Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 4 by Sirius Huang and Version 3 by Sirius Huang.
Biochar can be defined as the carbonaceous product that is obtained when biomass is subjected to heat treatment in an oxygen-limited environment (pyrolysis) and the charred product when applied to soil as an amendment. It is an important and popular carbon sequestration method to mitigate climate change.
- biochar
- carbon sequestration
- climate change
- shifting cultivation
- soil properties
1. Biochar and Its Importance
Biochar can be defined as the carbonaceous product that is obtained when biomass is subjected to heat treatment in an oxygen-limited environment (pyrolysis) and the charred product when applied to soil as an amendment [1]. Pyrolysis is the thermal depolymerization of biomass at elevated temperatures without the participation of oxygen. The end products of pyrolysis are syngas, bio-oil, and char [2][3]. The char can be used as an energy source, and acts as a soil amendment which is called biochar. It can be produced from a wide variety of organic materials including paper mill sludge, forestry and crop residues, and poultry waste [4]. Biochar applications gaining growing interest as a sustainable technology that helps in improving the weathered and degraded soils [5]. It enhances the soil’s physical (i.e., bulk density, water holding capacity, permeability, etc.), chemical (i.e., nutrient retention, nutrient availability, etc.”) and biological (microbial population, earthworm, enzyme activities, etc.) characteristics which thereby improve plant growth and development [6]. Its recalcitrant nature towards microbial decomposition guarantees a long-term benefit to soil fertility [7]. Apart from this, it also improves the saturated hydraulic conductivity of the topsoil of rice fields and xylem sap which results in higher crop yields and improved response to N and NP chemical fertilizer treatments [8]. They possess a negligible number of heavy metals or toxic elements such as As, Cd, Pb, and polycyclic aromatic hydrocarbons so contamination risk is very low. They have the potential to enhance soil fertility; crop productivity [9][10][11][12]; enhance nutrient and water use efficiencies [13], and mitigate emissions of N2O [14]. The increasing level of atmospheric CO2 can be mitigated by the long-term storage of C in soil. In this regard, biochar has emerged as a viable option for sequestering carbon in soil [9].
2. Properties of Biochar
The biochar properties are greatly influenced by the feedstock source and pyrolysis conditions [14][15]. In general, wood biochar has high total C; low ash content; low total N, P, K, S, Ca, Mg, Al, Na, and Cu contents; low potential cation exchange capacity (CEC); and exchangeable cations as compared with manure-based biochar. The increase in pyrolysis temperature increased the ash content, pH, and surface basicity and decreased the surface acidity [15]. In the case of fast pyrolysis, biomass is rapidly heated to 400–550 °C and the main product is bio-oil while in slow pyrolysis, the biomass is slowly heated to the desired peak temperature and the main products are biochar and syngas [16]. Some of the important physicochemical properties of biochar are higher surface area and porosity, low bulk density, higher cation exchange capacity (CEC), neutral to high pH, and higher carbon content [17]. It also contains N, P, and basic cations such asCa, Mg, and K [18] which are essential plant nutrients for crop growth and development. Pyrolysis at low temperature yields higher biochar while biochar with higher C content, large surface area, high adsorption characteristics, greater porosity, and more stable C are obtained at higher temperatures [18]. At high pyrolysis temperature (>600 °C), the functional groups are gradually lost, leaving the material with a high degree of condensation and more recalcitrant with polycyclic aromatic structure [18][19]. The stability of biochar to sustain hundreds to thousands of years in the soil is attributed to a higher proportion of aromatic structures which in turn provides higher resistance against chemical and biological decomposition [20]. The concentrations of C and N for plant-based biochar increase with an increase in pyrolysis temperature while the concentrations of C and N in mineral-rich feedstock decrease with increasing pyrolysis temperature [14]. Some of the properties of biochar from different biomass sources are presented in Table 1.
Table 1. Properties of biochar derived from different sources.
