You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Cui Guo.
The aerosol contains major ions, heavy metals, and organic matters that are important external nutrients in upper oceans and potentially influence marine microbes and biogeochemical cycles. Therefore, the role of atmospheric deposition to oceans has received growing attention.
- atmospheric deposition
- East Asia
- northwest Pacific Ocean
1. Introduction
Atmospheric particulate matter (also known as aerosols) is microscopic particles of solid or liquid matter suspended in the air. These particles are transported from the atmosphere to terrestrial and aquatic ecosystems via a process referred to as atmospheric deposition. Since industrialization, intensifying human activities have created increasing emissions of atmospheric aerosols. It is estimated that about 420–480 Tg of aerosols are transported and deposited into the ocean every year globally [1,2][1][2]. Through this important process, substances in the aerosols, including inorganic nutrients, metal elements and organic pollutants, are transported to seas and oceans [3]. Atmospheric deposition has been shown to be one of the major external sources of nutrients in the ocean and has an important impact on marine microbial food webs and global biogeochemical cycles (Figure 1) [4,5,6][4][5][6]. Thus, determining how atmospheric deposition affects marine ecosystems has become a key and urgent topic in the field of oceanography. In the SOLAS program (Surface Ocean–Lower Atmosphere Study) research plan from 2015 to 2025, “atmospheric deposition and marine biogeochemistry” have been listed as one of five core themes, focusing on the response of marine biogeochemical and biological processes to atmospheric deposition from anthropogenic and natural sources. International research programs such as GEOTRACES (an international study of marine biogeochemical cycles of trace elements and their isotopes) and IMBeR (Integrated Marine Biosphere Research) also include the impact of atmospheric deposition on the ocean as an important topic.
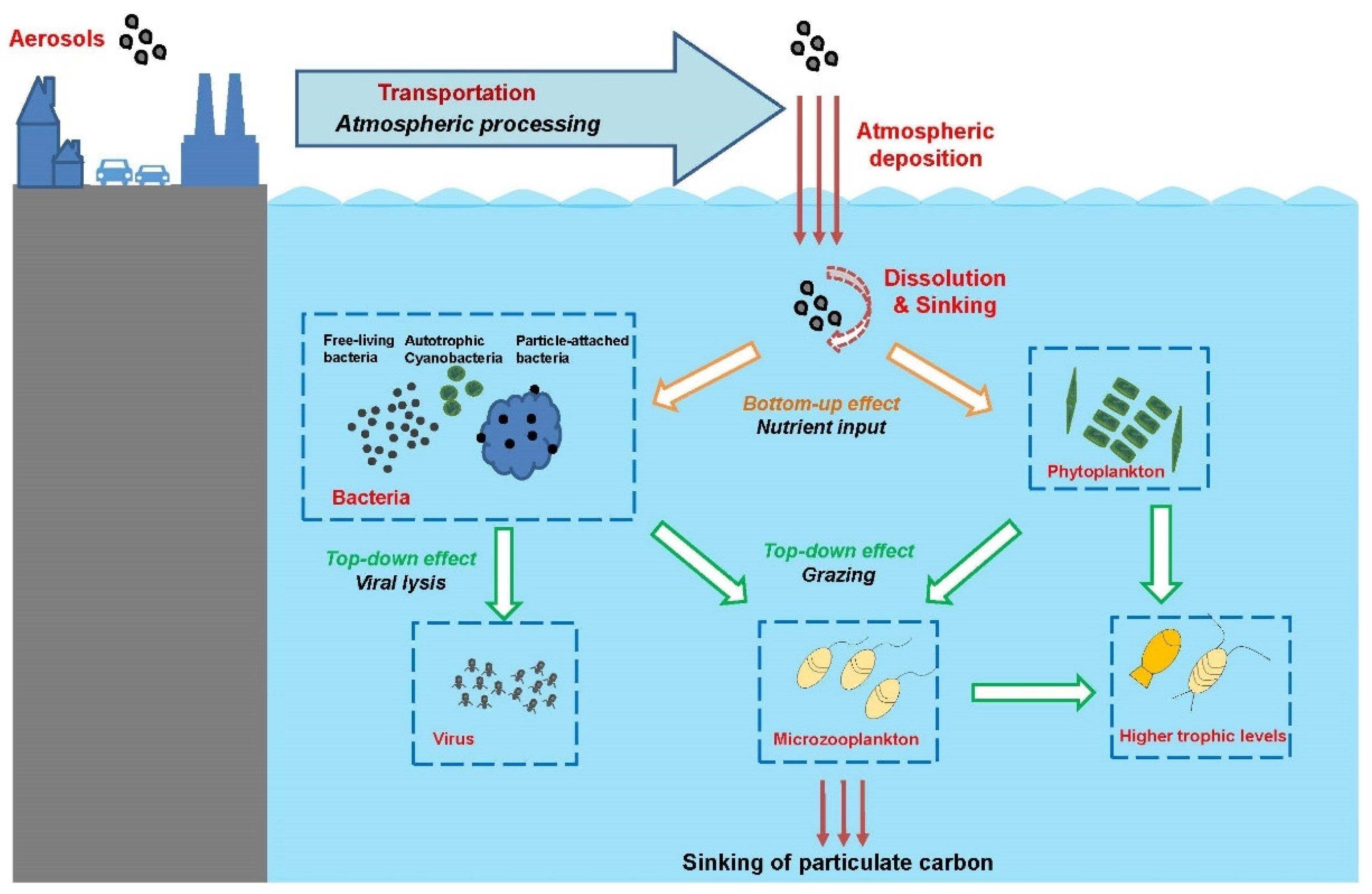
Figure 1. Impacts of aerosol deposition on the marine food web. Aerosols from natural and anthropogenic sources are transported to ocean regions. The solubility of nutrients in the aerosols can be enhanced by atmospheric processes such as acidification, photochemical, or cloud processes before being deposited to the seawater. In the ocean, the dissolved aerosol nutrients can be cycled through microbial food web, influencing microbial metabolism, community composition, and biogeochemical cycles, and/or sink in the deep ocean.
