Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Dean Liu and Version 2 by Dean Liu.
Antioxidants are substances that can prevent damage to cells caused by free radicals. Production of reactive oxygen species and the presence of oxidative stress play an important role in cardiac arrhythmias.
- antioxidants
- atrial fibrillation
- ventricular arrhythmia
- vitamin C
- vitamin E
1. Introduction
Oxidative stress (OS) is involved in the pathogenesis of cardiovascular diseases, such as hypertension, heart failure, atherosclerosis and ischaemia/reperfusion injury (IRI) [1][2][3][4][5][6]. Reactive oxygen species (ROS) production is also associated with cardiac arrhythmias [7][8][9]. So far, scientific studies have mainly focused on the role of OS in atrial fibrillation (AF). Little is known about the role of ROS and OS in the development of ventricular fibrillation (VF), ventricular tachycardia (VT) or other ventricular arrhythmias (VAs). VT and VF are the most common life-threatening arrhythmias leading to sudden cardiac arrest [10]. The use of antioxidants could contribute to better treatment of cardiac arrhythmias. A significant group of patients are people with heart failure (HF) and left ventricle ejection fraction (LVEF) <35% with implanted cardiac resynchronisation therapy devices with defibrillators (CRT-D) or implantable cardioverter-defibrillators (ICDs). ICDs are used for primary and secondary prevention in patients with HF. In these patients, OS is a critical pathophysiological pathway in the development and progression of HF [3][11] and possibly arrhythmias. The exact role of OS in arrhythmia development in these patients is complicated, since the intervention of the ICD also influences ROS production. Electric shocks can cause cellular myocardium damage and abnormalities in OS and calcium regulation, and can stimulate pro-inflammatory factors, which can paradoxically promote reinduction of VAs [12].
2. Antioxidants in Arrhythmia Treatment
2.1. Vitamins C and E in Atrial Fibrillation and Other Supraventricular Arrhythmias
Vitamin E exerts important antiarrhythmic, antioxidant and cardioprotective effects by inhibiting epinephrine-induced lipid peroxidation. This observation may be of particular importance in patients treated in intensive care units with high doses of catecholamines, or in patients with catecholaminergic polymorphic ventricular tachycardia (CPVT). Antioxidants can reduce OS in inflammation or cardiac ischaemia after surgery, which is one of the POAF development mechanisms. Mirhoseini et al. presented antioxidant supplementation and atrial arrhythmias in critically ill trauma patients, representing a very diverse group [13]. AF is the most common arrhythmia and is independently associated with higher mortality in trauma patients [14]. One of the studies used a high dose of an antioxidant (vitamin C—1000 mg orally, per tube or intravenously every 8 h, vitamin E—1000 units orally or per tube every 8 h and selenium—200 mcg orally, per tube or intravenously, daily for 7 days in total) during a two-week hospitalisation or before discharge, and showed the differences between the antioxidant and control groups. No differences were found in the hospital mortality rates, but the use of antioxidants was associated with a longer expected survival time [13]. Antioxidants may have antiarrhythmic effects, but they also reduce the inflammatory reaction severity, an important arrhythmogenic factor often found in injuries and polytrauma. Vitamin C decreases the spontaneous activity of the pulmonary vein and attenuates the arrhythmogenic effects of H2O2 in rabbits due to the intensification of ectopic beats [15]. This effect is related to the increase in Ca2+ release and can be important when performing ablation procedures. The meta-analysis of 15 clinical trials with 1738 subjects indicated that antioxidants, such as ascorbic acid, α-tocopherol, N-acetylcysteine and a mix of omega-3 polyunsaturated fatty acid (PUFA) + ascorbic acid + tocopherol, reduced POAF (21% vs. 34% in the control group) incidence after coronary artery bypass grafting (CABG) or valve replacement [16]. A meta-analysis of 11 randomised controlled trials with 3137 patients revealed that the use of n-3 PUFA without antioxidants did not reduce the incidence of POAF compared with the control group. However, the combination therapy with vitamins C and E decreased the incidence of POAF by 68% [17].2.2. Vitamins C and E in Ventricular Arrhythmias