| Materials Used for Producing Biochar | pH | Total C (%) |
Total N (%) |
C: N Ratio |
Ca (cmol kg −1) | Mg (cmol kg −1) | P (cmol kg −1) | K (cmol kg −1) |
Cation Exchange Capacity (cmol kg −1) |
|---|---|---|---|---|---|---|---|---|---|
| Paper mill waste 1 (waste woodchip) | 9.4 | 50.0 | 0.48 | 104 | 6.2 | 1.20 | - | 0.22 | 9.00 |
| Paper mill waste 2 (waste wood chip) | 8.2 | 52.0 | 0.31 | 168 | 11.0 | 2.60 | - | 1.00 | 18.00 |
| Green waste (grass, cotton trash and plant prunings) | 9.4 | 36.0 | 0.18 | 200 | 0.4 | 0.56 | - | 21.00 | 24.00 |
| Eucalyptus biochar | - | 82.4 | 0.57 | 145 | - | - | 1.87 | - | 4.69 |
| Cooking biochar | - | 72.9 | 0.76 | 96 | - | - | 0.42 | - | 11.19 |
| Poultry litter (450 °C) | 9.9 | 38.0 | 2.00 | 19 | - | - | 37.42 | - | 11 |
| Poultry litter (550 °C) | 13 | 33.0 | 0.85 | 39 | - | - | 5.81 | - | 11 |
| Wood biochar | 9.2 | 72.9 | 0.76 | 120 | 0.83 | 0.20 | 0.10 | 1.19 | 11.90 |
| Hardwood sawdust | - | 66.5 | 0.3 | 221 | - | - | - | - | - |
3. Benefits of Biochar
3.1. Interaction of Biochar in Soil
Biochar is known to sequester carbon and improve soil functions. Within a short period, the interaction between biochar, soils, microbes, and plant roots occurs after its incorporation into the soil [1]. The factors influencing the types of interactions are (i) feedstock composition, in particular, the total percentage and specific composition of the mineral fraction; (ii) pyrolysis process conditions; (iii) biochar particle size and delivery system; and (iv) soil properties and local environmental conditions. The aging of biochar starts before addition to soils and once incorporated, the rate is partly governed by the soil moisture and temperature condition [21]. Immediately after the application of biochar amendment, the evolution of biochar-derived carbon can be observed within the first 2 weeks and decreases exponentially with time [22]. Water plays a major role in mineral weathering processes such as hydrolysis, dissolution, carbonation and decarbonation, hydration, and redox reactions. The rate of these reactions depends on the type of biochar, nature of reactions, and pedoclimatic conditions. The dissolution and leaching of soluble salts (e.g., K and Na carbonates and oxides) present in the biochar is the first reaction among all the interactions. The dissolution makes the pH increase in the water film around the biochar particles [23]. The biochar converted from biomass is still thermodynamically unstable under the oxidative state of most surface soils [24]. Low-temperature biochar has a considerable fraction of non-aromatic C, which makes the biochar more susceptible to microbial attack [25] and subsequent oxidation than high-temperature biochar [26]. Despite the high stability of aromatic C, it has redox activity and functions as a reducing agent, O2 being the most common electron-acceptor species. The electron-donating properties of an area with a high density of π-electrons boosted the abiotic reaction and initiated the oxidation of biochar [27]. The number of free radicles in biochar is dependent on the pyrolysis process [28] and thereby increases the reactivity towards the oxidation [29]. Biochar particles can have both acidic and basic properties and are greatly influenced by the moisture condition and the surface retention of ions through electrostatic interactions [30]. They usually co-exist, with the oxidative processes the concentration of basic sites decreases as the biochar particle weathers [31][32]. The biochar which has higher mineral content have interaction with organic matter and clay mineral surfaces depending on the type of clay (2:1, 1:1), distribution of functional groups on the clays (siloxane, OH), and organic matter (COOH, C=O, C–O, CN), the polarity of these compounds and the composition and concentration of cations and anions in solution [33]. There are also complex interactions between biochar with plant roots and microorganisms. Biochar interacts with the soils along with the root hairs. Once the root system encounters the biochar particle, the root hairs can penetrate the water-filled macropores of the particle and the organic compounds (including low- and high-molecular-weight compounds such as free exudates and mucilage); sloughed-out cells and tissues; and lysates from the growing root can be absorbed by biochar surfaces [34]. The fauna (such as worms, termites, larvae, and other insects) present in soil ingest or live inside biochar by breaking it up or coating it with organic compounds. Bioturbation by earthworms plays an important role in the physical mixing of the soil profile with the biochar. Over time, the downward movement of biochar increases within the soil profile where the soil microbial activity is lower [35].