2. Effect of East Asian Aerosol on Phytoplankton in NWPO and Its Marginal Seas
2.1. Fertilizing Effect
As an important source of nutrients to the ocean, atmospheric deposition has been recognized to exert an important role in regulating primary production and phytoplankton growth (Figure 1). Assessments based on modeling and remote sensing data suggest that the aerosol input has a close connection with biological production and coastal eutrophication. By analyzing more than 10 years of satellite records of Asian dust events and remotely sensed chlorophyll a concentrations, many studies have identified significant correlations between chlorophyll a concentrations and aerosol optical depth, a proxy for atmospheric dust input and nutrient supply, in NWPO and the adjacent China Seas [49,73,74,75,76,77][7][8][9][10][11][12]. Strong dust events could enhance phytoplankton biomass by more than 2-fold (estimated by increase in chlorophyll a concentrations) that could account for up to 70% increase in ocean primary production and trigger phytoplankton blooms in the northern SCS and NWPO [49,76,77][7][11][12]. They also found that the stimulation effect of atmospheric aerosol was greater in the central basin where other sources of nutrient inputs (e.g., river runoff or upwelling) were lower [78][13]. It is suggested that atmospheric Fe input has a fundamental effect on phytoplankton growth in China Seas and could explain 5–68% of the phytoplankton growth [79,80,81][14][15][16]. Atmospheric N deposition can support >10% of the annual export production in nearshore regions along the Japanese coast and the SCS [49,82][7][17]. Using sediment trap measurement and a biogeochemical model, it has been shown that the seasonal variability of deep-ocean POC export is largely driven by the atmospheric Fe and N deposition that cause seasonal change of phytoplankton community composition and micro- and meso-zooplankton grazing pressure [83][18]. In addition to direct stimulation by atmospheric nutrients, strong winds accompanying the dust storms can also induce vertical mixing of the water column and the supply of nutrients into the mixed layer from the subsurface [49[7][19],84], the effect of which may occasionally overwhelm the effect of atmospheric input of aerosol nutrients [73][8].
Bottle incubation-based microcosm assays provide solutions to evaluate direct effects of atmospheric deposition of Asian dust on phytoplankton growth and identify specific contributions of aerosol nutrients. Amendment of dust, haze particles or rainwater into seawater samples caused significant increase in chlorophyll a concentration by up to 4-fold in NWPO and its marginal Seas. The stimulation effect was more profound in oligotrophic than mesotrophic waters [64[20][21],85], while aerosol addition had little or no effect on phytoplankton growth in some eutrophic waters [86,87,88][22][23][24]. However, considering the fast nutrient dispersion and high sinking rates of aerosols in the in situ seawater, the effect of aerosols in the real marine environment may be less significant than that in the microcosm experiments.
2.2. Stimulation of N2 Fixation
Response of marine N2 fixation to aerosol deposition is also of particular interest because growth of nitrogen-fixing organisms could be limited by Fe and P in ocean ecosystems [36][25]. Most studies focus on the effects of aerosol Fe, because N2 fixing diazotrophs require a high amount of cellular Fe as an important cofactor of the nitrogenase enzyme that catalyzes N2 fixation [89][26]. Meanwhile, dissolved Fe is present at extremely low concentrations (<0.1 nM) in surface waters of the open ocean [56][27] that could limit growth of marine phytoplankton including diazotrophs [90,91][28][29]. N2 fixation rate was significantly enhanced by addition of Saharan mineral dust in the Mediterranean Sea [92,93,94][30][31][32] and North Atlantic [36][25] and the stimulation effect was attributed to the supply of Fe and P in the dust. The availability of N, P, and Fe and their ratios in the aerosol and ambient seawater could largely determine the trend and the extent to which aerosol addition could influence N2 fixation in the ocean. For example, addition of Saharan mineral dust with a lower N:P ratio into the Eastern Mediterranean stimulated N2 fixation rates more prominently compared to anthropogenic European aerosols with a higher N:P ratio [94][32]. A recent study reported supply ratio of Fe:N from subsurface layers is the most important factor in regulating diazotroph abundances and N2 fixation rates across the tropical NWPO, while phosphate availability sets an upper limit of total amount of fixed N [95][33]. As the atmospheric P inputs were strongly depleted relative to N and Fe in the context of the stoichiometry of phytoplankton Fe, N, P requirements, especially in anthropogenic aerosols [96][34], deposition of anthropogenic East Asian aerosol may fuel diazotrophs with more stoichiometrically available Fe than P in the NWPO region and its marginal seas by providing anthropogenic East Asian aerosols, causing the switch of N2 fixation from Fe to P limitation. In the northern SCS receiving East Asian aerosols, co-limitation of N2 fixation by both Fe and P have been demonstrated by nutrient addition assays [95][33]. However, the effect of atmospheric input on N2 fixation in NWPO is still unknown.