Ventricular arrhythmias generated in the reperfusion phase may be life-threatening, and their treatment with standard antiarrhythmic drugs is also not entirely safe. Vitamins C and E may improve safety in patients with AMI or congenital or acquired long QT syndrome. The use of antioxidants or ROS-scavengers with drugs that prolong QT may be a promising therapeutic option in reducing the risk of side effects. Interesting conclusions have been reached concerning patients with chronic Chagas disease, who have a higher prevalence of PVCs caused by increased oxidative stress [18]. Forty-one patients received antioxidants, such as vitamins C (500 mg/day) and E (800 UI/day), for six months. A 24-hour Holter monitoring device was used to detect arrhythmias. The prevalence of PVCs (incidents) in patients with advanced heart disease was 5391 before antioxidant supplementation vs. 1185 after supplementation. The reduction in PVCs was accompanied by a decrease in serum markers of oxidative stress. The activities of SOD, GPx, and glutathione reductase were decreased in all patients in whom vitamins C and E were used [18]. Rarely, frequent PVCs can lead to chaotic, dangerous heart rhythms and possibly sudden cardiac death, and therefore require treatment. Therefore, a new and safe therapy for reducing oxidative stress is highly desirable. It appears that antioxidant therapy could benefit the treatment of sudden cardiac arrest in the VT/VF mechanism. The above data from studies in animal models and patients of the cardiology departments indicate that the use of natural antioxidants, e.g., vitamin C or E, may benefit patients with supraventricular and ventricular arrhythmias (Figure 1). Using antioxidants contained among other nutrients in food, could be a simple and safe method of preventing or reducing the risk of developing, for instance, the most commonly treated arrhythmia (AF) in patients after cardiac surgery or of the recurrence of arrhythmias after cardioversion. Post-operative arrhythmias represent a significant source of morbidity and mortality. Better therapies are essential for patients taking antiarrhythmic drugs, which show only moderate antiarrhythmic efficacy, with a concurrent risk of proarrhythmia and adverse organ effects. Patients on a PUFA-rich diet can also benefit.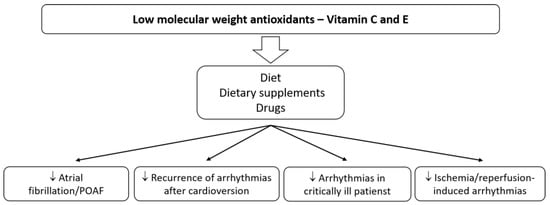
Figure 1. The main effects of vitamins C and E on cardiac arrhythmias.
2.3. Resveratrol and Atrial Fibrillation
As AF is the most common arrhythmia, much research has focused on the use of antioxidants in AF. A study on rabbit hearts demonstrated that RSV and piceatannol (a derivative of RSV) reduce AF incidence after administration of acetylcholine and isoproterenol [19]. Thus, RSV and vitamins C and E can improve the outcomes of patients with supraventricular and ventricular arrhythmias, including those in a critical condition, with risk factors and with diseases resulting in myocardial remodelling and structural changes. Conversely, human studies looking at wine (alcohol) consumption as a source of RSV produce conflicting results. A large cohort study of 79,019 Swedish men and women indicated that consuming >14 drinks per week of wine was associated with an increased risk of AF [20]. These findings indirectly call into question theories on the antiarrhythmic effects of resveratrol in humans. However, it should be remembered that the conflicting studies were designed differently, and it is often impossible to unequivocally compare the doses of alcohol and/or RSV consumed. The effect of RVS may be different when used alone, e.g., as an extract, from when it is present in an alcoholic drink. More clinical trials of RSV supplementation are needed, but the initial results are promising. Grapes, especially their skins, and blackberries and blackcurrants are the main RSV sources. The use of RSV and/or the Mediterranean diet as supportive therapy may positively influence the outcome of AF treatment.2.4. Resveratrol and Ventricular Arrhythmias
Beneficial antiarrhythmic effects of RSV were demonstrated more than 20 years ago in animal studies, among others. Male Sprague–Dawley rats (200–300 g) underwent myocardial ischaemia by occlusion of the left descending coronary artery and were infused with a bolus of resveratrol’s different doses 15 min before coronary occlusion [21]. Pretreatment with RSV did not affect ischaemia-induced arrhythmias (100% of the rats had VT) and mortality (33% with RSV vs. 38% without RSV). Instead, a drastic reduction in mortality and in the incidence and duration of VT/VF during the reperfusion phase in animals infused with RSV was observed. Using the highest dose (2.3 × 10−5 g/kg), no animal had VF, 20% had VT, and the mortality was 0% [21]. A study of diabetic rats treated with RSV (5 mg/kg/day, intraperitoneally) revealed a decreased frequency and duration of VT/VF and a lowered incidence of other arrhythmia types in the ischaemia–reperfusion model. After 6 min of left coronary artery occlusion and 6 min of reperfusion, there were no rats with VT/VF, and the survival rate was 100% compared to 25.5% VF and 62.5% VT in the control group [22]. Menezes-Rodriguez et