3.2. Role of Biochar in Carbon Sequestration
An increase in ambient temperature has now been unequivocally proven and reported to increase at an unprecedented rate [36]. Since the late nineteenth century, global surface temperatures have increased by 0.88 °C [37]. Carbon dioxide (CO2), methane (CH4), and nitrous oxides (NO2) are considered to be the important anthropogenic GHGs, which are released into the atmosphere through the burning of fossil and biomass fuels as well decomposition of above- and belowground organic matter. As per the report, carbon dioxide (CO2) concentration has increased from 280 ppmv in 1850 to 380 ppmv in 2005 (up to 31% increase) [37]. An increase in the concentrations of methane (CH4) and nitrous oxide (N2O) have also been observed over the same period but at a steady rate [36][37][38]. According to Pacala and Socolow [39], approximately 7 PgCyr−1 (1 Pg = 1015 g) is emitted by fossil fuel combustion and around 1.6 PgCyr−1 through deforestation, land-use change, and soil cultivation which in turn plays an important role in contributing to climate change leading to global warming. Thus, there lies a strong quest for mitigating the risks of global warming by stabilizing the GHGs present in the atmosphere [40][41][42]. As per Lal [43], three strategies can be adopted to lower CO2 emissions viz. (i) reducing global energy use, (ii) developing low- or no-carbon fuel, and (iii) sequestering CO2 from point sources or the atmosphere through natural and engineering techniques [42]. From the view of CO2 sequestration, there is a wide range of processes and technological options available in agricultural, industrial, and natural ecosystems which include biotic and abiotic sequestration [43]. Studies have considered the potential of bio-based carbon materials for gas capture and storage, and biochar has emerged as one of the important tools among different carbon sequestration techniques [9][44][45]. The application of biochar in the soil poses a novel approach to establishing a significant long-term sink of atmospheric carbon dioxide (CO2) in terrestrial ecosystems. With the use of a wide variety of biochar application programs, an estimation of 9.5 BTof carbon can be potentially stored in the soils by the year 2100 [9]. About 50% of the carbon can be sequestered during the conversion of biomass carbon to biochar as compared to only 3% carbon retention in soil by burning and less than 10–20% (after 5–10 years) through biological decomposition, thereby giving higher yields of stable soil carbon in soil upon application [9]. The recalcitrance mechanism in biochar is considered to be one of the most important phenomena for sequestering carbon for a longer period [18]. Long-term carbon sinks of biochar are also due to slow microbial decomposition and chemical transformation [20]. Biochar amended at 2, 5, 10, 20, 40, and 60% w/w levels corresponding to field application rate of 24–720 Mg ha−1 has been reported to reduce CO2 production as well as significant suppression of the ambient CH4 oxidation and N2O production at all levels as compared to unamended soils [44]. Thus, biochar can offer both large and long-term C sink in the soil making it one of the desirable choices for carbon sequestration for mitigating climate change. The figure (Figure 1) below represents the mechanism through which biochar acts as a carbon sink. Thus, biochar production from biowaste can not only act as a promising precursor for CO2 sequestration but also has also emerged as a sustainable strategy for solid waste management [45].
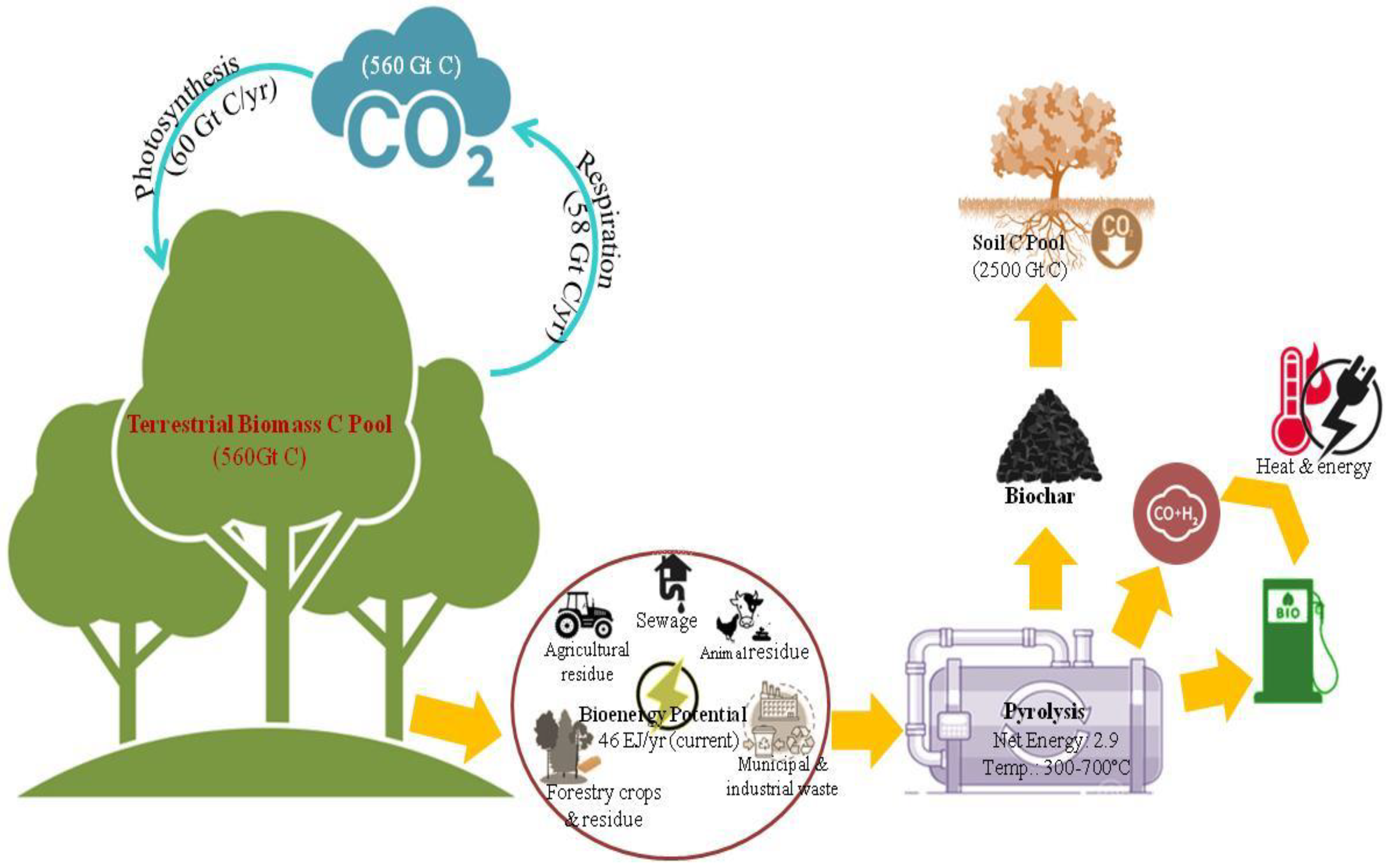
Figure 1. Schematic diagram of biochar-induced carbon sequestration.
3.3. Impact of Biochar on Soil Physical, Chemical, and Microbial Properties