Growth of marine diazotrophs, particularly the prominent genus Trichodesmium, benefit from aerosol additions [93][31]. Trichodesmium can actively acquire nutrients from airborne dust by multiple pathways and strategies, including efficient dust capturing and centering in the colony [97][35], sensing particle composition and selective collection of nutrient-rich (i.e., Fe-rich, P-rich) particles [98[36][37],99], and mutualistic interactions between Trichodesmium and associated bacteria for utilization of iron from dust [100][38]. In addition, the heterotrophic bacterial N2 fixers [94][32] and other N2-fixing unicellular cyanobacteria [101][39] have also been reported to prevail after aerosol addition.
2.3. Change of Nutrient Stoichiometry
A number of bottle incubation experiments have been conducted to demonstrate the detailed response of phytoplankton to atmospheric deposition in NWPO. From these nutrient enrichment experiments, it was found that the addition of inorganic N and aerosols both caused a significant increase in phytoplankton biomass, although the promotion effect of aerosol or dust addition was usually greater when the same amount of inorganic N was added to oligotrophic seawaters [64,87][20][23]. This suggests that the East Asian aerosols stimulate phytoplankton growth by supplying not only N but also other components, possibly Fe, in LNLC regions [64][20]. However, by providing excess N but negligible amounts of P, the atmospheric input may increase the N:P ratio and cause P limitation in the oligotrophic seawater. Thus, adding additional P with aerosols sometimes stimulated a larger increase in chlorophyll a concentration than by adding aerosols alone [86][22], especially in coastal or estuarine regions where the N:P ratio is usually higher. In the oligotrophic seawaters of the SCS and the subtropical gyre of NWPO, a combination of N, P, and Fe addition was observed to have the strongest stimulation effect in multiple nutrient addition experiments [85,87][21][23].
2.4. Shift of Community Composition and Struture
Alleviation of nutrient limitation and change of nutrient stoichiometry by atmospheric input can further drive changes in phytoplankton community composition and physiological state. Bioassays showing the change of phytoplankton biomass and community structure change with dry and wet deposition amendment in the NWPO and its marginal seas are summarized, and the study sites are shown in Figure 2. In general, the larger micro-phytoplankton (20–200 μm) derive more benefit from the input of atmospheric nutrients than pico- (0.2–2 μm) and nano-sized cells (2–20 μm), leading to a shift in size structure of the phytoplankton community [64,87,102,103][20][23][40][41]. However, the beneficial phytoplankton taxa were not consistent across NWPO, due to differences in nutrient stoichiometry of the experimental sites and different sources of aerosols. After the addition of East Asian aerosols to the oligotrophic SCS and Kuroshio extension region, the phytoplankton community composition shifted to diatoms (N:P < 16), while it shifted to dinoflagellates in the ECS (N:P 16), due to the different nutrient requirement of the two taxa [64,88][20][24]. Using the amplicon sequencing of the rbcL gene method, Meng et al. [104][42] observed different changes in the phytoplankton community structure after adding aerosols from different sources: mineral dust resulted in a significant increase in the relative abundance of Haptophyceae, while aerosols with the highest N led to the largest increase in Bacillariophyceae (diatoms), Dinophyceae (dinoflagellates), and Cryptophyceae. Among the diatom species, Pseudo-nitzschia, Nitzschia, and Chaetoceros usually accounted for the largest increases in response to aerosol addition [64,85][20][21].
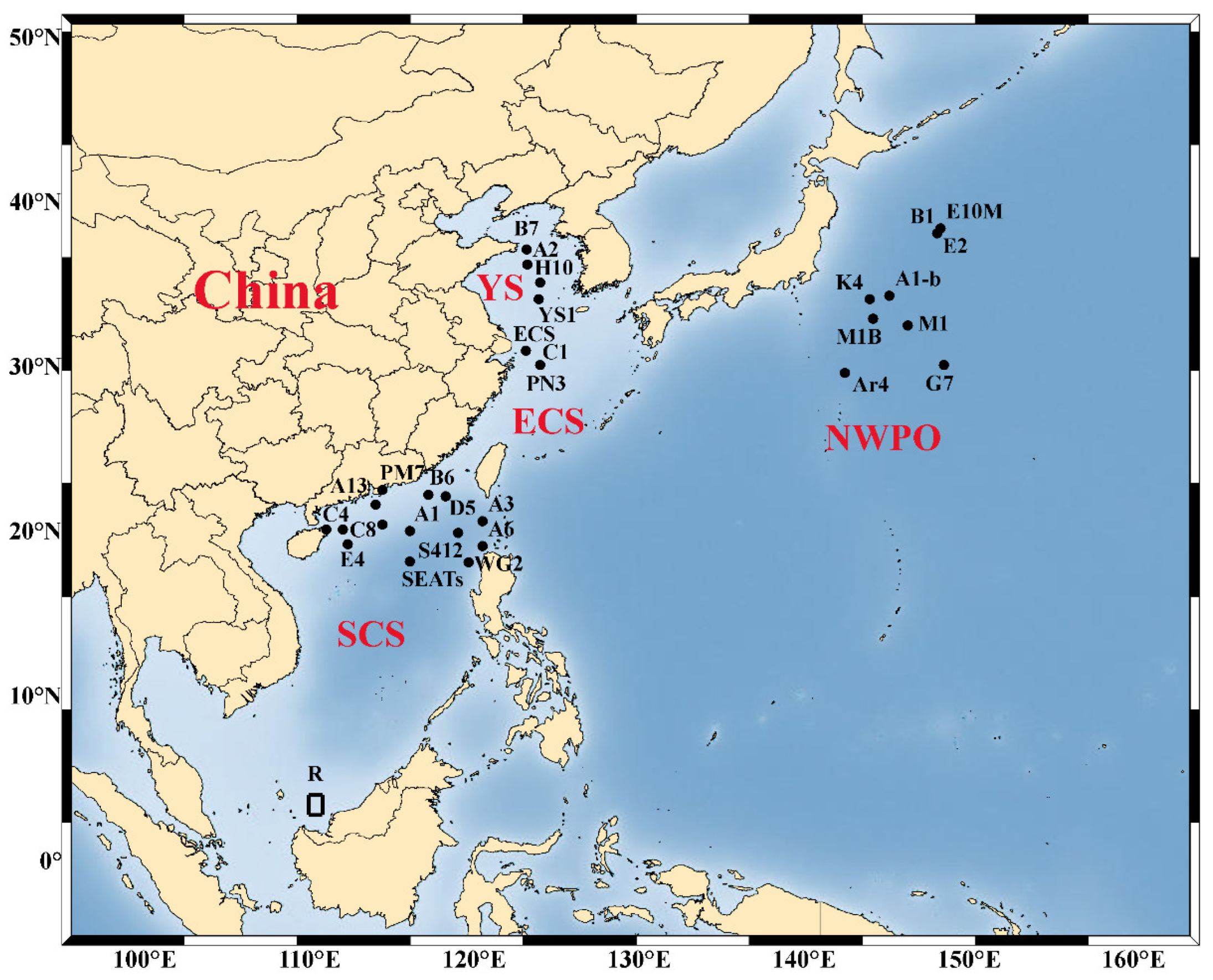
Figure 2. Sampling sites for aerosol amendment bioassays conducted in northwest Pacific Ocean (NWPO) and its marginal seas. ECS, East China Sea; SCS, South China Sea; YS, Yellow Sea.