al. indicated that in an animal study with 10 min of cardiac ischaemia (occlusion of the left anterior descending coronary artery) and 75 min of recirculation, RSV (1 mg/kg/day, administered orally) significantly reduced the incidence of atrioventricular (AV) block and lethality [23]. Incidence of VAs in the RSV group was 10% lower than in the control group (70% vs. 80%), and mortality was 37.5% lower (25.0% vs. 62.5%). AV block was much less common in the RSV group [23]. In a model with isolated hearts perfused using the Langendorff technique and with 30 min of global ischaemia and 120 min of reperfusion, the RSV also showed a beneficial effect [24]. VF incidence was significantly lower in the RSV group compared to control (median 2.5 vs. 11.5). The incidence of single arrhythmias was reduced from 121 to 36 (median of incidence). CAT, SOD and GPx activity, MDA concentration and nitrite level were significantly higher in the RSV group [24]. Another rat study (an ischaemia–reperfusion model) used dealcoholised Malbec wine (1.1 mg/L RSV) and ethanolic RSV solution (0.5 mL/L) [25]. Heart perfusion with Malbec wine prevented the onset of reperfusion arrhythmias. However, it did not reduce them after the onset, as in the control group. The hearts with arrhythmias that were perfused with RSV recovered sinus rhythm in less than 1 min. Interestingly, the study pointed out that Malbec wine prevented action potential shortening and RSV preserved the resting potential during ischaemia. Overall, arrhythmia in the control group occurred in approximately 90% of cases. In the group with wine and RES, it occurred in about 50% of cases [25]. In rats, RSV significantly increased the doses of aconitine and ouabain required to induce PVCs, VT and VF [26]. It also showed a protective effect on coronary ligation-induced rat arrhythmia and shortened the duration of arrhythmias. Affecting ion channels, RSV decreased ICa current, selectively increased IKs and had no effect on IKr [26]. This action may explain the antiarrhythmic effect. The above-mentioned studies clearly indicate the possibility of using RSV to treat SVAs, VAs and ischaemia–reperfusion induced arrhythmias (Figure 2). Molecular mechanisms underlying IRI injury in striated muscles involve the production of high levels of ROS. Patients undergoing revascularisation procedures may benefit from reducing the risk of life-threatening arrhythmias or shortening of the arrhythmia in the reperfusion phase.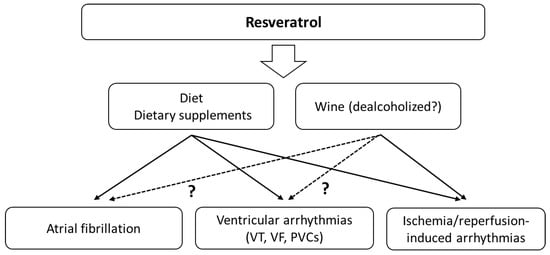
Figure 2.
The main effects of resveratrol on cardiac arrhythmias. The effects may also depend on the simultaneous consumption of alcohol.
2.5. Statins and Atrial Fibrillation
Statins are the most commonly prescribed drugs for dyslipidemia and show antioxidant activity. A meta-analysis from 2021 indicates that statin treatment significantly increases the circulating concentrations of GPx and SOD, suggesting an antioxidant effect of these agents [27]. A few randomised trials pose that statin therapy may result in sudden cardiac death (SCD) reduction as an effect of the antiarrhythmic properties of the drugs [28]. Many mechanisms describe the influence of statins on the levels of oxidants and antioxidants [29][30]. They can increase endothelial nitric oxide synthase (eNOS) transcription and the availability of tetrahydrobiopterin (BH4) and eNOS expression through stimulation of protein kinase B (Akt), inhibiting endothelial senescence induced by OS [31][32]. Statins can also affect nicotinamide adenine dinucleotide phosphate oxidases (NADPH) and stimulate antioxidant defences [29]. For example, atorvastatin increased the expression of catalase and manganese superoxide dismutase (MnSOD) [31]. Statins also reduce arrhythmic risk by lowering lipid peroxidation and affect various molecules that play an essential role in both OS and inflammation (Figure 3). The relationship between OS and inflammation is also very strong. OS activates different transcription factors (e.g., NF-κB) that induce the expression of pro-inflammatory cytokines, adhesion molecules and chemokines [33]. Moreover, ROS production is typical of activated inflammatory cells. The direct and indirect associations between the inflammatory process, cellular pathways and arrhythmia are complex. Statins can also reduce oxidized low-density lipoprotein (oxLDL), MDA and thiobarbituric acid reactive substance (TBARS) levels and affect the activity of antioxidant enzymes—SOD, GPx and catalase [34][35][36]. Thus, they can influence the development of arrhythmias.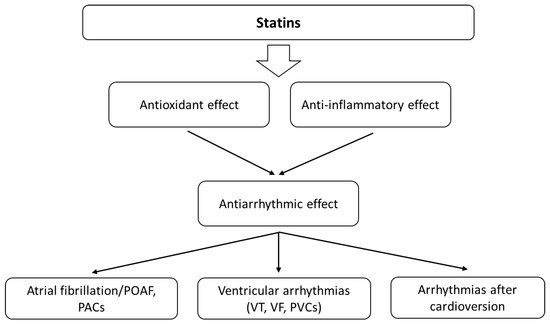
Figure 3. The main effects of statins on cardiac arrhythmias. Their antiarrhythmic activity is probably related to the influence on oxidative stress and the bioavailability of ROS, as well as modulation of the inflammatory response.