Improved soil physical, chemical, and biological properties are desirable for optimum plant growth and development. Applications of biochar in soil are known to have a significant impact on various properties of soil [46][47][48]. The high porosity of biochar tends to improve a wide range of soil physical properties such as total porosity, soil density, soil moisture content, water holding capacity, and hydraulic conductivity [46][49][50]. Improvement in water retention capacity of the soil is mainly attributed to improved soil texture and aggregation posed by higher surface area and porosity of biochar [8][51][52]. Biochar applications at higher rates significantly increase the field capacity of the soil [53]. The effects can be more pronounced in non-irrigated regions with an increase in available water for crop growth as well as reducing the occurrence of water stress in between the events of rainfall. Addition of biochar decreases soil bulk density [48][54] which affects the infiltration rate in soil. Improved bulk density due to increased soil porosity will have a positive impact on soil aeration which is desirable for root and microbial respiration. Higher organic carbon content [55], as well as surface charge [56] in biochar, is another aspect that plays a crucial role in enhancing soil aggregation and its stability [55]. The stable soil aggregates change the structure of soil and thus improve soil moisture retaining capacity, infiltration, run-off reduction, and erosion [55].
Biochar plays a significant role in improving soil’s chemical properties which includes raising pH, organic carbon, and exchangeable cations [10]. Most studies reported an increase in soil pH upon biochar additions [48][51]. An increase in cation exchange capacity (CEC) is confirmed by Lehmann et al. [57] which is an important property to prevent leaching loss of nutrients and thus can increase the fertilizer use efficiency (FUE). The higher CEC of biochar is reported to possibly enhance soil aggregation by aiding in forming certain complexes between organic matter and other minerals with that biochar [56]. However, it is observed that the effective cation exchange capacity is reported to increase with time after being incorporated into the soil [56]. This is so because the surfaces of biochar tend to get oxidized after getting in contact with moisture (water) and air [31][56][58]. The advantages of an increase in pH value on biochar application are more pronounced, especially in acidic soils that are associated with heavy metal toxicity or nutrient deficiencies. Depending on the pH-buffering capacity of the soil, biochar is reported with typical high liming equivalence in raising the pH value in acidic soils [59]. The increase in pH due to liming effect of biochar can play a significant role in the availability of essential nutrients in the soil. Important macro (N, P, K, Ca, Mg) and micro-nutrients (Cu, Fe, Mn, Zn,) which are essential for plant growth and development are reported to increase upon application of biochar in soil [10][12][60][61]. Apart from this, biochar due to its high affinity to hold nutrients reduces nutrient loss through leaching which in turn increases fertilizer use efficiency by the plant [57]. Several investigations have confirmed that volatilization of NH4− decreases significantly with a high biochar application rate (10% or 20%, w/w) due to high CEC [57] however, biochar with high N content may lead to a higher leaching of NO3− [14]. Biochar particles are assumed to act like clay and thus hold large amounts of immobile water even at increased matric potentials. Several other studies also reveal that the addition of biochar significantly increases the nodulations of rhizobia [62] thereby confirming the improvement in nitrogen fixation [63]. As per Biederman and Harpole [62], the increase in N-fixation following biochar application was reported to be 72%. In terrestrial ecosystems, biochar is also observed to act as a habitat for mycorrhizal fungi. The porous structure of biochar provides a habitat for microbes in soil and protects them from predation [47]. The habitat leads to a ubiquitous symbiotic association between them and favors the soils to carry out various ecosystem services in contributing to sustainable plant production and ecosystem restoration [47].