The shift of phytoplankton size and community structure from pico- to nano- and micro-phytoplankton in response to aerosol addition has important biogeochemical implications in the NWPO. For example, in the oligotrophic SCS that is dominated by picophytoplankton, the community composition shift to diatoms may contribute more to vertical carbon export through sinking of senescent cells [64][20]. Meanwhile, increased phytoplankton biomass and change of community composition can stimulate grazing activities from higher trophic levels and thus enhance the carbon export through downward zooplankton fecal pellets or detritus [64,102][20][40]. All these changes can enhance the biological pump and potentially change the carbon budget in the oligotrophic SCS.
2.5. Inhibitory Effect
Atmospheric deposition has also been demonstrated to have an inhibitory effect on phytoplankton growth, especially in pico- and nano-phytoplankton. The negative effect was mostly attributed to the toxicity of some trace metals in the aerosols, such as Cu and Cd [27,108][43][44]. However, the current understanding of the toxic effects of East Asian atmospheric deposition on phytoplankton is very limited. Metal toxicity has been found across many phytoplankton taxa with different abilities to tolerate toxic metals and different toxicity thresholds [109,110,111][45][46][47]. Generally, phytoplankton with a small cell size are more sensitive to metal toxicity as they have a larger surface area to volume ratio and higher nutrient uptake efficiency [110][46]. It has been reported that cyanobacteria are most sensitive to Cu and Cd toxicity, diatoms are the least sensitive, and coccolithophores and dinoflagellates are intermediate in sensitivity [42][48]. Indeed, a significant decline in Prochlorococcus in response to East Asian aerosol amendment has been observed in the oligotrophic seawater of the SCS [64,106,112][20][49][50]. In the coastal regions of the SCS and Yellow Sea, negative responses to aerosol or rainwater addition have also observed in Synechococcus and pico-eukaryotes [102,105,106][40][49][51]. It has been reported that the intracellular trace metal concentrations in size-fractionated plankton of the surface water of the NWPO have been significantly elevated relative to their biological requirements due to anthropogenic aerosol deposition [113][52]. The stronger toxic effect on small phytoplankton may also contribute to the phytoplankton size structure shift to larger phytoplankton.
Combined metal-to-metal and metal-to-nutrient interactions further complicate the effects of aerosols. For example, Cu toxicity in phytoplankton may be influenced by other metals (e.g., Fe) and nutrient status [111][47]. In the ECS, phytoplankton growth was more inhibited after the addition of aerosol with high Cu than that with both high Cu and Fe [114][53]. Coastal strains of some phytoplankton, i.e., Synechococcus, exhibit higher Cu tolerance and lower stress response than open-ocean strains [115][54]. Moreover, although the final yield and growth rate of cyanobacteria decreases in response to aerosol amendment, their cell size and chlorophyll a content increases [64[20][50],112], which may be due to an uncoupling between photosynthesis and cell division [116][55].
The negative effect of East Asian haze particles on total phytoplankton biomass has only been observed at the very high deposition loadings of 2 mg L−1 [103][41], when the inhibition impact exceeded the fertilization effect, while a stimulation effect was always reported at low and medium loadings of 0.03-0.6 mg L−1 [64,103][20][41]. Considering that realistic loadings of haze particles is far less than 2 mg L−1, the overall effect of atmospheric deposition on phytoplankton biomass should be promotion.
3. Effect of East Asian Aerosol on Bacteria
Recent studies on aerosol impacts have begun to focus on the responses of heterotrophic bacteria following aerosol additions. In the oligotrophic ocean, bacterial biomass and production are often limited by dissolved organic carbon. The shortage of inorganic nutrients will also affect bacterial growth directly or indirectly by limiting phytoplankton growth [117][56]. Therefore, the supply of nutrients and organic matter transported by atmospheric deposition can alleviate the nutritional limitation of bacteria and affect the bacterial activity and diversity. Saharan dust deposition in the Mediterranean Sea and Atlantic Ocean has been shown by microcosm or mesocosm experiments to affect bacterial abundance, production, and community composition [71,103,118,119,120,121,122,123,124][41][57][58][59][60][61][62][63][64]. Far fewer studies have been conducted in the NW Pacific region with East Asian aerosol deposition.
