Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Naeem Khan.
The field of immunotherapy has undergone radical conceptual changes over the last decade. There are various examples of immunotherapy, including the use of monoclonal antibodies, cancer vaccines, tumor-infecting viruses, cytokines, adjuvants, and autologous T cells carrying chimeric antigen receptors (CARs) that can bind cancer-specific antigens known as adoptive immunotherapy.
- B cells
- IgM
- IgG
- B cell receptor
1. Introduction
In recent years, immunotherapy has made extraordinary advances and brought longstanding survival benefits to patients with cancer. However, many patients do not respond to immunotherapy, or their responses are temporary, indicating immune resistance. While a lot has been achieved in the field of T-cell immunotherapy, much less has been spoken about the role of its contemporary—B cells in the tumor microenvironment. Most immunotherapies at present target T cells through checkpoint inhibitors and mechanistically work by reactivating anti-tumor immunity [1]. T cells can mediate their tumor-killing function directly, and immunotherapy strategies that take advantage of this include CAR-T-cell therapy, checkpoint inhibitors, and T-cell-based cancer vaccines. T cells can also mediate tumor killing indirectly via cytokines (cytokine therapy), monoclonal antibodies, oncolytic viruses, and adjuvants [2,3][2][3].
Of late, immunotherapy based on immune checkpoint blockade has garnered a lot of attention [1]. However, these therapies have limitations, such as a low frequency of mutation, reduced immune cell infiltration in tumors, and the suppressive nature of the tumor microenvironment [4], and, therefore, there is a need for an alternative approach using other immune cells.
Bursa-derived lymphocytes (B cells) can generate immunoglobulins (antibodies) and play key roles in humoral immunity. Generally, B-cell receptors (BCRs) identify antigens, and this leads to B-cell activation and differentiation into plasma cells (Figure 1) [5]. B cells can be divided into three categories: (i) B1B cells, present in the pleural cavities and peritoneum; (ii) follicular B (FOB) cells or B2 B cells, found in the lymph nodes, spleen, and Peyer’s patches; and (iii) marginal zone B (MZB) cells, located in the marginal sinus of the spleen [5]. Both B1B cells and FOB cells produce antibodies