However, changes in soil properties as discussed above depend on biochar application rate, type of feedstock, soil type, pyrolysis parameters, and various other conditions prevailed [10][64][65]. Several studies revealed that biochar, when applied at sufficiently high rates tends to improve soil’s physical properties [10][59][66][67]. Chan et al. [11] reported that the increasing rate of biochar application tends to increase the field capacity but, the significant changes could only be observed at higher rates of biochar application, i.e., 50 and 100 Mg ha−1. Soil amended with biochar made from green waste with application rates of 50 and 100 Mg ha−1 to an alfisol has shown significant retention of water at field capacity compared to control [10]. In another case, the addition of mixed hardwood biochar at 1 and 2% (w/w) to a mollisol, did not detect any effect on moisture retention at soil water potential of −0.33 bars and −15 bar however, significant increases in moisture retention were observed at −1 and −5 bars soil water potential compared to control [48]. An increase in pH value after application of biochar is reported to be higher in sandy and loamy soils as compared to clayey soils [68] however; buffering capacity is reported to be higher in finely textured soil compared to that of coarse-textured soil. Nutrient retention capacity is found to be higher in aged biochar when compared to fresh biochar [31], which suggests that CEC increases in soil over time following biochar application. It can act as an alternative to fertilizer. Thus, biochar can be effectively used as a soil amendment to improve its overall quality in a sustainable, economic, and environmentally friendly way. The overall effect of biochar on various soil properties has been represented in Figure 2.
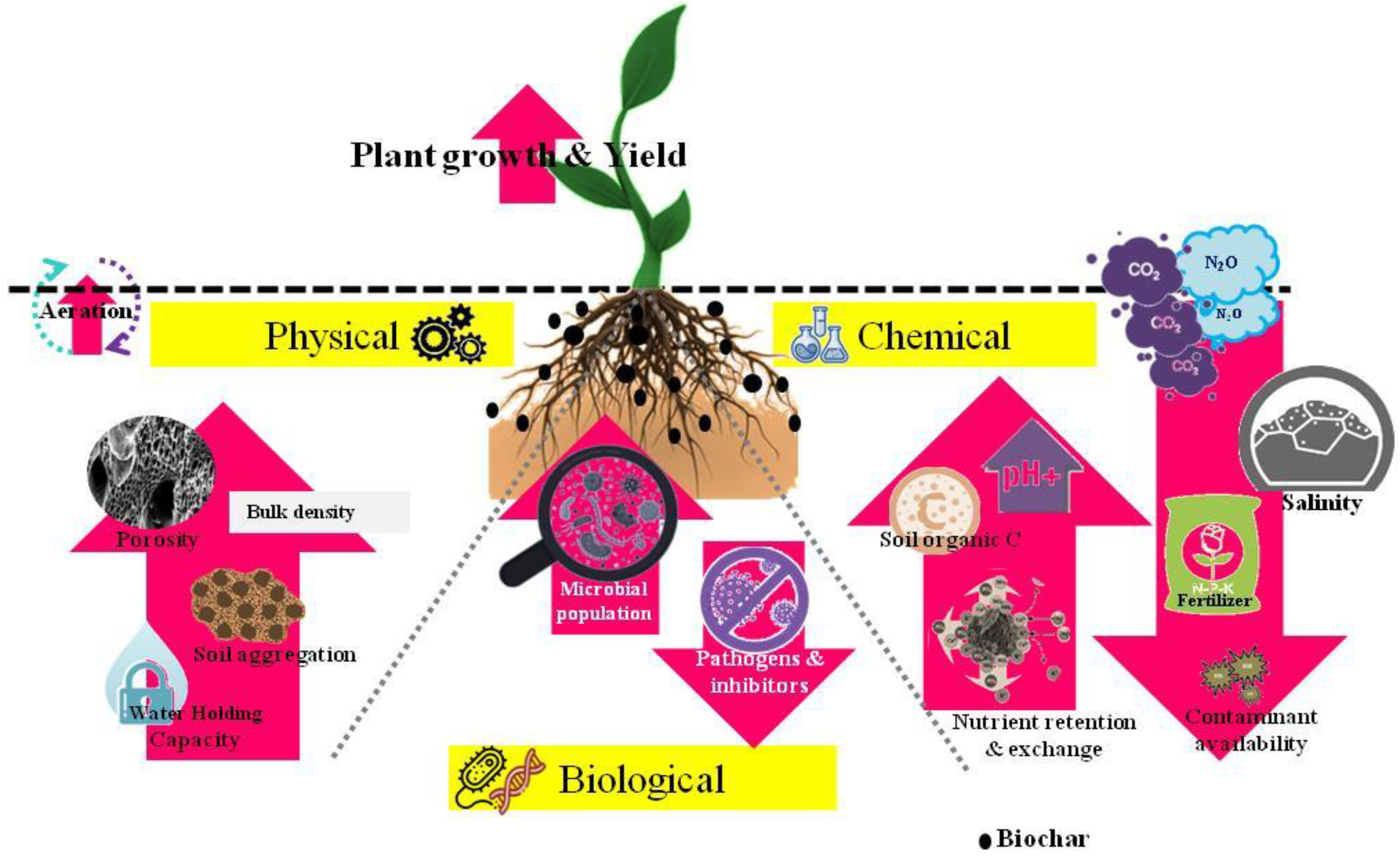
Figure 2. Graphical representation of the overall effect of Biochar after soil application.