2.6. Statins and Ventricular Arrhythmias
Unfortunately, there is a lack of research on the antioxidant effect of statins in VAs. One in vitro study analysed the effect of oxLDL on intracellular Ca2+ handling in adult rat ventricular cardiomyocytes [37]. It revealed that oxLDL could cause SR-Ca2+ leak and spontaneous Ca2+ sparks and could induce a pro-arrhythmogenic profile. The same study also showed that people with well-controlled stable coronary artery disease, usually treated with statins, had significantly lower oxLDL levels than subjects at high cardiovascular risk [37]. This indicates that the antioxidant properties of statins may be useful in treating arrhythmias. Ma et al. [38] reported similar results, demonstrating that atorvastatin could inhibit the ICa,L current via suppressing ROS production in neonatal rat ventricular myocytes. Some studies demonstrate that statins decrease VAs in ICD patients. A very interesting study of 304 subjects with ICD indicated that patients on statins had a significantly lower level of derivatives of ROS (DROMs) (a measure of lipid peroxides), and the DROMs and statins were strongly associated with ICD events [39]. Importantly, DROMs were the dominant predictor of the ICD event rate [39]. This indicates that statins exert an antiarrhythmic effect by influencing the oxidative status. Exciting research was published in 2016 by Chen et al. regarding the effects of statin on arrhythmia in young, healthy persons with sleep deprivation [40]. Seventy-two young, healthy participants were recruited from the army and remained awake in the sleep laboratory for 48 h. Participants were randomised to statin or placebo groups. Patients in the statin group were given 20 mg of atorvastatin (daily) 3 days before sleep deprivation and for 2 days during sleep deprivation. The study assessed, inter alia, the activity or concentration of OS parameters (SOD and MDA) and frequency of arrhythmia—premature atrial complexes (PACs) and PVCs—simultaneously. After 48 h of sleep deprivation, the frequency of PACs and PVCs was significantly decreased in the statin group (seven PACs/hour, and five PVCs/hour vs. fourteen PACs and nine PVCs in the control group). MDA concentration was 2.51 nmol/mL lower in the statin group. SOD activity was also lower, but without statistical significance. The study concluded that a possible mechanism of the influence of statins on arrhythmias is related to OS [40]. There is a relationship between redox status and the antioxidant effect of statins, not only in life-threatening ventricular arrhythmias but also in mild ones, including PVCs.2.7. Other Studies with Antioxidants
In addition to the OS modulators mentioned above, other antioxidants have also been investigated. For instance, it was shown that mitochondrial OS plays a central role in gap junction remodelling and arrhythmia [41]. An animal model of treatment with MitoTEMPO ((2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride, a specific scavenger of mitochondrial superoxide) revealed decreased spontaneous PVCs and VT inducibility and reduced SCD by reducing mitochondrial ROS levels. MitoTEMPO treatment also increased the Cx-43 level at the gap junctions to 62% of the control value and increased the gap junction conduction [41]. Cx43 is the major structural protein of ventricular gap junctions. A significant decrease in Cx43 expression causes sudden arrhythmic death [42]. In a guinea pig model, MitoTEMPO eliminated SCD by decreasing dispersion of repolarisation (indexed by the QT variability index) and VAs [9]. Moreover, treatment with MitoTEMPO preserved cardiac function and prevented the onset of HF [9]. A model of I/R-induced arrhythmia in cats demonstrated that injections of NOS inhibitor—L-NAME (NG-nitro-L-arginine methyl ester)—decreased the incidence of arrhythmias by 40% and eliminated reperfusion-induced VT/VF [43]. L-NAME can also counteract the proarrhythmic effects of carbon monoxide [44]. Unfortunately, there are not many new studies on NOS inhibitors, and the data on L-NAME are inconclusive. Some studies indicate a possible proarrhythmic effect of L-NAME. Inhibition of NO increases VF in isolated rat hearts subjected to I/R [45]. MitoTEMPO and L-NAME are not used in clinical practice (especially MitoTEMPO), and most results come from animal model studies. Although it is traditionally not classified as an antioxidant, L-NAME affects NO synthesis and redox balance. Magnolol, an active component of Magnolia officinalis is about 1000-times more potent than α-tocopherol in inhibiting lipid peroxidation. It was shown in 1996 that magnolol significantly reduced the incidence and duration of I/R-induced VT/VF in rats during 30 min of coronary ligation and 10 min of reperfusion [46]. A similar effect was observed using honokiol (polyphenolic compound, similar to magnolol traditionally used in medical practice) [47]. Other antioxidants, found in pomegranates (Punica granatum L.), may cause a significant reduction in the occurrence of arrhythmias [48]. Rat hearts perfused with Krebs solution with pomegranate lyophilised juice (1.8 mg/mL, 2% v/v) had a lower incidence and shorter duration