multisystem disease, complicating 3–8% of pregnancies, and simultaneously constitutes one of the most significant causes of maternal mortality [17,18][12][13]. It is defined as new-onset hypertension after the 20th week of gestation with a systolic blood pressure (BP) >140 mmHg or diastolic BP ≥90 mmHg, and significant proteinuria amounting to 300 mg of protein within 24 h. Pathophysiological consequences are impaired placental perfusion followed by the involvement of the whole organism, including renal, hematologic, hepatological, and neurological complications, and fetal growth restriction. Known risk factors for atherosclerosis constitute risk factors for PE. One of the everyday objectives of clinicians comprises the prediction of the onset of PE for the optimal therapeutic modality and prevention of the PE effects on the mother and the fetus. According to the ASPRE study, aspirin use in high PE risk patients decreases the prevalence of preterm preeclampsia by 50% [19][14]. Additionally, assessment of risk factors and modern treatment of hypertension in pregnancy remain fundamental in prevention and early diagnosis of PE. However, a lack of noninvasive methods, which might improve PE screening, is evident. Therefore, extrapolating cardiological experience with IMT measurements to perinatology might contribute to developing an upgraded PE screening protocol involving the ultrasound IMT measurement. In the course of pregnancy, endothelium adapts by increasing the endothelium-dependent vasodilatation. Yet, this adaptation is less expressed if complications of pregnancy occur [16][11]. An increased IMT is also associated with the onset of an incident of hypertension in the population without the previously diagnosed hypertension. Endothelial dysfunction, the basis of the onset of atherothrombosis, is the process underlying PE and contributing to the severity of PE [15]. Predicting PE onset for optimal therapeutic modality and prevention of the effects of PE in the mother and fetus, is a goal in the daily work of the obstetrician. Placental dysfunction is considered the leading cause of preeclampsia and FGR. Insufficiency of placental circulation may lead to mothers’ gestational hypertension, although ultrasound observations indicate that vascular changes occur on a more profound level. Fetal growth restriction (FGR) is observed when the fetus cannot achieve its genetic growth potential, primarily as a consequence of placental dysfunction [29][16]. It is vital to note that placental insufficiency affects both the mother and the fetus, thus, an increased cIMT is observed in the mother, whereas an increased aIMT is found in the fetus [30,31][17][18]. Moreover, although the use of non-invasive, high-resolution ultrasound-based imaging allows for the detection of atherosclerosis, it is still inconclusive whether PE and fetal growth impairment can be predicted on the basis of IMT in an asymptomatic population.4. Fetal IMT
The most accessible fetal artery for IMT measurement is the abdominal aorta. Most authorscholars measure fetal aIMT in the infrarenal segment—between renal arteries and iliac bifurcation [24,32,33,34][19][20][21][22]. An increased thickness of the fetal aorta causes an increase in the resistance of flow in the artery, which may be expressed by an increased value of pulsatility index (PI). This phenomenon may have further consequences for the disturbed flow in the smaller arteries [33][21]. The change in the diameter of the systolic and diastolic abdominal aorta is greater in aortas with higher intima-media thickness, which can be attributed to the increased vessel stiffness. Additionally, the disturbed flow through the aorta with an increased IMT may cause vessel wall dysfunction. Fetal growth restriction (FGR) is one of the most common conditions affecting ongoing pregnancies. A substantial body of evidence has reported a broad spectrum of unfavorable perinatal and lifelong effects associated with FGR, such as changes in metabolism, cardiovascular system, and brain structure [35,36,37][23][24][25]. Fetal cardiovascular adaptation to hypoxia and undernutrition is a central adaptive mechanism that induces cardiac and vascular remodeling. Vascular intima-media thickness (IMT) is a standard diagnostic procedure in assessing cardiovascular risk in asymptomatic adults. Recently, an inverse relationship between the aortic IMT (aIMT), arterial stiffness, and low birth weight have been reported [24,33][19][21]. The growth-restricted fetus showed evidence of abdominal aortic intima-media thickening detected by both ultrasound and histology, neither one of which were present in the abdominal aorta of the non-growth-restricted fetus [38][26]. Cardiovascular programming among SGA fetuses challenge the opinion of the healthy, but small, fetuses. Another theory is that IMT measurement is more accurate and may be applied in detecting subclinical impairment in the earlier stages of fetal development. According to the research, vascular remodeling beginning in utero accounts for the risk factors for cardiovascular diseases and type 2 diabetes in adulthood, whereas cardiovascular changes observed in fetuses persist and are still present at six months of age [35,36][23][24]. In addition, fetal aIMT is associated with a placenta-to-fetus weight ratio, which implies that an increased IMT in fetal vessels impairs placental function and nutrient distribution from the maternal circulation [42][27].5. Treatment Options
It is possible that endothelial repair therapy or micro-scale anti-inflammatory treatment might be effective in preventing cardiovascular remodeling. Skilton et al. conducted a study in which they postnatally used ω-3 supplementation to prevent growth restriction and thickening of cIMT. The supplementation continued for five years and statistically significantly reduced growth restriction, as well as an increased cIMT in the pediatric population [45][28]. Another promising perspective was the study on protease-activated receptor-2 (PAR2), which modulates inflammatory responses, obesity, and vasodilation. According to the mentioned study on a rat model, PAR2 antagonists inhibited adipose gain and metabolic dysfunction. Additionally, vasodilation activity was observed in endothelial dysfunction, which might contribute to preventing future complications of prenatal exposure to risk factors [46][29].References
- Willeit, P.; Tschiderer, L.; Allara, E.; Reuber, K.; Seekircher, L.; Gao, L.; Liao, X.; Lonn, E.; Gerstein, H.C.; Yusuf, S.; et al. Carotid Intima-Media Thickness Progression as Surrogate Marker for Cardiovascular Risk: Meta-Analysis of 119 Clinical Trials Involving 100 667 Patients. Circulation 2020, 142, 621–642.