References
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management; Earthscan: London, UK, 2009; Volume 1.
- Antal, M.J.; Grønli, M. The art, science, and technology of charcoal production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640.
- Joseph, S.D.; Camps-Arbestain, M.; Lin, Y.; Munroe, P.; Chia, C.H.; Hook, J.; Van Zwieten, L.; Kimber, S.; Cowie, A.; Singh, B.P.; et al. An investigation into the reactions of biochar in soil. Soil Res. 2010, 48, 501–515.
- Chan, K.Y.; Xu, Z. Biochar: Nutrient properties and their enhancement. Biochar Environ. Manag. Sci. Technol. 2009, 1, 67–84.
- Lehmann, J.; Rondon, M. Bio-char soil management on highly weathered soils in the humid tropics. Biol. Approaches Sustain. Soil Syst. 2006, 113, 530.
- Yamato, M.; Okimori, Y.; Wibowo, I.F.; Anshori, S.; Ogawa, M. Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci. Plant Nutr. 2006, 52, 489–495.
- Steiner, C.; Teixeira, W.G.; Lehmann, J.; Nehls, T.; de Macêdo, J.L.V.; Blum, W.E.; Zech, W. Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 2007, 291, 275–290.
- Asai, H.; Samson, B.K.; Stephan, H.M.; Songyikhangsuthor, K.; Homma, K.; Kiyono, Y.; Inoue, Y.; Shiraiwa, T.; Horie, T. Biochar amendment techniques for upland rice production in Northern Laos: 1. Soil physical properties, leaf SPAD and grain yield. Field Crop. Res. 2009, 111, 81–84.
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems—A review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 403–427.
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of green waste biochar as a soil amendment. Soil Res. 2007, 45, 629–634.
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Using poultry litter biochars as soil amendments. Soil Res. 2008, 46, 437–444.
- Steiner, C.; Glaser, B.; Geraldes Teixeira, W.; Lehmann, J.; Blum, W.E.; Zech, W. Nitrogen retention and plant uptake on a highly weathered central Amazonian Ferralsol amended with compost and charcoal. J. Plant Nutr. Soil Sci. 2008, 171, 893–899.
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230.
- Singh, B.; Singh, B.P.; Cowie, A.L. Characterisation and evaluation of biochars for their application as a soil amendment. Soil Res. 2010, 48, 516–525.
- Mukherjee, A.; Zimmerman, A.R. Organic carbon and nutrient release from a range of laboratory- produced biochars and biochar—Soil mixtures. Geoderma 2013, 193–194, 122–130.
- Berek, A.K. Exploring the potential roles of biochars on land degradation mitigation. J. Degrad. Min. Lands Manag. 2014, 1, 149–158.
- Major, J.; Rondon, M.; Molina, D.; Riha, S.J.; Lehmann, J. Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 2010, 333, 117–128.
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero, M.A.; Sonoki, T. Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 2014, 11, 6613–6621.
- Song, W.; Guo, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2012, 94, 138–145.
- Schulz, H.; Glaser, B. Effects of biochar compared to organic and inorganic fertilizers on soil quality and plant growth in a greenhouse experiment. J. Plant Nutr. Soil Sci. 2012, 175, 410–422.
- Nguyen, B.T.; Lehmann, J. Black carbon decomposition under varying water regimes. Org. Geochem. 2009, 40, 846–853.