of VF during ischaemia and reperfusion phases. Single salvo VT was significantly lower in the pomegranate group compared to the control. Antioxidant enzymes, such as SOD, GPx and catalase, demonstrated a significant increase in the group with pomegranate juice. The addition of L-NAME reduced these effects [48]. Arctigenin (a natural lignan compound extracted from Arctium lappa) also shows antiarrhythmic activity. In an I/R injured rat heart model, arctigenin significantly reduced the incidence and duration of VT, VF and ventricular ectopic beats [49]. Rats treated with arctigenin had no VF during reperfusion and markedly enhanced activities of SOD and GPx, as well as reduced MDA levels. Administration of arctigenin (50 mg/kg/day and 200 mg/kg/ day) significantly increased the expression of Nrf2 (nuclear factor erythroid 2-related factor 2), Trx1 (thioredoxin-1) and Nox1 (NADPH Oxidase 1) [49]. Interesting research also concerns melatonin, which appears as a key molecule in heart ageing. Melatonin was shown to have an antiarrhythmic effect through a decrease in OS, the stimulation of antioxidant enzymes [50] and a decrease in fibrosis and apoptosis associated with AT1 (angiotensin 1) reduction and HSP70-VDR (heat shock protein 70—vitamin D receptor) increase [51]. Melatonin cardioprotection against OS and arrhythmias could also be explained by modulation of ion channels and Cx43 expression [52]. A detailed description of the molecular mechanisms can be found in Segovia-Roldan et al. [53]. One of the latest studies from 2021 demonstrates that melatonin protects against the development of early VF in the myocardial infarction animal model [54]. A randomised controlled trial indicated that melatonin might help modulate OS and the duration of AF following CABG surgery [55]. The feasibility of using antioxidative effects was also noticed in ICD patients, who form a specific group. It often includes people with HF who avoid physical exercises and have increased OS parameters. Some observations indicate that patients with chronic HF who also had ICDs or cardiac resynchronisation therapy-defibrillators (CRT-D), and who performed different stretching exercises (30 s per stretching exercise on a floor mat or chair, followed by 20 s of relaxation, with each exercise being performed twice and with all 7 stretches amounting to 20 min in total), had significantly decreased ROS and malondialdehyde-modified low-density lipoprotein cholesterol (MDA-LDL) level [56]. Exercise causes the increase in intracellular antioxidants in skeletal muscles and attenuation of oxidative stress. During the study, patients had no ICD shocks. This may indicate a positive role of antioxidants in this group of patients.References
- Rodrigo, R.; González, J.; Paoletto, F. The Role of Oxidative Stress in the Pathophysiology of Hypertension. Hypertens. Res. 2011, 34, 431–440.
- Korsager Larsen, M.; Matchkov, V.V. Hypertension and Physical Exercise: The Role of Oxidative Stress. Medicina 2016, 52, 19–27.
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating Oxidative Stress in Heart Failure: Past, Present and Future. Eur. J. Heart Fail. 2019, 21, 425–435.
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial Dysfunction and Oxidative Stress in Heart Disease. Exp. Mol. Med. 2019, 51, 1–3.
- Yang, X.; Li, Y.; Li, Y.; Ren, X.; Zhang, X.; Hu, D.; Gao, Y.; Xing, Y.; Shang, H. Oxidative Stress-Mediated Atherosclerosis: Mechanisms and Therapies. Front. Physiol. 2017, 8, 600.
- Olejnik, A.; Krzywonos-Zawadzka, A.; Banaszkiewicz, M.; Bil-Lula, I. Ameliorating Effect of Klotho Protein on Rat Heart during i/r Injury. Oxid. Med. Cell. Longev. 2020, 2020, 6427284.
- Sovari, A.A.; Dudley, S.C. Reactive Oxygen Species-Targeted Therapeutic Interventions for Atrial Fibrillation. Front. Physiol. 2012, 3, 311.
- Tanaka, M.; Nakamura, K.; Kusano, K.F.; Morita, H.; Ohta-Ogo, K.; Miura, D.; Miura, A.; Nakagawa, K.; Tada, T.; Murakami, M.; et al. Elevated Oxidative Stress is Associated with Ventricular Fibrillation Episodes in Patients with Brugada-Type Electrocardiogram without SCN5A Mutation. Cardiovasc. Pathol. 2011, 20, e37–e42.
- Dey, S.; DeMazumder, D.; Sidor, A.; Brian Foster, D.; O’Rourke, B. Mitochondrial ROS Drive Sudden Cardiac Death and Chronic Proteome Remodeling in Heart Failure. Circ. Res. 2018, 123, 356–371.
- Kauppila, J.P.; Hantula, A.; Kortelainen, M.L.; Pakanen, L.; Perkiömäki, J.; Martikainen, M.; Huikuri, H.V.; Junttila, M.J. Association of Initial Recorded Rhythm and Underlying Cardiac Disease in Sudden Cardiac Arrest. Resuscitation 2018, 122, 76–78.
- Romuk, E.; Wojciechowska, C.; Jacheć, W.; Nowak, J.; Niedziela, J.; Malinowska-Borowska, J.; Głogowska-Gruszka, A.; Birkner, E.; Rozentryt, P. Comparison of Oxidative Stress Parameters in Heart Failure Patients Depending on Ischaemic or Nonischaemic Aetiology. Oxid. Med. Cell. Longev. 2019, 2019, 7156038.
- Clementy, N.; Bodin, A.; Bisson, A.; Teixeira-Gomes, A.P.; Roger, S.; Angoulvant, D.; Labas, V.; Babuty, D. The Defibrillation Conundrum: New Insights into the Mechanisms of Shock-Related Myocardial Injury Sustained from a Life-Saving Therapy. Int. J. Mol. Sci. 2021, 22, 5003.