In the SCS, small or insignificant increases in bacterial abundance in response to anthropogenic East Asian aerosol (collected from Hong Kong and Qingdao) input were demonstrated by microcosm experiments [112[50][65],125], whereas significant increases were observed after the addition of dust particles (collected from Mt. Tateyama and Loess Plateau) [126,127][66][67]. Bacterial production was enhanced by ~2 to 4-fold, although the increase in bacterial biomass was much smaller [112][50]. Greater responses in bacterial production than in bacterial abundance have also been reported from the central Atlantic and Mediterranean Sea [118,121][58][61]. It is probable that enhanced grazing pressure and viral infection after aerosol addition contribute to maintain a constant bacterial abundance (Figure 1) [102,112,124,128][40][50][64][68]. Therefore, it has been suggested that the atmospheric input may change the microbial ecosystem from a bottom-up limited to a top-down controlled bacterial community [129][69]. Alternatively, a shift in bacterial community composition towards one with more active bacteria with higher nucleic acid content after dust addition may also be closely associated with the enhancement of bacterial production [118][58].
Clear changes in bacterial diversity and community composition in NWPO were also detected in response to East Asian aerosol input, although the detailed changes following aerosol additions were site-specific. Generally, the relative abundance of copiotrophs, such as Rhodobacteraceae and Flavobacteriaceae, increased, while the proportion of oligotrophs, such as SAR 11 clade, Prochlorococcus, AEGEAN-169 marine group, decreased, leading to a slight increase in bacterial diversity in the oligotrophic SCS [112][50]. Both bacterial production and the community composition shift exhibited significant relationships with the hydrographic conditions of the different locations. Stronger promotion effects of the East Asian aerosols on bacterial production and community shift from oligotrophs to copiotrophs were demonstrated at the more oligotrophic sites with lower chlorophyll a concentration [112][50].
References
- Jickells, T.D.; An, Z.S.; Andersen, K.K.; Baker, A.R.; Bergametti, G.; Brooks, N.; Cao, J.; Boyd, P.W.; Duce, R.A.; Hunter, K.A.; et al. Global iron connections between desert dust, ocean biogeochemistry, and climate. Science 2005, 308, 67–71.
- Shao, Y.; Wyrwoll, K.-H.; Chappell, A.; Huang, J.; Lin, Z.; McTainsh, G.H.; Mikami, M.; Tanaka, T.Y.; Wang, X.; Yoon, S. Dust cycle: An emerging core theme in Earth system science. Aeolian Res. 2011, 2, 181–204.
- Jickells, T.D.; Moore, C.M. The importance of atmospheric deposition for ocean productivity. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 481–501.
- Duce, R.A.; LaRoche, J.; Altieri, K.; Arrigo, K.R.; Baker, A.R.; Capone, D.G.; Cornell, S.; Dentener, F.; Galloway, J.; Ganeshram, R.S.; et al. Impacts of atmospheric anthropogenic nitrogen on the open ocean. Science 2008, 320, 893–897.
- Kanakidou, M.; Duce, R.A.; Prospero, J.M.; Baker, A.R.; Benitez-Nelson, C.; Dentener, F.J.; Hunter, K.A.; Liss, P.S.; Mahowald, N.; Okin, G.S.; et al. Atmospheric fluxes of organic N and P to the global ocean. Glob. Biogeochem. Cycles 2012, 26, GB3026.
- Mahowald, N.M.; Scanza, R.; Brahney, J.; Goodale, C.L.; Hess, P.G.; Moore, J.K.; Neff, J. Aerosol deposition impacts on land and ocean carbon cycles. Curr. Clim. Change Rep. 2017, 3, 16–31.
- Shen, C.; Zhao, H.; Chen, F.; Xiao, H. The distribution of aerosols and their impacts on chlorophyll—A distribution in the South China Sea. J. Geophys. Res. Biogeosciences 2020, 125, e2019JG005490.
- Shiozaki, T.; Chen, Y.-L.L. Different mechanisms controlling interannual phytoplankton variation in the South China Sea and the western North Pacific subtropical gyre: A satellite study. Adv. Space Res. 2013, 52, 668–676.
- Tan, S.-C.; Yao, X.; Gao, H.-W.; Shi, G.-Y.; Yue, X. Variability in the correlation between Asian dust storms and chlorophyll a concentration from the north to equatorial Pacific. PLoS ONE 2013, 8, e57656.
- Tan, S.C.; Shi, G.Y.; Shi, J.H.; Gao, H.W.; Yao, X. Correlation of Asian dust with chlorophyll and primary productivity in the coastal seas of China during the period from 1998 to 2008. J. Geophys. Res. 2011, 116, G02029.
- Wang, S.H.; Hsu, N.C.; Tsay, S.C.; Lin, N.H.; Sayer, A.M.; Huang, S.J.; Lau, W.K. Can Asian dust trigger phytoplankton blooms in the oligotrophic northern South China Sea? Geophys. Res. Lett. 2012, 39, L05811.
- Yoon, J.E.; Kim, K.; Macdonald, A.M.; Park, K.T.; Kim, H.C.; Yoo, K.C.; Yoon, H.I.; Yang, E.J.; Jung, J.; Lim, J.H. Spatial and temporal variabilities of spring Asian dust events and their impacts on chlorophyll—A concentrations in the western North Pacific Ocean. Geophys. Res. Lett. 2017, 44, 1474–1482.