with a high affinity and specificity. MZB cells produce antibodies for blood-borne pathogens in an early phase of infection, their mostly T-independent antibody response, and a low-affinity IgM antibody [6,7][6][7]. Naïve B cells activate upon interactions with their cognate receptors, generate extrafollicular responses in the early course of infection, and differentiate into short-lived plasma cells. Later on, a few B cells undergo the germinal center reaction and differentiate into either long-lived plasma cells or memory B cells. Plasma cells secrete antibodies [8]. More importantly, B cells also secrete cytokines that can affect T-cell function, dendritic cell (DC) function, and lymphoid tissue reorganization [9,10][9][10].
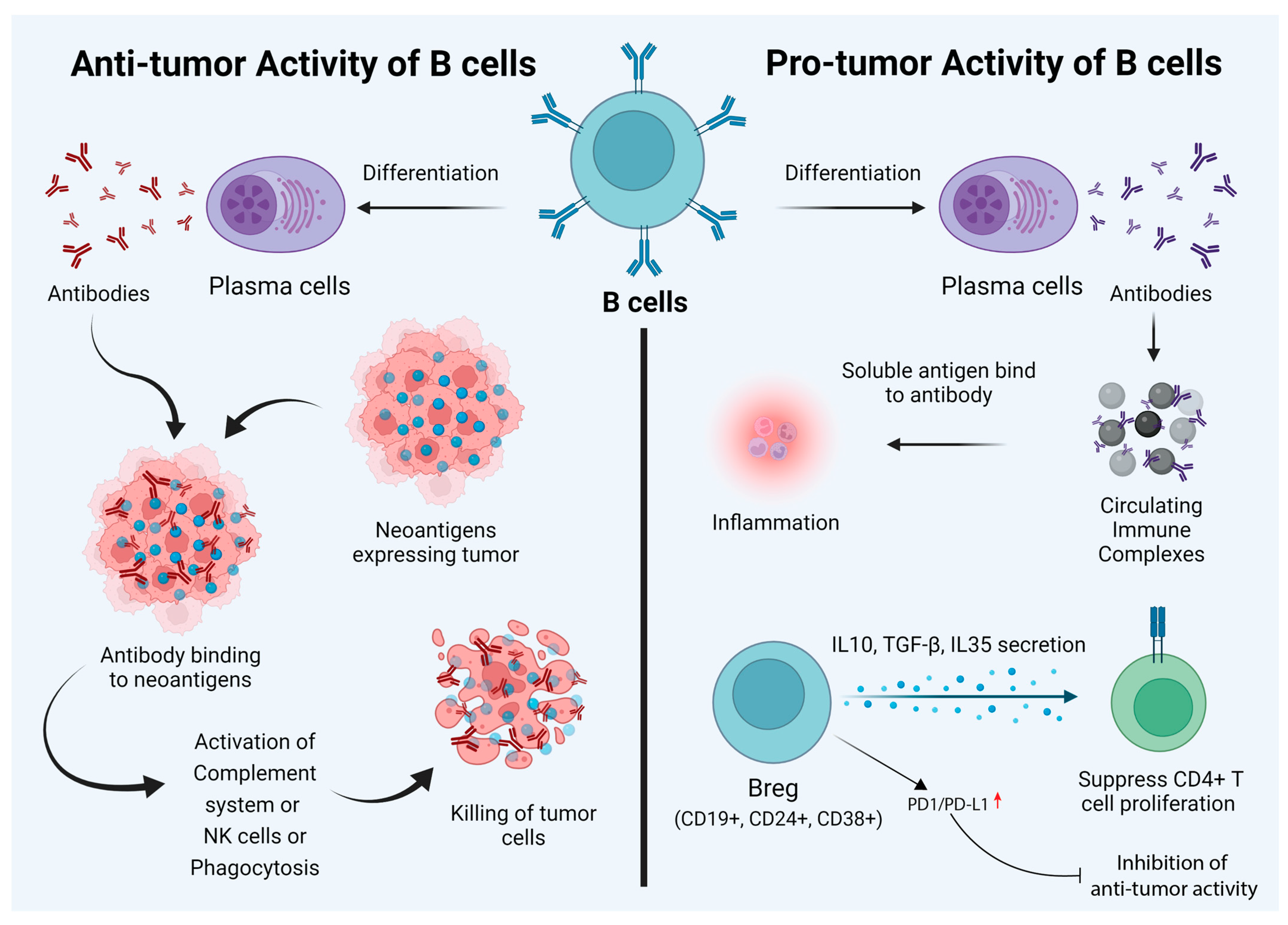
Figure 1. Dual nature of B cells in the cancer tumor microenvironment. Their anti-tumor characteristics can be utilized to empower immunotherapy goals. While behaving as anti-tumorigenic (left panel), B cells can recognize tumor-specific “neoantigens” and can stimulate antibody production, thus killing oncogenic cells. B cells can also have a pro-tumorigenic effect and promote tumor growth. Circulating immune complexes (CICs) and specific types of B cells (e.g., CD19+, CD24+, and CD38+) are the main factors behind this. These Breg cells differentiate due to inflammation and various other factors. They are responsible for immune tolerance and enhance Foxp3 expression in Treg cells. However, in some hepatocellular carcinomas, the expression of PD1/PD-L1 can suppress the anti-tumor activity of Bregs.
The use of immunotherapeutic interventions based on B cells and T cells would be an effective method of combating tumors. Furthermore, there is evidence that B cells infiltrate into tumor tissues; such B cells are called tumor-infiltrating B (TIB) cells, and these cells can differentiate into other B-cell subtypes [11]. Regulatory B cells (Bregs) are part of TIBs and have a direct association with tumor immunosuppression [2]. TIBs can modulate the immune response through interactions with other immune cells, such as Treg cells, NK cells, and CD4+ T cells [12]. Reports suggest that B cells play an important role in various cancers, such as breast cancer [13[13][14][15],14,15], epithelial ovarian cancer [16], melanoma [17], non-small-cell lung cancer [18[18][19],19], and renal cell carcinoma [20].