- Singh, B.; Cowie, A. A novel approach, using 13C natural abundance, for measuring decomposition of biochars in soil. In Carbon and Nutrient Management in Agriculture; Fertilizer and Lime Research Centre, Massey University: Palmerston North, New Zealand, 2008.
- Yao, F.X.; Arbestain, M.C.; Virgel, S.; Blanco, F.; Arostegui, J.; Maciá-Agulló, J.A.; Macìas, F. Simulated geochemical weathering of a mineral ash-rich biochar in a modified Soxhlet reactor. Chemosphere 2010, 80, 724–732.
- Macías, F.; Camps Arbestain, M. Soil carbon sequestration in a changing global environment. Mitig. Adapt. Strateg. Glob. Chang. 2010, 15, 511–529.
- McBeath, A.V.; Smernik, R.J. Variation in the degree of aromatic condensation of chars. Org. Geochem. 2009, 40, 1161–1168.
- Nguyen, B.T.; Lehmann, J.; Hockaday, W.C.; Joseph, S.; Masiello, C.A. Temperature sensitivity of black carbon decomposition and oxidation. Environ. Sci. Technol. 2010, 44, 3324–3331.
- Contescu, A.; Vass, M.; Contescu, C.; Putyera, K.; Schwarz, J.A. Acid buffering capacity of basic carbons revealed by their continuous pK distribution. Carbon 1998, 36, 247–258.
- Feng, J.W.; Zheng, S.; Maciel, G.E. EPR investigations of the effects of inorganic additives on the charring and char/air interactions of cellulose. Energy Fuels 2004, 18, 1049–1065.
- Amonette, J.E.; Joseph, S. Physical properties of biochar. In Biochar for Environmental Management; Earthscan: London, UK; Sterling, VA, USA, 2009; pp. 13–29.
- Boehm, H.P. Carbon Surface Chemistry in Graphite and Precursors; Delhaes, P., Ed.; CRC: Amsterdam, The Netherlands, 2001; pp. 141–178.
- Cheng, C.H.; Lehmann, J.; Engelhard, M.H. Natural oxidation of black carbon in soils: Changes in molecular form and surface charge along a climosequence. Geochim. Cosmochim. Acta 2008, 72, 1598–1610.
- Cheng, C.H.; Lehmann, J. Ageing of black carbon along a temperature gradient. Chemosphere 2009, 75, 1021–1027.
- Sun, K.; Jin, J.; Keiluweit, M.; Kleber, M.; Wang, Z.; Pan, Z.; Xing, B. Polar and aliphatic domains regulate sorption of phthalic acid esters(PAEs) to biochars. Bioresour. Technol. 2012, 118, 120–127.
- Violante, A.; Gianfreda, L. Formation and Reactivity Toward. Soil Biochem. 2000, 10, 207.
- Major, J.; Lehmann, J.; Rondon, M.; Goodale, C. Fate of soil applied black carbon: Downward migration, leaching and soil respiration. Glob. Chang. Biol. 2010, 16, 1366–1379.
- IPCC. 2007 Climate Change. Climate Change Impacts, Adaptation and Vulnerability; Working Group II; IPCC: Geneva, Switzerland, 2007.
- Change, O.C. Intergovernmental Panel on Climate Change; World Meteorological Organization: Geneva, Switzerland, 2007; Volume 52.
- Ehhalt, D.; Prather, M.; Dentener, F.; Derwent, R.; Dlugokencky, E.; Holland, E.; Isaksen, I.; Katima, J.; Kirchhoff, V.; Matson, P.; et al. Atmospheric Chemistry and Greenhouse Gases. In Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Houghton, J.T., Ding, Y., Griggs, D.J., Noguer, M., van der Linden, P.J., Dai, X., Maskell, K., Johnson, C.A., Eds.; Cambridge University Press: Cambridge, UK, 2001; p. 881.
- Pacala, S.; Socolow, R. Stabilization wedges: Solving the climate problem for the next 50 years with current technologies. Science 2004, 305, 968–972.
- Kerr, R.A. Climate Change: Scientists Tell Policymakers We’re All Warming the World. Science 2007, 315, 754–757.
- Kluger, J. What now? Our feverish planet badly needs a cure. Time 2007, 169, 50–60.
- Schrag, D.P. Preparing to capture carbon. Science 2007, 315, 812–813.
- Lal, R. Carbon sequestration. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 815–830.
- Spokas, K.A.; Koskinen, W.C.; Baker, J.M.; Reicosky, D.C. Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 2009, 77, 574–581.