- Lin, I.I.; Wong, G.T.F.; Lien, C.-C.; Chien, C.-Y.; Huang, C.-W.; Chen, J.-P. Aerosol impact on the South China Sea biogeochemistry: An early assessment from remote sensing. Geophys. Res. Lett. 2009, 36, L17605.
- Shi, J.-H.; Gao, H.-W.; Zhang, J.; Tan, S.-C.; Ren, J.-L.; Liu, C.-G.; Liu, Y.; Yao, X. Examination of causative link between a spring bloom and dry/wet deposition of Asian dust in the Yellow Sea, China. J. Geophys. Res. 2012, 117, D17304.
- Tan, S.; Li, J.; Gao, H.; Wang, H.; Che, H.; Chen, B. Satellite-observed transport of dust to the East China Sea and the North Pacific Subtropical Gyre: Contribution of dust to the increase in chlorophyll during spring 2010. Atmosphere 2016, 7, 152.
- Tan, S.-C.; Wang, H. The transport and deposition of dust and its impact on phytoplankton growth in the Yellow Sea. Atmos. Environ. 2014, 99, 491–499.
- Onitsuka, G.; Uno, I.; Yanagi, T.; Yoon, J.-H. Modeling the effects of atmospheric nitrogen input on biological production in the Japan Sea. J. Oceanogr. 2009, 65, 433–438.
- Xiu, P.; Chai, F. Impact of atmospheric deposition on carbon export to the deep ocean in the subtropical northwest Pacific. Geophys. Res. Lett. 2021, 48, e2020GL089640.
- Hung, C.-C.; Gong, G.-C.; Chung, W.-C.; Kuo, W.-T.; Lin, F.-C. Enhancement of particulate organic carbon export flux induced by atmospheric forcing in the subtropical oligotrophic northwest Pacific Ocean. Mar. Chem. 2009, 113, 19–24.
- Guo, C.; Yu, J.; Ho, T.Y.; Wang, L.; Song, S.; Kong, L.; Liu, H. Dynamics of phytoplankton community structure in the South China Sea in response to the East Asian aerosol input. Biogeosciences 2012, 9, 1519–1536.
- Chu, Q.; Liu, Y.; Shi, J.; Zhang, C.; Gong, X.; Yao, X.; Guo, X.; Gao, H. Promotion Effect of Asian Dust on Phytoplankton Growth and Potential Dissolved Organic Phosphorus Utilization in the South China Sea. J. Geophys. Res. 2018, 123, 1101–1116.
- Zhang, C.; Gao, H.; Yao, X.; Shi, Z.; Shi, J.; Yu, Y.; Meng, L.; Guo, X. Phytoplankton growth response to Asian dust addition in the northwest Pacific Ocean versus the Yellow Sea. Biogeosciences 2018, 15, 749–765.
- Zhang, C.; He, J.; Yao, X.; Mu, Y.; Guo, X.; Ding, X.; Yu, Y.; Shi, J.; Gao, H. Dynamics of phytoplankton and nutrient uptake following dust additions in the northwest Pacific. Sci. Total Environ. 2020, 739, 139999.
- Zhang, C.; Ito, A.; Shi, Z.; Aita, M.N.; Yao, X.; Chu, Q.; Shi, J.; Gong, X.; Gao, H. Fertilization of the northwest Pacific Ocean by East Asia air pollutants. Glob. Biogeochem. Cycles 2019, 33, 690–702.
- Mills, M.M.; Ridame, C.; Davey, M.; Roche, J.; Geider, R.J. Iron and phosphorus co-limit nitrogen fixation in the eastern tropical North Atlantic. Nature 2004, 429, 292–294.
- Berman-Frank, I.; Cullen, J.T.; Shaked, Y.; Sherrell, R.M.; Falkowski, P.G. Iron availability, cellular iron quotas, and nitrogen fixation in Trichodesmium. Limnol. Oceanogr. 2001, 46, 1249–1260.
- Moore, C.M.; Mills, M.M.; Arrigo, K.R.; Berman-Frank, I.; Bopp, L.; Boyd, P.W.; Galbraith, E.D.; Geider, R.J.; Guieu, C.; Jaccard, S.L.; et al. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 2013, 6, 701–710.
- Chappell, P.D.; Moffett, J.W.; Hynes, A.M.; Webb, E.A. Molecular evidence of iron limitation and availability in the global diazotroph Trichodesmium. ISME J. 2012, 6, 1728–1739.
- Sohm, J.A.; Webb, E.A.; Capone, D.G. Emerging patterns of marine nitrogen fixation. Nat. Rev. Microbiol. 2011, 9, 499–508.
- Ridame, C.; Guieu, C.; L’Helguen, S. Strong stimulation of N2 fixation in oligotrophic Mediterranean Sea: Results from dust addition in large in situ mesocosms. Biogeosciences 2013, 10, 7333–7346.
- Ridame, C.; Le Moal, M.; Guieu, C.; Ternon, E.; Biegala, I.C.; L’Helguen, S.; Pujo-Pay, M. Nutrient control of N2 fixation in the oligotrophic Mediterranean Sea and the impact of Saharan dust events. Biogeosciences 2011, 8, 2773–2783.
- Rahav, E.; Cheung, S.-Y.; Guo, C.; Liu, H.; Tsagaraki, T.M.; Giannakourou, A.; Tsiola, A.; Psarra, S.; Lagaria, A.; Mulholland, M.R.; et al. Evaluating the impact of atmospheric depositions on springtime dinitrogen fixation in the Cretan Sea (Eastern Mediterranean)—A mesocosm approach. Front. Mar. Sci. 2016, 3, 180.