2. Dual Role of B Cells
The role of B cells in tumor immunotherapy is controversial and is not widely discussed compared to that of T cells. Based on existing reports, it is safe to say that B cells play a dual role in cancer immunotherapy. Besides secreting antibodies, B cells also regulate T cells and innate immune cell responses. B cells process and present antigens, and the balance of B-cell subtypes and their functions affect pro- and anti-tumorigenic functions [21]. Given this background, it is important to note that there are disparate reports regarding the prognostic value of B cells in tumor immunity. Here, wresearchers provide a summary of the role of B cells in pro- and anti-tumor immunity (Figure 1).3. Anti-Tumor Activity of B Cells
The antibody response in the tumor microenvironment is triggered by the expression of specific new antigens called neoantigens (as a result of mutations); the overexpression of genes; aberrant post-translational modifications; the expression of a specific differentiation marker, for example, CD20 in leukemia; and the expression of a marker normally found in other tumors, for example, the expression of cancer–testis antigens in melanoma and other tumor types [22]. An important thing to note is that CD20 is not the exclusive marker for leukemic cells but is also expressed in normal B cells from the stage of pro-B cells. CD20 also functions as a B-cell co-receptor to modulate the levels of B-cell signaling via Src-family kinases [23]. Antibodies produced by B cells can lead to the killing of tumor cells through the activation of the complement system, direct killing by NK cells, or phagocytosis by macrophages [24] (Figure 2). There are several studies to suggest that the antibodies produced by B cells against tumor cells lead to the efficient control of tumor growth. For example, a study conducted by Li et al. showed that an injection of tumor-specific antibodies leads to complement activation and tumor regression in a model of large-cell lung carcinoma [25]. Carmi et al., using an allogenic tumor model, showed that antibodies produced by B cells activate dendritic cells, which, in turn, activate the cytotoxic T-cell response leading to the control of tumor growth [26,27][26][27]. B cells can also directly target tumor cells. Tao et al. showed that CD19+ B cells from tumor-draining lymph nodes express FAS ligand (FAS-L) and, upon interaction with FAS, lead to the apoptosis of 4T1 murine breast cancer cells [28]. Another example of direct killing mediated by B cells is that of CpG-activated B cells, which can directly target cancer cells through the TRAIL/Apo-2L pathway [29] (Figure 2). B cells can also produce granzymes upon IL-21 stimulation and can mediate the killing of non-stimulated ones [30]. In addition, B cells that express B220, CD19, and CD11c can act as antigen-presenting cells (APCs). In ovarian cancers, B cells have been found in close proximity to T cells, indicating that they can act as APCs [31]. The role of B cells has also been studied in non-small-cell lung cancer, where B cells have been found to act as APCs to CD4+ T cells. Activated B cells classified as CD69+ HLA-DR+ CD27+ CD21+ could induce Th-1 differentiation, whereas exhausted B cells categorized as CD69+ HLA DR+ CD27− CD21− induced the generation of Tregs [32].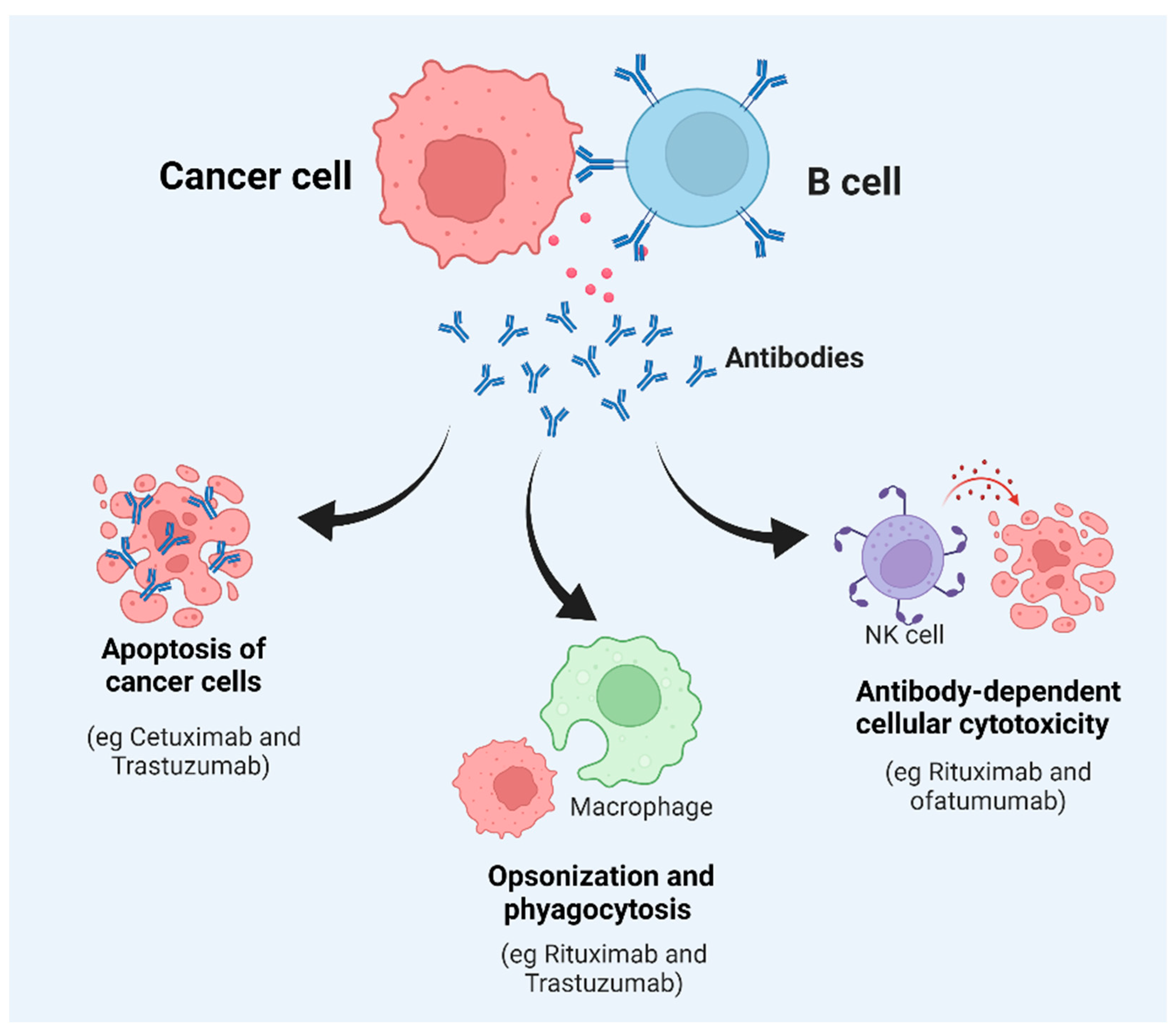
Figure 2. Key factors affecting B-cell function and tumor survival. Once a B cell interacts with tumor cells, it can generate different types of responses, including the secretion of antibodies. This can result in apoptosis, phagocytosis, opsonization, or direct killing of the target cancerous cells or tumors.