- Prospective Study on the Prognostic Value of Repeated Carotid Intima-Media Thickness Assessment in Patients with Coronary and Extra Coronary Steno-Occlusive Arterial Disease. Available online: https://www.mp.pl/paim/issue/article/4407 (accessed on 28 November 2021).
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of Clinical Cardiovascular Events with Carotid Intima-Media Thickness: A Systematic Review and Meta-Analysis. Circulation 2007, 115, 459–467.
- Van den Oord, S.C.H.; Sijbrands, E.J.G.; ten Kate, G.L.; van Klaveren, D.; van Domburg, R.T.; van der Steen, A.F.W.; Schinkel, A.F.L. Carotid Intima-Media Thickness for Cardiovascular Risk Assessment: Systematic Review and Meta-Analysis. Atherosclerosis 2013, 228, 1–11.
- Lamotte, C.; Iliescu, C.; Libersa, C.; Gottrand, F. Increased Intima-Media Thickness of the Carotid Artery in Childhood: A Systematic Review of Observational Studies. Eur. J. Pediatr. 2011, 170, 719–729.
- Kadoglou, N.P.E.; Iliadis, F.; Liapis, C.D. Exercise and Carotid Atherosclerosis. Eur. J. Vasc. Endovasc. Surg. 2008, 35, 264–272.
- Markopoulou, P.; Papanikolaou, E.; Analytis, A.; Zoumakis, E.; Siahanidou, T. Preterm Birth as a Risk Factor for Metabolic Syndrome and Cardiovascular Disease in Adult Life: A Systematic Review and Meta-Analysis. J. Pediatr. 2019, 210, 69–80.e5.
- Lespagnol, E.; Dauchet, L.; Pawlak-Chaouch, M.; Balestra, C.; Berthoin, S.; Feelisch, M.; Roustit, M.; Boissière, J.; Fontaine, P.; Heyman, E. Early Endothelial Dysfunction in Type 1 Diabetes Is Accompanied by an Impairment of Vascular Smooth Muscle Function: A Meta-Analysis. Front. Endocrinol. 2020, 11, 203.
- Ryckman, K.K.; Spracklen, C.N.; Smith, C.J.; Robinson, J.G.; Saftlas, A.F. Maternal Lipid Levels during Pregnancy and Gestational Diabetes: A Systematic Review and Meta-Analysis. BJOG 2015, 122, 643–651.
- Lain, K.Y.; Catalano, P.M. Metabolic Changes in Pregnancy. Clin. Obstet. Gynecol. 2007, 50, 938–948.
- Lopes van Balen, V.A.; van Gansewinkel, T.a.G.; de Haas, S.; van Kuijk, S.M.J.; van Drongelen, J.; Ghossein-Doha, C.; Spaanderman, M.E.A. Physiological Adaptation of Endothelial Function to Pregnancy: Systematic Review and Meta-Analysis. Ultrasound Obstet. Gynecol. 2017, 50, 697–708.
- Carty, D.M.; Delles, C.; Dominiczak, A.F. Preeclampsia and Future Maternal Health. J. Hypertens. 2010, 28, 1349–1355.
- Sibai, B.; Dekker, G.; Kupferminc, M. Pre-Eclampsia. Lancet 2005, 365, 785–799.
- Rolnik, D.L.; Wright, D.; Poon, L.C.Y.; Syngelaki, A.; O’Gorman, N.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. ASPRE Trial: Performance of Screening for Preterm Pre-Eclampsia. Ultrasound Obstet. Gynecol. 2017, 50, 492–495.