- Mirhoseini, M.F.; Hamblin, S.E.; Moore, W.P.; Pouliot, J.; Jenkins, J.M.; Wang, W.; Chandrasekhar, R.; Collier, B.R.; Patel, M.B. Antioxidant Supplementation and Atrial Arrhythmias in Critically Ill Trauma Patients. J. Surg. Res. 2018, 222, 10–16.
- Hadjizacharia, P.; O’Keeffe, T.; Brown, C.V.R.; Inaba, K.; Salim, A.; Chan, L.S.; Demetriades, D.; Rhee, P. Incidence, Risk Factors, and Outcomes for Atrial Arrhythmias in Trauma Patients. Am. Surg. 2011, 77, 634–639.
- Lin, Y.K.; Lin, F.Z.; Chen, Y.C.; Cheng, C.C.; Lin, C.I.; Chen, Y.J.; Chen, S.A. Oxidative Stress on Pulmonary Vein and Left Atrium Arrhythmogenesis. Circ. J. 2010, 74, 1547–1556.
- Violi, F.; Pastori, D.; Pignatelli, P.; Loffredo, L. Antioxidants for Prevention of Atrial Fibrillation: A Potentially Useful Future Therapeutic Approach? A Review of the Literature and Meta-Analysis. Europace 2014, 16, 1107–1116.
- Guo, X.Y.; Yan, X.L.; Chen, Y.W.; Tang, R.B.; Du, X.; Dong, J.Z.; Ma, C.S. Omega-3 Fatty Acids for Postoperative Atrial Fibrillation: Alone or in Combination with Antioxidant Vitamins? Heart Lung Circ. 2014, 23, 743–750.
- Barbosa, J.L.; Thiers, C.A.; Pereira, B.D.B.; Do Nascimento, E.M.; Ribeiro Frazon, C.M.; Budni, P.; Filho, D.W.; Pedrosa, R.C. Impact of the Use of Benznidazole Followed by Antioxidant Supplementation in the Prevalence of Ventricular Arrhythmias in Patients with Chronic Chagas Disease: Pilot Study. Am. J. Ther. 2016, 23, e1474–e1483.
- Frommeyer, G.; Wolfes, J.; Ellermann, C.; Kochhäuser, S.; Dechering, D.G.; Eckardt, L. Acute Electrophysiologic Effects of the Polyphenols Resveratrol and Piceatannol in Rabbit Atria. Clin. Exp. Pharmacol. Physiol. 2019, 46, 94–98.
- Larsson, S.C.; Drca, N.; Wolk, A. Alcohol Consumption and Risk of Atrial Fibrillation: A Prospective Study and Dose-Response Meta-Analysis. J. Am. Coll. Cardiol. 2014, 64, 281–289.
- Hung, L.M.; Chen, J.K.; Huang, S.S.; Lee, R.S.; Su, M.J. Cardioprotective Effect of Resveratrol, a Natural Antioxidant Derived from Grapes. Cardiovasc. Res. 2000, 47, 549–555.
- Kaya, S.T.; Bozdogan, O.; Ozarslan, T.O.; Taskin, E.; Eksioglu, D.; Erim, F.; Firat, T.; Yasar, S. The Protection of Resveratrol and Its Combination with Glibenclamide, but Not Berberine on the Diabetic Hearts against Reperfusion-Induced Arrhythmias: The Role of Myocardial K ATP Channel. Arch. Physiol. Biochem. 2019, 125, 114–121.
- Menezes-Rodrigues, F.S.; Errante, P.R.; Araújo, E.A.; Fernandes, M.P.P.; da Silva, M.M.; Pires-Oliveira, M.; Scorza, C.A.; Scorza, F.A.; Taha, M.O.; Caricati-Neto, A. Cardioprotection Stimulated by Resveratrol and Grape Products Prevents Lethal Cardiac Arrhythmias in an Animal Model of Ischemia and Reperfusion. Acta Cir. Bras. 2021, 36, e360306.
- Kazemirad, H.; Kazerani, H.R. Cardioprotective Effects of Resveratrol Following Myocardial Ischemia and Reperfusion. Mol. Biol. Rep. 2020, 47, 5843–5850.
- Prado, N.J.; Perdicaro, D.J.; Parra, M.; Carrión, A.M.; Miatello, R.M.; Renna, N.F.; Ponce, Z.A.Z.; Vazquez, P.M.A.; Diez, E.R. Antiarrhythmic Mechanisms of Malbec Wine and Resveratrol in Isolated Rat Heart. Cardiovasc. Disord. Med. 2017, 3, 1–7.
- Zhang, Y.; Liu, Y.; Wang, T.; Li, B.; Li, H.; Wang, Z.; Yang, B. Resveratrol, a Natural Ingredient of Grape Skin: Antiarrhythmic Efficacy and Ionic Mechanisms. Biochem. Biophys. Res. Commun. 2006, 340, 1192–1199.