4. The Pro-Tumor Activity of B Cells
While B cells can mediate tumor cell killing, these cells can also promote tumor growth. Circulating immune complexes (CICs) are made of antibodies attached to multiple soluble antigens. CICs are formed by IgG antibodies bound to tumor antigens, such as globulin, viral RNA antigens released from cell debris, and apoptotic and necrotic cells. These complexes can induce inflammation by recognizing the Fc region [33]. In a model of epithelium carcinogenesis (K14-HPV16 mice), it was shown that CICs produced by B cells induced chronic inflammation through the activation of myeloid cells via engagement of the FcR (Figure 1). Antibody production in the tumor-draining lymph nodes of melanoma-bearing mice augments tumor growth [34]. Tumor-promoting abilities are also maintained by diverse B-cell populations known as regulatory B cells or Bregs. Breg cells are a subpopulation of B-cells (both mature and immature) with immunoinhibitory abilities. There are multiple factors playing a role in the development of Breg cells, such as CD40, activation, inflammation, Toll-like receptors (TLRs), and various transcription factors (Figure 1) [35]. These cells mediate immune tolerance and are defined as CD5+ CD24hi CD27+ CD38hi B cells [36]. Bregs produce IL-10, IL-35, and TGF-β. Bregs suppress CD4+ T-cell proliferation and lead to Foxp3 expression in Tregs by producing IL-10, IL-35, and TGF-β. In patients with acute myeloid leukemia (AML), Bregs are categorized as CD19+, CD24+, and CD38+, and the presence of these cells is correlated with poor prognosis [37] (Figure 1). Moreover, it has been reported that the expression of checkpoint inhibitors, such as PD-1 and PD-L1, by Bregs in hepatocellular carcinoma (HCC) samples leads to the suppression of anti-tumor activity [38] (Figure 1). There are several studies that have used mice models to study cancer. Fibrosarcoma cells when injected into B6 mice showed pro-tumor activity, while the depletion of B cells via the administration of anti-IgM antibodies in a xenograft mice model led to a reduction in tumor growth, metastasis, and anti-tumor activity [12]. The administration of human papillomavirus type 16 (HPV-16) transgenic cells into Rag-/- mice and CD4-/- or CD8-/- mice led to a reduction in skin tumor growth [39]. The transfer of B16/F10 cells into B6 mice enhanced melanoma [12]. Cancers such as EL4 thymoma, MC38 colon carcinoma in B6 mice, and EMT-6 breast carcinoma in BALB/c mice led to a reduction in tumor growth in mice lacking B cells compared to wild-type mice [40]. Mouse models have been used to study various cancer types. Conditional mouse models harboring floxed-Myd88L252P CD19-Cre and Myd88L252P-IRES-Yfp; CD19-Cre have been used to study plasma cell neoplasms. To understand the role of TRAF3 in cancer, TRAF3-deficient mice and TRAF3xBCL2 tg mice have been used, as they develop a distinct type of cancer. Similarly, c-myc-driven mouse models have been used to study lymphoma. Eµ-TCL-1 transgenic mice and Traf2DN/BCL2-double-tg mice have been used in a chronic lymphocytic leukemia (CLL) study [41]. The above examples shed light on the duality of B cells in cancers. Antibodies can be pro-tumorigenic when they form circulating immune complexes (CICs). CICs bind to Fcγ receptors on immunosuppressive myeloid cells and promote angiogenesis. However, antibodies have also shown anti-tumorigenic function. Antibodies against tumor antigens show Fc-mediated effector functions, such as complement-dependent cytotoxicity (CDC), ADCC, FcR-driven phagocytosis, and antigen presentation by dendritic cells [27]. Similarly, FAS/FAS-L interaction also shows both pro- and anti-tumor activities. The FAS/FAS-L interaction in Bregs induces apoptosis in CD4+ T cells, while the same interaction also helps in killing tumor cells. Granzyme B also shows dual activity. It causes pro-tumorigenic activity by degrading the T-cell receptor (TCR) ε chain without apoptosis; however, it induces apoptosis in B-CLL cancer [42]. Mechanistic insights into the role of B cells in Immunotherapy The role of B cells in immunotherapy is rather controversial and complicated. Depending on the state of activation, B cells have been reported to play divergent roles in T-cell differentiation and effector functions in various tumor models. B cells can execute their regulatory functions through the release of cytokines. One of the mechanisms by which B cells exert their effect is through the production of IL-10 and their interaction with Tregs. However, all of these studies have been performed in mice that lack B cells. Using wild-type mice, DiLillo et al. demonstrated that B