- Wen, Z.; Browning Thomas, J.; Cai, Y.; Dai, R.; Zhang, R.; Du, C.; Jiang, R.; Lin, W.; Liu, X.; Cao, Z.; et al. Nutrient regulation of biological nitrogen fixation across the tropical western North Pacific. Sci. Adv. 2022, 8, eabl7564.
- Baker, A.R.; Weston, K.; Kelly, S.D.; Voss, M.; Streu, P.; Cape, J.N. Dry and wet deposition of nutrients from the tropical Atlantic atmosphere: Links to primary productivity and nitrogen fixation. Deep Sea Res. Part I Oceanogr. Res. Pap. 2007, 54, 1704–1720.
- Rubin, M.; Berman-Frank, I.; Shaked, Y. Dust- and mineral-iron utilization by the marine dinitrogen-fixer Trichodesmium. Nat. Geosci. 2011, 4, 529–534.
- Kessler, N.; Armoza-Zvuloni, R.; Wang, S.; Basu, S.; Weber, P.K.; Stuart, R.K.; Shaked, Y. Selective collection of iron-rich dust particles by natural Trichodesmium colonies. ISME J. 2020, 14, 91–103.
- Wang, S.; Koedooder, C.; Zhang, F.; Kessler, N.; Eichner, M.; Shi, D.; Shaked, Y. Colonies of the marine cyanobacterium Trichodesmium optimize dust utilization by selective collection and retention of nutrient-rich particles. iScience 2022, 25, 103587.
- Basu, S.; Gledhill, M.; de Beer, D.; Prabhu Matondkar, S.G.; Shaked, Y. Colonies of marine cyanobacteria Trichodesmium interact with associated bacteria to acquire iron from dust. Commun. Biol. 2019, 2, 284.
- Bonnet, S.; Guieu, C.; Chiaverini, J.; Ras, J.; Stock, A. Effect of atmospheric nutrients on the autotrophic communities in a low nutrient, low chlorophyll system. Limnol. Oceanogr. 2005, 50, 1810–1819.
- Guo, C.; Liu, H.; Yu, J.; Zhang, S.; Wu, C.J. Role of microzooplankton grazing in regulating phytoplankton biomass and community structure in response to atmospheric aerosol input. Mar. Ecol. Prog. Ser. 2014, 507, 69–79.
- Zhang, C.; Yao, X.; Chen, Y.; Chu, Q.; Yu, Y.; Shi, J.; Gao, H. Variations in the phytoplankton community due to dust additions in eutrophication, LNLC and HNLC oceanic zones. Sci. Total Environ. 2019, 669, 282–293.
- Meng, X.; Chen, Y.; Wang, B.; Ma, Q.W.; Wang, F.J. Responses of phytoplankton community to the input of different aerosols in the East China Sea. Geophys. Res. Lett. 2016, 43, 7081–7088.
- Paytan, A.; Mackey Katherine, R.M.; Chen, Y.; Lima Ivan, D.; Doney Scott, C.; Mahowald, N.; Labiosa, R.; Post Anton, F. Toxicity of atmospheric aerosols on marine phytoplankton. Proc. Natl. Acad. Sci. USA 2009, 106, 4601–4605.
- Mackey, K.R.; Buck, K.N.; Casey, J.R.; Cid, A.; Lomas, M.W.; Sohrin, Y.; Paytan, A. Phytoplankton responses to atmospheric metal deposition in the coastal and open-ocean Sargasso Sea. Front. Microbiol. 2012, 3, 359.
- Echeveste, P.; Agusti, S.; Tovar-Sanchez, A. Toxic thresholds of cadmium and lead to oceanic phytoplankton: Cell size and ocean basin-dependent effects. Environ. Toxicol. Chem. 2012, 31, 1887–1894.
- Mann, E.; Ahlgren, N.; Moffett, J.; Chisholm, S. Copper toxicity and cyanobacteria ecology in the Sargasso Sea. Limnol. Oceanogr. 2002, 47, 976–988.
- Yang, T.; Chen, Y.; Zhou, S.; Li, H. Impacts of aerosol copper on marine phytoplankton: A review. Atmosphere 2019, 10, 414.
- Brand, L.E.; Sunda, W.G.; Guillard, R.R.L. Reduction of marine phytoplankton reproduction rates by copper and cadmium. J. Exp. Mar. Biol. Ecol. 1986, 96, 225–250.
- Zhou, W.; Li, Q.P.; Wu, Z. Coastal phytoplankton responses to atmospheric deposition during summer. Limnol. Oceanogr. 2020, 66, 1298–1315.
- Duan, X.; Guo, C.; Zhang, C.; Li, H.; Zhou, Y.; Gao, H.; Xia, X.; He, H.; McMinn, A.; Wang, M. Effect of East Asian atmospheric particulate matter deposition on bacterial activity and community structure in the oligotrophic Northwest Pacific. Environ. Pollut. 2021, 283, 117088.
- Liu, Y.; Zhang, T.R.; Shi, J.H.; Gao, H.W.; Yao, X.H. Responses of chlorophyll a to added nutrients, Asian dust, and rainwater in an oligotrophic zone of the Yellow Sea: Implications for promotion and inhibition effects in an incubation experiment. J. Geophys. Res. 2013, 118, 1763–1772.
- Liao, W.-H.; Yang, S.-C.; Ho, T.-Y. Trace metal composition of size-fractionated plankton in the Western Philippine Sea: The impact of anthropogenic aerosol deposition. Limnol. Oceanogr. 2017, 62, 2243–2259.