- Zinellu, A.; Mangoni, A.A. A Systematic Review and Meta-Analysis of the Effect of Statins on Glutathione Peroxidase, Superoxide Dismutase, and Catalase. Antioxidants 2021, 10, 1841.
- De Sutter, J.; Firsovaite, V.; Tavernier, R. Prevention of Sudden Death in Patients with Coronary Artery Disease: Do Lipid-Lowering Drugs Play a Role? Prev. Cardiol. 2002, 5, 177–182.
- Costa, S.; Reina-Couto, M.; Albino-Teixeira, A.; Sousa, T. Statins and Oxidative Stress in Chronic Heart Failure. Rev. Port. Cardiol. 2016, 35, 41–57.
- Davignon, J.; Jacob, R.F.; Mason, R.P. The Antioxidant Effects of Statins. Coron. Artery Dis. 2004, 15, 251–258.
- Ota, H.; Eto, M.; Kano, M.R.; Kahyo, T.; Setou, M.; Ogawa, S.; Iijima, K.; Akishita, M.; Ouchi, Y. Induction of Endothelial Nitric Oxide Synthase, SIRT1, and Catalase by Statins Inhibits Endothelial Senescence through the Akt Pathway. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2205–2211.
- Aoki, C.; Nakano, A.; Tanaka, S.; Yanagi, K.; Ohta, S.; Jojima, T.; Kasai, K.; Takekawa, H.; Hirata, K.; Hattori, Y. Fluvastatin Upregulates Endothelial Nitric Oxide Synthase Activity via Enhancement of Its Phosphorylation and Expression and via an Increase in Tetrahydrobiopterin in Vascular Endothelial Cells. Int. J. Cardiol. 2012, 156, 55–61.
- Vonderlin, N.; Siebermair, J.; Kaya, E.; Köhler, M.; Rassaf, T.; Wakili, R. Critical Inflammatory Mechanisms Underlying Arrhythmias. Herz 2019, 44, 121–129.
- Sezer, E.D.; Sozmen, E.Y.; Nart, D.; Onat, T. Effect of Atorvastatin Therapy on Oxidantantioxidant Status and Atherosclerotic Plaque Formation. Vasc. Health Risk Manag. 2011, 7, 333–343.
- Hadi, N.R.; Abdelhussein, M.A.; Rudha, A.R.M.; Jamil, D.A.; Al-Aubaidy, H.A. Simvastatin Use in Patients with Type 2 Diabetes Mellitus: The Effects on Oxidative Stress. Oman Med. J. 2015, 30, 237–240.
- Mohamadin, A.M.; Elberry, A.A.; Abdel Gawad, H.S.; Morsy, G.M.; Al-Abbasi, F.A. Protective Effects of Simvastatin, a Lipid Lowering Agent, against Oxidative Damage in Experimental Diabetic Rats. J. Lipids 2011, 2011, 167958.
- Rodríguez-Sánchez, E.; Navarro-García, J.A.; González-Lafuente, L.; Aceves-Ripoll, J.; Vázquez-Sánchez, S.; Poveda, J.; Mercado-García, E.; Corbacho-Alonso, N.; Calvo-Bonacho, E.; Fernández-Velasco, M.; et al. Oxidized Low-Density Lipoprotein Associates with Ventricular Stress in Young Adults and Triggers Intracellular Ca2+ Alterations in Adult Ventricular Cardiomyocytes. Antioxidants 2020, 9, 1213.
- Ma, Y.; Kong, L.; Qi, S.; Wang, D. Atorvastatin Blocks Increased L-Type Ca2+ Current and Cell Injury Elicited by Angiotensin II via Inhibiting Oxide Stress. Acta Biochim. Biophys. Sin. 2016, 48, 378–384.
- Bloom, H.L.; Shukrullah, I.; Veledar, E.; Gutmann, R.; London, B.; Dudley, S.C. Statins Decrease Oxidative Stress and ICD Therapies. Cardiol. Res. Pract. 2010, 1, 253803.
- Chen, W.R.; Liu, H.; Bin; Sha, Y.; Shi, Y.; Wang, H.; Yin, D.W.; Chen, Y.D.; Shi, X.M. Effects of Statin on Arrhythmia and Heart Rate Variability in Healthy Persons with 48-Hour Sleep Deprivation. J. Am. Heart Assoc. 2016, 5, e003833.
- Sovari, A.A.; Rutledge, C.A.; Jeong, E.M.; Dolmatova, E.; Arasu, D.; Liu, H.; Vahdani, N.; Gu, L.; Zandieh, S.; Xiao, L.; et al. Mitochondria Oxidative Stress, Connexin43 Remodeling, and Sudden Arrhythmic Death. Circ. Arrhythmia Electrophysiol. 2013, 6, 623–631.
- Van Norstrand, D.W.; Asimaki, A.; Rubinos, C.; Dolmatova, E.; Srinivas, M.; Tester, D.J.; Saffitz, J.E.; Duffy, H.S.; Ackerman, M.J. Connexin43 Mutation Causes Heterogeneous Gap Junction Loss and Sudden Infant Death. Circulation 2012, 125, 474–481.