cells are essential for optimal CD4+ and CD8+ immunity induction. Specifically, in a B16 melanoma model, tumor growth increased in a B-cell-depleted host. As opposed to resting B cells, several reports have denoted the importance of activated B cells in cellular immunotherapy. Many of these reports focus on the role of activated B cells as effective antigen-presenting cells (APCs) for T cells. It was recently reported that the adoptive transfer of activated B cells specific for 4T1 tumors into tumor-bearing hosts resulted in the initiation of T-cell-mediated immunity to 4T1 tumors in the peripheral blood and the spleen. Furthermore, B cells play a major role in the production of antibodies specific to tumor-associated epitopes. Antibodies exert their anti-tumor effects by various means. One mechanism is antibody-dependent cell-mediated cytotoxicity (ADCC), which is mediated by neutrophils, T cells, macrophages, and natural killer cells. Another mechanism for tumor lysis involves complement-dependent cytotoxicity (CDC). While antibodies by themselves are not effective in causing lysis of target cells, antibodies of the IgM and IgG classes can activate the complement system and cause cell lysis [43]. However, B-cell-mediated tumor immunity is further complicated by tumor evasion strategies. Tumors under ADCC attack develop mechanisms to evade NK cell attack. A well-known evasion strategy is through the shedding of the endogenous MHC-class-I-related chain molecule (MIC), which binds the activation receptor NKG2D on NK cells and results in the internalization of NK2GD and reduced NK cell activity. Therefore, the shedding of MIC has been established as a mechanism to evade NK cell immunosurveillance. A recent study found that, in tumors with MICA amplification, the presence of high levels of IgG1/3 B cells was associated with better survival in breast cancer and melanoma. In sharp contrast, the levels of IgG1/3 level did not influence survival for tumors without MICA amplification. These results suggest intricate interactions between B-cell-mediated immune responses and tumor ADCC pathway defects [44]. Factors affecting B-cell function in the tumor microenvironment As described previously, B cells can have both tumor-promoting and anti-tumorigenic effects. Multiple factors in the tumor microenvironment influence B-cell function, such as immune cells, the direct action of tumor cells on B cells, immune checkpoint stimulation on B cells, and hypoxia [42].4.1. Immune Cells
B cells present in the microenvironment of a solid tumor express granzyme B and are located close to IL21-secreting Treg cells. IL21 induces granzyme-B-expressing human Breg cells [45]. Moreover, in vitro studies have shown that activated CD4+ CD25+ Tregs can suppress B-cell proliferation by inducing granzyme-dependent cell death [46]. MDSC can induce Bregs in a mouse model of breast cancer. These Bregs express PD-L1 and PD-1. MDSCs can impair B-cell function through the secretion of IL-7, which is correlated with reduced antibody production [47].4.2. Cytokines and Metabolites
Metabolites such as leukotriene B4 (LTB4) can activate the peroxisome proliferator-activated receptor α (PPARα) in B cells inducing Breg differentiation [48]. Human breast cancer cells also express the CXCL-13 receptor, which induces the migration of CXCR-5-expressing B cells, and the coculture of CXCR-5-expressing B cells with cancer cells such as MCF-7 can induce the apoptosis of B cells and the appearance of a Breg population [49].4.3. Expression of Immune Checkpoint on B Cells
Checkpoint inhibitors (CPIs) work via T-cell modulation. Various mAbs have been approved till date, including Ipilimumab (anti-CLTA-4), Pembrolizumab (anti-PD-1), Nivolumab (anti-PD-1), and Atezolizumab (anti-PD-L1) that function through inhibiting the interaction of the checkpoint inhibitor with their ligands [50]. PD-1 expression on B cells prevents signal transduction through the B-cell receptor (BCR) via the recruitment and phosphorylation of protein tyrosine phosphatase non-receptor type 11 (PTPN11). PTPN11 deactivates spleen tyrosine kinase (SYK), preventing the downstream signaling cascade [51].4.4. Hypoxia
Hypoxia is a hallmark of cancer. Due to intense cell proliferation, as well as immune cell infiltration, there is vascular disorganization leading to hypoxia and the activation of hypoxia-inducible factors (HIFs) at the tumor site. Reports indicate that the deletion of glucose transporter 1 (Glut1), a target of HIF-1α, leads to reduced B-cell proliferation and decreased antibody production capacities [52].References
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167.