- Wang, F.J.; Chen, Y.; Guo, Z.G.; Gao, H.W.; Mackey, K.R.; Yao, X.H.; Zhuang, G.S.; Paytan, A. Combined effects of iron and copper from atmospheric dry deposition on ocean productivity. Geophys. Res. Lett. 2017, 44, 2546–2555.
- Stuart, R.K.; Dupont, C.L.; Johnson, D.A.; Paulsen, I.T.; Palenik, B. Coastal strains of marine Synechococcus species exhibit increased tolerance to copper shock and a distinctive transcriptional response relative to those of open-ocean strains. Appl. Environ. Microbiol. 2009, 75, 5047–5057.
- Fisher, N.S.; Jones, G.J.; Nelson, D.M. Effects of copper and zinc on growth, morphology, and metabolism of Asterionella japonica (Cleve). J. Exp. Mar. Biol. Ecol. 1981, 51, 37–56.
- Mills, M.M.; Moore, C.M.; Langlois, R.; Milne, A.; Achterberg, E.; Nachtigall, K.; Lochte, K.; Geider, R.J.; La, R.J. Nitrogen and phosphorus co-limitation of bacterial productivity and growth in the oligotrophic subtropical North Atlantic. Limnol. Oceanogr. 2008, 53, 824–834.
- Marin, I.; Nunes, S.; Sanchez-Perez, E.D.; Aparicio, F.L.; Estrada, M.; Marrase, C.; Moreno, T.; Wagener, T.; Querol, X.; Peters, F. Anthropogenic versus mineral aerosols in the stimulation of microbial planktonic communities in coastal waters of the northwestern Mediterranean Sea. Sci. Total Environ. 2017, 574, 553–568.
- Guo, C.; Xia, X.; Pitta, P.; Herut, B.; Rahav, E.; Berman-Frank, I.; Giannakourou, A.; Tsiola, A.; Tsagaraki, T.M.; Liu, H. Shifts in microbial community structure and activity in the ultra-oligotrophic eastern Mediterranean Sea driven by the deposition of Saharan dust and European aerosols. Front. Mar. Sci. 2016, 3, 170.
- Laghdass, M.; Blain, S.; Besseling, M.; Catala, P.; Guieu, C.; Obernosterer, I. Effects of Saharan dust on the microbial community during a large in situ mesocosm experiment in the NW Mediterranean Sea. Aquat. Microb. Ecol. 2011, 62, 201–213.
- Lekunberri, I.; Lefort, T.; Romero, E.; Vazquez-Dominguez, E.; Romera-Castillo, C.; Marrase, C.; Peters, F.; Weinbauer, M.; Gasol, J.M. Effects of a dust deposition event on coastal marine microbial abundance and activity, bacterial community structure and ecosystem function. J. Plankton Res. 2010, 32, 381–396.
- Marañén, E.; Fernández, A.; Mouriño-Carballido, B.; MartÍnez-GarcÍa, S.; Teira, E.; Cermeño, P.; Chouciño, P.; Huete-Ortega, M.; Fernández, E.; Calvo-DÍaz, A.; et al. Degree of oligotrophy controls the response of microbial plankton to Saharan dust. Limnol. Oceanogr. 2010, 55, 2339–2352.
- Marin-Beltran, I.; Logue, J.B.; Andersson, A.F.; Peters, F. Atmospheric deposition impact on bacterial community composition in the NW Mediterranean. Front. Microbiol. 2019, 10, 858.
- Rahav, E.; Belkin, N.; Paytan, A.; Herut, B. Phytoplankton and bacterial response to desert dust deposition in the coastal waters of the southeastern Mediterranean Sea: A four-year in situ survey. Atmosphere 2018, 9, 305.
- Reche, I.; Ortega-Retuerta, E.; Romera, O.; Villena, E.P.; Baquero, R.M.; Casamayor, E.O. Effect of Saharan dust inputs on bacterial activity and community composition in Mediterranean lakes and reservoirs. Limnol. Oceanogr. 2009, 54, 869–879.
- Guo, C.; Jing, H.; Kong, L.; Liu, H. Effect of East Asian aerosol enrichment on microbial community composition in the South China Sea. J. Plankton Res. 2013, 35, 485–503.
- Maki, T.; Ishikawa, A.; Kobayashi, F.; Kakikawa, M.; Aoki, K.; Mastunaga, T.; Hasegawa, H.; Iwasaka, Y. Effects of Asian dust (KOSA) deposition event on bacterial and microalgal communities in the Pacific Ocean. Asian J. Atmos. Environ. 2011, 5, 157–163.
- Maki, T.; Ishikawa, A.; Mastunaga, T.; Pointing, S.B.; Saito, Y.; Kasai, T.; Watanabe, K.; Aoki, K.; Horiuchi, A.; Lee, K.C.; et al. Atmospheric aerosol deposition influences marine microbial communities in oligotrophic surface waters of the western Pacific Ocean. Deep Sea Res. Part I Oceanogr. Res. Pap. 2016, 118, 37–45.
- Rahav, E.; Paytan, A.; Mescioglu, E.; Bar-Zeev, E.; Martínez Ruiz, F.; Xian, P.; Herut, B. Bio-aerosols negatively affect Prochlorococcus in oligotrophic aerosol-rich marine regions. Atmosphere 2020, 11, 540.
- Dinasquet, J.; Bigeard, E.; Gazeau, F.; Azam, F.; Guieu, C.; Maranon, E.; Ridame, C.; Wambeke, F.; Obernosterer, I.; Baudoux, A.C. Impact of dust addition on the microbial food web under present and future conditions of pH and temperature. Biogeosciences 2022, 19, 1303–1319.
More