- Kukushkina, O.I.; Fedotkina, L.K.; Balashov, V.P.; Balykova, L.A.; Sosunov, A.A. Effect of NO-Synthase Inhibitor L-NAME on Occlusion and Reperfusion Arrhythmias in Cats. Bull. Exp. Biol. Med. 1999, 127, 460–462.
- Dallas, M.L.; Yang, Z.; Boyle, J.P.; Boycott, H.E.; Scragg, J.L.; Milligan, C.J.; Elies, J.; Duke, A.; Thireau, J.; Reboul, C.; et al. Carbon Monoxide Induces Cardiac Arrhythmia via Induction of the Late Na+ Current. Am. J. Respir. Crit. Care Med. 2012, 186, 648–656.
- Pabla, R.; Curtis, M.J. Effects of NO Modulation on Cardiac Arrhythmias in the Rat Isolated Heart. Circ. Res. 1995, 77, 984–992.
- Hong, C.Y.; Huang, S.S.; Tsai, S.K. Magnolol Reduces Infarct Size and Suppresses Ventricular Arrhythmia in Rats Subjected to Coronary Ligation. Clin. Exp. Pharmacol. Physiol. 1996, 23, 660–664.
- Tsai, S.K.; Huang, C.H.; Huang, S.S.; Hung, L.M.; Hong, C.Y. Antiarrhythmic Effect of Magnolol and Honokiol during Acute Phase of Coronary Occlusion in Anesthetized Rats: Influence of L-NAME and Aspirin. Pharmacology 1999, 59, 227–233.
- Kazemirad, H.; Kazerani, H.R. The Anti-Arrhythmic Effects of Pomegranate (Punica Granatum) are Mainly Mediated by Nitric Oxide. J. Berry Res. 2020, 10, 573–584.
- Yang, J.; Yin, H.S.; Cao, Y.J.; Jiang, Z.A.; Li, Y.J.; Song, M.C.; Wang, Y.F.; Wang, Z.H.; Yang, R.; Jiang, Y.F.; et al. Arctigenin Attenuates Ischemia/Reperfusion Induced Ventricular Arrhythmias by Decreasing Oxidative Stress in Rats. Cell. Physiol. Biochem. 2018, 49, 728–742.
- Ding, K.; Wang, H.; Xu, J.; Li, T.; Zhang, L.; Ding, Y.; Zhu, L.; He, J.; Zhou, M. Melatonin Stimulates Antioxidant Enzymes and Reduces Oxidative Stress in Experimental Traumatic Brain Injury: The Nrf2-ARE Signaling Pathway as a Potential Mechanism. Free Radic. Biol. Med. 2014, 73, 1–11.
- Prado, N.J.; Casarotto, M.; Calvo, J.P.; Mazzei, L.; Ponce Zumino, A.Z.; García, I.M.; Cuello-Carrión, F.D.; Fornés, M.W.; Ferder, L.; Diez, E.R.; et al. Antiarrhythmic Effect Linked to Melatonin Cardiorenal Protection Involves AT1 Reduction and Hsp70-VDR Increase. J. Pineal Res. 2018, 65, e12513.
- Benova, T.; Viczenczova, C.; Radosinska, J.; Bacova, B.; Knezl, V.; Dosenko, V.; Weismann, P.; Zeman, M.; Navarova, J.; Tribulova, N. Maladaptation of Connexin-43 and Propensity of the Heart to Lethal. Can. J. Physiol. Pharmacol. 2013, 91, 633–639.
- Segovia-Roldan, M.; Diez, E.R.; Pueyo, E. Melatonin to Rescue the Aged Heart: Antiarrhythmic and Antioxidant Benefits. Oxid. Med. Cell. Longev. 2021, 2021, 8876792.
- Tsvetkova, A.S.; Bernikova, O.G.; Mikhaleva, N.J.; Khramova, D.S.; Ovechkin, A.O.; Demidova, M.M.; Platonov, P.G.; Azarov, J.E. Melatonin Prevents Early but Not Delayed Ventricular Fibrillation in the Experimental Porcine Model of Acute Ischemia. Int. J. Mol. Sci. 2021, 22, 328.
- Xu, N.W.; Chen, Y.; Liu, W.; Chen, Y.J.; Fan, Z.M.; Liu, M.; Li, L.J. Inhibition of JAK2/STAT3 Signaling Pathway Suppresses Proliferation of Burkitt’s Lymphoma Raji Cells via Cell Cycle Progression, Apoptosis, and Oxidative Stress by Modulating HSP70. Med. Sci. Monit. 2018, 24, 6255–6263.
- Kato, M.; Masuda, T.; Ogano, M.; Hotta, K.; Takagi, H.; Tanaka, S.; Kamada, Y.; Akiyama, A.; Kamekawa, D.; Shimizu, R.; et al. Stretching Exercises Improve Vascular Endothelial Dysfunction through Attenuation of Oxidative Stress in Chronic Heart Failure Patients with an Implantable Cardioverter Defibrillator. J. Cardiopulm. Rehabil. Prev. 2017, 37, 130–138.
More