- Tokunaga, R.; Naseem, M.; Lo, J.H.; Battaglin, F.; Soni, S.; Puccini, A.; Berger, M.D.; Zhang, W.; Baba, H.; Lenz, H.-J. B cell and B cell-related pathways for novel cancer treatments. Cancer Treat. Rev. 2019, 73, 10–19.
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367.
- Maggs, L.; Cattaneo, G.; Dal, A.E.; Moghaddam, A.S.; Ferrone, S. CAR T Cell-Based Immunotherapy for the Treatment of Glioblastoma. Front. Neurosci. 2021, 15, 535.
- Kriegsmann, K.; Kriegsmann, M.; Cremer, M.; Schmitt, M.; Dreger, P.; Goldschmidt, H.; Müller-Tidow, C.; Hundemer, M. Cell-based immunotherapy approaches for multiple myeloma. Br. J. Cancer 2019, 120, 38–44.
- Appelgren, D.; Eriksson, P.; Ernerudh, J.; Segelmark, M. Marginal-Zone B-Cells Are Main Producers of IgM in Humans, and Are Reduced in Patients With Autoimmune Vasculitis. Front. Immunol. 2018, 9, 2242.
- Cerutti, A.; Cols, M.; Puga, I. Marginal zone B cells: Virtues of innate-like antibody-producing lymphocytes. Nat. Rev. Immunol. 2013, 13, 118–132.
- Suan, D.; Sundling, C.; Brink, R. Plasma cell and memory B cell differentiation from the germinal center. Curr. Opin. Immunol. 2017, 45, 97–102.
- Mesin, L.; Ersching, J.; Victora, G.D. Germinal Center B Cell Dynamics. Immunity 2016, 45, 471–482.
- Zou, W. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 2006, 6, 295–307.
- Martin, F.; Chan, A.C. B cell immunobiology in disease: Evolving concepts from the clinic. Annu. Rev. Immunol. 2006, 24, 467–496.
- Fremd, C.; Schuetz, F.; Sohn, C.; Beckhove, P.; Domschke, C. B cell-regulated immune responses in tumor models and cancer patients. OncoImmunology 2013, 2, e25443.
- Coronella-Wood, J.A.; Hersh, E.M. Naturally occurring B-cell responses to breast cancer. Cancer Immunol. Immunother. 2003, 52, 715–738.
- Chung, W.; Eum, H.H.; Lee, H.-O.; Lee, K.-M.; Lee, H.-B.; Kim, K.-T.; Ryu, H.S.; Kim, S.; Lee, J.E.; Park, Y.H.; et al. Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer. Nat. Commun. 2017, 8, 15081.
- Azizi, E.; Carr, A.J.; Plitas, G.; Cornish, A.E.; Konopacki, C.; Prabhakaran, S.; Nainys, J.; Wu, K.; Kiseliovas, V.; Setty, M.; et al. Single-Cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment. Cell 2018, 174, 1293–1308.e36.
- Milne, K.; Köbel, M.; Kalloger, S.E.; Barnes, R.O.; Gao, D.; Gilks, C.B.; Watson, P.; Nelson, B.H. Systematic Analysis of Immune Infiltrates in High-Grade Serous Ovarian Cancer Reveals CD20, FoxP3 and TIA-1 as Positive Prognostic Factors. PLoS ONE 2009, 4, e6412.
- Tirosh, I.; Izar, B.; Prakadan, S.M.; Wadsworth, M.H., II; Treacy, D.; Trombetta, J.J.; Rotem, A.; Rodman, C.; Lian, C.; Murphy, G.; et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 2016, 352, 189–196.
- Lambrechts, D.; Wauters, E.; Boeckx, B.; Aibar, S.; Nittner, D.; Burton, O.; Bassez, A.; Decaluwé, H.; Pircher, A.; Van den Eynde, K.; et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 2018, 24, 1277–1289.
- Lavin, Y.; Kobayashi, S.; Leader, A.; Amir, E.D.; Elefant, N.; Bigenwald, C.; Remark, R.; Sweeney, R.; Becker, C.D.; Levine, J.H.; et al. Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell 2017, 169, 750–765.e17.