- Barbour, L.A.; McCurdy, C.E.; Hernandez, T.L.; Kirwan, J.P.; Catalano, P.M.; Friedman, J.E. Cellular Mechanisms for Insulin Resistance in Normal Pregnancy and Gestational Diabetes. Diabetes Care 2007, 30 (Suppl. 2), S112–S119.
- Nardozza, L.M.M.; Caetano, A.C.R.; Zamarian, A.C.P.; Mazzola, J.B.; Silva, C.P.; Marçal, V.M.G.; Lobo, T.F.; Peixoto, A.B.; Araujo Júnior, E. Fetal Growth Restriction: Current Knowledge. Arch. Gynecol. Obstet. 2017, 295, 1061–1077.
- Stergiotou, I.; Bijnens, B.; Cruz-Lemini, M.; Figueras, F.; Gratacos, E.; Crispi, F. Maternal Subclinical Vascular Changes in Fetal Growth Restriction with and without Pre-Eclampsia. Ultrasound Obstet. Gynecol. 2015, 46, 706–712.
- Gomez-Roig, M.D.; Mazarico, E.; Valladares, E.; Guirado, L.; Fernandez-Arias, M.; Vela, A. Aortic Intima-Media Thickness and Aortic Diameter in Small for Gestational Age and Growth Restricted Fetuses. PLoS ONE 2015, 10, e0126842.
- Stergiotou, I.; Crispi, F.; Valenzuela-Alcaraz, B.; Cruz-Lemini, M.; Bijnens, B.; Gratacos, E. Aortic and Carotid Intima-Media Thickness in Term Small-for-Gestational-Age Newborns and Relationship with Prenatal Signs of Severity. Ultrasound Obstet. Gynecol. 2014, 43, 625–631.
- Evanoff, N.G.; Dengel, D.R.; Narasimhan, S. Assessing Vascular Characteristics of the Fetal Descending Aorta: A Feasibility Study. J. Clin. Ultrasound 2020, 48, 211–215.
- Visentin, S.; Londero, A.P.; Calanducci, M.; Grisan, E.; Bongiorno, M.C.; Marin, L.; Cosmi, E. Fetal Abdominal Aorta: Doppler and Structural Evaluation of Endothelial Function in Intrauterine Growth Restriction and Controls. Ultraschall Med. 2019, 40, 55–63.
- Galjaard, S.; Pasman, S.A.; Ameye, L.; Timmerman, D.; Devlieger, R. Intima-Media Thickness Measurements in the Fetus and Mother during Pregnancy: A Feasibility Study. Ultrasound Med. Biol. 2014, 40, 1949–1957.
- Cruz-Lemini, M.; Crispi, F.; Valenzuela-Alcaraz, B.; Figueras, F.; Sitges, M.; Bijnens, B.; Gratacós, E. Fetal Cardiovascular Remodeling Persists at 6 Months in Infants with Intrauterine Growth Restriction. Ultrasound Obstet. Gynecol. 2016, 48, 349–356.
- Visentin, S.; Grumolato, F.; Nardelli, G.B.; Di Camillo, B.; Grisan, E.; Cosmi, E. Early Origins of Adult Disease: Low Birth Weight and Vascular Remodeling. Atherosclerosis 2014, 237, 391–399.
- Robbins, C.L.; Hutchings, Y.; Dietz, P.M.; Kuklina, E.V.; Callaghan, W.M. History of Preterm Birth and Subsequent Cardiovascular Disease: A Systematic Review. Am. J. Obstet. Gynecol. 2014, 210, 285–297.
- Skilton, M.R.; Celermajer, D.S.; Cosmi, E.; Crispi, F.; Gidding, S.S.; Raitakari, O.T.; Urbina, E.M. Natural History of Atherosclerosis and Abdominal Aortic Intima-Media Thickness: Rationale, Evidence, and Best Practice for Detection of Atherosclerosis in the Young. J. Clin. Med. 2019, 8, 1201.
- Iwashima, S.; Ishikawa, T.; Akira, O.; Itou, H. Association of Abdominal Aortic Wall Thickness in the Newborn with Maternal Factors. Am. J. Perinatol. 2012, 29, 441–448.
- Skilton, M.R.; Ayer, J.G.; Harmer, J.A.; Webb, K.; Leeder, S.R.; Marks, G.B.; Celermajer, D.S. Impaired Fetal Growth and Arterial Wall Thickening: A Randomized Trial of ω-3 Supplementation. Pediatrics 2012, 129, e698–e703.
- Kagota, S.; Maruyama, K.; McGuire, J.J. Characterization and Functions of Protease-Activated Receptor 2 in Obesity, Diabetes, and Metabolic Syndrome: A Systematic Review. Biomed. Res. Int. 2016, 2016, 3130496.