- Karimi, M.; Shirzad, M.; Silva, J.A.; Rodrigues, A.E. Biomass/Biochar carbon materials for CO2 capture and sequestration by cyclic adsorption processes: A review and prospects for future directions. J. CO2 Util. 2022, 57, 101890.
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18.
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal responses to biochar in soil–concepts and mechanisms. Plant Soil 2007, 300, 9–20.
- Laird, D.A.; Fleming, P.; Davis, D.D.; Horton, R.; Wang, B.; Karlen, D.L. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 2010, 158, 443–449.
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82.
- Van Zwieten, L.; Kammann, C.; Cayuela, M.L.; Singh, B.P.; Joseph, S.; Kimber, S.; Donne, S.; Clough, T.; Spokas, K.A. Biochar effects on nitrous oxide and methane emissions from soil. In Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Routledge: London, UK, 2015; pp. 521–552.
- Brockhoff, S.R.; Christians, N.E.; Killorn, R.J.; Horton, R.; Davis, D.D. Physical and mineral-nutrition properties of sand-based turf grass root zones amended with biochar. Agron. J. 2010, 102, 1627–1631.
- Verheijen, F.; Jeffery, S.; Bastos, A.C.; Van der Velde, M.; Diafas, I. Biochar application to soils. In A Critical Scientific Review of Effects on Soil Properties, Processes, and Functions; Office for the Official Publications of the European Communities: Luxembourg; Rome, Italy, 2010; Volume 24099, p. 162.
- Alburquerque, J.A.; Calero, J.M.; Barrón, V.; Torrent, J.; del Campillo, M.C.; Gallardo, A.; Villar, R. Effects of biochars produced from different feedstocks on soil properties and sunflower growth. J. Plant Nutr. Soil Sci. 2014, 177, 16–25.
- Oguntunde, P.G.; Abiodun, B.J.; Ajayi, A.E.; Van De Giesen, N. Effects of charcoal production on soil physical properties in Ghana. J. Plant Nutr. Soil Sci. 2008, 171, 591–596.
- Gwenzi, W.; Chaukura, N.; Mukome, F.N.; Machado, S.; Nyamasoka, B. Biochar production and applications in sub-Saharan Africa: Opportunities, constraints, risks and uncertainties. J. Environ. Manag. 2015, 150, 250–261.
- Cheng, C.H.; Lehmann, J.; Thies, J.E.; Burton, S.D.; Engelhard, M.H. Oxidation of black carbon by biotic and abiotic processes. Org. Geochem. 2006, 37, 1477–1488.
- Lehmann, J.; Pereira da Silva, J.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357.
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730.
- Mukherjee, A.; Lal, R. The biochar dilemma. Soil Res. 2014, 52, 217–230.
- Baldock, J.A.; Smernik, R.J. Chemical composition and bioavailability of thermally altered Pinus resinosa (Red pine) wood. Org. Geochem. 2002, 33, 1093–1109.
- Noguera, D.; Rondón, M.; Laossi, K.R.; Hoyos, V.; Lavelle, P.; de Carvalho, M.H.C.; Barot, S. Contrasted effect of biochar and earthworms on rice growth and resource allocation in different soils. Soil Biol. Biochem. 2010, 42, 1017–1027.
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214.
- Rondon, M.; Ramirez, J.; Lehmann, J. Charcoal additions reduce net emissions of greenhouse gases to the atmosphere. In Proceedings of the 3rd USDA Symposium on Greenhouse Gases and Carbon Sequestration, Baltimore, MD, USA, 21–24 March 2005; p. 208.
- Enders, A.; Hanley, K.; Whitman, T.; Joseph, S.; Lehmann, J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour. Technol. 2012, 114, 644–653.
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M.A. Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci. 2009, 174, 105–112.
- Novak, J.M.; Busscher, W.J.; Watts, D.W.; Amonette, J.E.; Ippolito, J.A.; Lima, I.M.; Gaskin, J.; Das, K.C.; Steiner, C.; Ahmedna, M.; et al. Biochars impact on soil-moisture storage in an ultisol and two aridisols. Soil Sci. 2012, 177, 310–320.
- Streubel, J.D.; Collins, H.P.; Garcia-Perez, M.; Tarara, J.; Granatstein, D.; Kruger, C.E. Influence of contrasting biochar types on five soils at increasing rates of application. Soil Sci. Soc. Am. J. 2011, 75, 1402–1413.
- De Gryze, S.; Cullen, M.; Durschinger, L.; Lehmann, J.; Bluhm, D.; Six, J.; Suddick, E. Evaluation of the opportunities for generating carbon offsets from soil sequestration of biochar. In An Issues Paper Commissioned by the Climate Action Reserve; Final Version; Terra Global Capital: San Francisco, CA, USA, 2010; pp. 1–99.
More