- Chevrier, S.; Levine, J.H.; Zanotelli, V.R.T.; Silina, K.; Schulz, D.; Bacac, M.; Ries, C.H.; Ailles, L.; Jewett, M.A.S.; Moch, H.; et al. An Immune Atlas of Clear Cell Renal Cell Carcinoma. Cell 2017, 169, 736–749.e18.
- Wouters, M.C.A.; Nelson, B.H. Prognostic Significance of Tumor-Infiltrating B Cells and Plasma Cells in Human Cancer. Clin. Cancer Res. 2018, 24, 6125–6135.
- Duarte, J.D.G.; Peyper, J.M.; Blackburn, J.M. B cells and antibody production in melanoma. Mamm. Genome 2018, 29, 790–805.
- Kläsener, K.; Geoffroy Andrieux, J.J.; Reth, M. CD20 as a gatekeeper of the resting state of human B cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2021342118.
- Yuen, G.J.; Demissie, E.; Pillai, S. B Lymphocytes and Cancer: A Love–Hate Relationship. Trends Cancer 2016, 2, 747–757.
- Li, Q.; Teitz-Tennenbaum, S.; Donald, E.J.; Li, M.; Chang, A.E. In vivo sensitized and in vitro activated B cells mediate tumor regression in cancer adoptive immunotherapy. J. Immunol. 2009, 183, 3195–3203.
- Carmi, Y.; Spitzer, M.; Linde, I.; Burt, B.M.; Prestwood, T.R.; Perlman, N.; Davidson, M.G.; Kenkel, J.A.; Segal, E.; Pusapati, G.; et al. Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity. Nature 2015, 521, 99–104.
- Fridman, W.H.; Petitprez, F.; Meylan, M.; Chen, T.W.-W.; Sun, C.-M.; Roumenina, L.T.; Sautes-Fridman, C. B cells and cancer: To B or not to B? J. Exp. Med. 2021, 218, e20200851.
- Tao, H.; Lu, L.; Xia, Y.; Dai, F.; Wang, Y.; Bao, Y.; Lundy, S.; Ito, F.; Pan, Q.; Zhang, X.; et al. Antitumor effector B cells directly kill tumor cells via the Fas/FasL pathway and are regulated by IL-10. Eur. J. Immunol. 2015, 45, 999–1009.
- Kemp, T.J.; Moore, J.M.; Griffith, T.S. Human B cells express functional TRAIL/Apo-2 ligand after CpG-containing oligodeox-ynucleotide stimulation. J. Immunol. 2004, 173, 892–899.
- Jahrsdörfer, B.; Blackwell, S.E.; Wooldridge, J.E.; Huang, J.; Andreski, M.W.; Jacobus, L.S.; Taylor, C.M.; Weiner, G.J. B-chronic lymphocytic leukemia cells and other B cells can produce granzyme B and gain cytotoxic potential after interleukin-21-based activation. Blood 2006, 108, 2712–2719.
- Rubtsov, A.V.; Rubtsova, K.; Kappler, J.W.; Jacobelli, J.; Friedman, R.S.; Marrack, P. CD11c-Expressing B Cells Are Located at the T Cell/B Cell Border in Spleen and Are Potent APCs. J. Immunol. 2015, 195, 71–79.
- Bruno, T.C.; Ebner, P.J.; Moore, B.L.; Squalls, O.G.; Waugh, K.A.; Eruslanov, E.B.; Singhal, S.; Mitchell, J.D.; Franklin, W.A.; Merrick, D.T.; et al. Antigen-Presenting Intratumoral B Cells Affect CD4+ TIL Phenotypes in Non–Small Cell Lung Cancer Patients. Cancer Immunol. Res. 2017, 5, 898–907.
- Andreu, P.; Johansson, M.; Affara, N.I.; Pucci, F.; Tan, T.; Junankar, S.; Korets, L.; Lam, J.; Tawfik, D.; DeNardo, D.G.; et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell 2010, 17, 121–134.
- Pucci, F.; Garris, C.; Lai, C.P.; Newton, A.; Pfirschke, C.; Engblom, C.; Alvarez, D.; Sprachman, M.; Evavold, C.; Magnuson, A.; et al. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science 2016, 352, 242–246.
- Rosser, E.C.; Mauri, C. Regulatory B Cells: Origin, Phenotype, and Function. Immunity 2015, 42, 607–612.
- Iwata, Y.; Matsushita, T.; Horikawa, M.; DiLillo, D.J.; Yanaba, K.; Venturi, G.M.; Szabolcs, P.M.; Bernstein, S.H.; Magro, C.M.; Williams, A.D.; et al. Characterization of a rare IL-10–competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 2011, 117, 530–541.
- Lv, Y.; Wang, H.; Liu, Z. The Role of Regulatory B Cells in Patients with Acute Myeloid Leukemia. Med. Sci. Monit. 2019, 25, 3026–3031.
- Shalapour, S.; Lin, X.J.; Bastian, I.N.; Brain, J.; Burt, A.D.; Aksenov, A.A.; Vrbanac, A.F.; Li, W.; Perkins, A.; Matsutani, T.; et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 2017, 551, 340–345.
- de Visser, K.E.; Korets, L.V.; Coussens, L.M. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte de-pendent. Cancer Cell 2005, 7, 411–423.
- Zhang, Y.; Morgan, R.; Podack, E.R.; Rosenblatt, J. B cell regulation of anti-tumor immune response. Immunol. Res. 2013, 57, 115–124.
- Perez-Chacon, G.; Vincent-Fabert, C.; Zapata, J.M. Editorial: Mouse Models of B Cell Malignancies. Front. Immunol. 2021, 12, 4546.
- Largeot, A.; Pagano, G.; Gonder, S.; Moussay, E.; Paggetti, J. The B-Side of Cancer Immunity: The Underrated Tune. Cells 2019, 8, 449.
- Hu, X.; Zhang, J.; Wang, J.; Fu, J.; Li, T.; Zheng, X.; Wang, B.; Gu, S.; Jiang, P.; Fan, J.; et al. Landscape of B cell immunity and related immune evasion in human cancers. Nat. Genet. 2019, 51, 560–567.
- Kim, S.S.; Sumner, W.A.; Miyauchi, S.; Cohen, E.E.; Califano, J.A.; Sharabi, A.B. Role of B Cells in Responses to Checkpoint Blockade Immunotherapy and Overall Survival of Cancer Patients. Clin. Cancer Res. 2021, 27, 6075–6082.
- Lindner, S.; Dahlke, K.; Sontheimer, K.; Hagn, M.; Kaltenmeier, C.; Barth, T.F.; Beyer, T.; Reister, F.; Fabricius, D.; Lotfi, R.; et al. Interleukin 21–Induced Granzyme B–Expressing B Cells Infiltrate Tumors and Regulate T Cells. Cancer Res. 2013, 73, 2468–2479.
- Zhao, D.-M.; Thornton, A.M.; DiPaolo, R.J.; Shevach, E.M. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood 2006, 107, 3925–3932.
- Wang, Y.; Schafer, C.C.; Hough, K.; Tousif, S.; Duncan, S.R.; Kearney, J.F.; Ponnazhagan, S.; Hsu, H.-C.; Deshane, J.S. Myeloid-Derived Suppressor Cells Impair B Cell Responses in Lung Cancer through IL-7 and STAT. J. Immunol. 2018, 201, 278–295.
- Wejksza, K.; Lee-Chang, C.; Bodogai, M.; Bonzo, J.; Gonzalez, F.J.; Lehrmann, E.; Becker, K.; Biragyn, A. Cancer-produced metabolites of 5-lipoxygenase induce tumor-evoked regulatory B cells via peroxisome prolifera-tor-activated receptor alpha. J. Immunol. 2013, 190, 2575–2584.
- Pimenta, E.M.; De, S.; Weiss, R.; Feng, D.; Hall, K.; Kilic, S.; Bhanot, G.; Ganesan, S.; Ran, S.; Barnes, B.J. IRF5 is a novel regulator of CXCL13 expression in breast cancer that regulates CXCR5(+) B- and T-cell trafficking to tumor-conditioned media. Immunol. Cell Biol. 2015, 93, 486–499.
- Willsmore, Z.N.; Harris, R.J.; Crescioli, S.; Hussein, K.; Kakkassery, H.; Thapa, D.; Cheung, A.; Chauhan, J.; Bax, H.J.; Chenoweth, A.; et al. B Cells in Patients With Melanoma: Implications for Treatment With Checkpoint Inhibitor Antibodies. Front. Immunol. 2021, 11, 622442.
- Okazaki, T.; Maeda, A.; Nishimura, H.; Kurosaki, T.; Honjo, T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc. Natl. Acad. Sci. USA 2001, 98, 13866–13871.
- Caro-Maldonado, A.; Wang, R.; Nichols, A.G.; Kuraoka, M.; Milasta, S.; Sun, L.D.; Gavin, A.L.; Abel, E.D.; Kelsoe, G.; Green, D.R.; et al. Metabolic Reprogramming Is Required for Antibody Production That Is Suppressed in Anergic but Exaggerated in Chronically BAFF-Exposed B Cells. J. Immunol. 2014, 192, 3626–3636.
More
