Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Xiaozhe Bao and Version 3 by Dean Liu.
Arbuscular mycorrhizal fungi (AMF) and soil microbe interactions are among the most important and influential processes that occur, as they significantly influence the plant growth and soil structure properties. Their interactions may be of crucial importance to the sustainable, low-input productivity of paddy ecosystems.
- rice
- arbuscular mycorrhizal fungi
- mycorrhizosphere microbes
1. Major Groups of Bacteria Interacting with AMF
Bacteria populations have been found to be dominant in the rice rhizosphere, exerting a great influence over rice growth and production [1][36]. Different groups of soil bacteria have been reported to interact with AMF, including plant-growth-promoting bacteria (PGPR), endo-bacteria, mycorrhiza helper bacteria (MHB), and deleterious bacteria (DB), etc. [2][3][4][37,38,39] (Figure 1). Knowledge of such interactions is vital for enhancing the tripartite symbiosis between AMF, bacteria, and host plants.
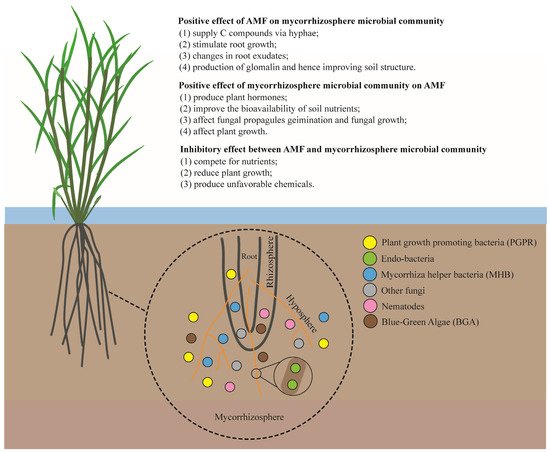
Figure 1. Schematic view of interactions between AMF and different components of the mycorrhizosphere.
1.1. PGPR
PGPR are among the most important soil bacteria, as they significantly enhance rice yield and reduce the need for chemical fertilizers [5][40]. The mechanisms involved include: (1) enhancing the solubility of soil nutrients by producing enzymes and siderophores; (2) producing phytohormones; (3) controlling pathogens and alleviating the adverse effects of stress; and (4) interacting with other soil microbes [6][7][8][9][41,42,43,44]. A variety of PGPR have been reported to interact with AMF, including Pseudomonas, Bacillus, Azospirillum, Herbaspirillum, Paenibacillus, rhizobia, etc. [6][10][11][12][41,45,46,47]. In paddy fields, there are several different enhanced combinations of bacterial strains and AMF species that could promote rice growth and development (Table 1). Bacterial strains include Bacillus, Pseudomonas, Azospirillum, and Herbaspirillum, while AMF species include Rhizophagus irregularis (renamed from Glomus intraradices) and Funneliformis mosseae [13][14][15][16][48,49,50,51]. The abbreviations for AMF and PGPR can be seen in Table 2. Their synergistic stimulating effects include the solubilization of phosphate, nitrogen fixing, rice growth promotion, and the suppression of pathogens [6][41]. For example, Hoseinzade et al. [17][52] observed that the interaction of AMF (F. mosseae) with nitrogen-fixing bacteria (Herbaspirillum seropedicae) together with urea (nitrogen source) and triple super phosphate (phosphorus source) fertilizers promoted the growth of rice plants. Norouzinia et al. [16][51] showed that the co-inoculation of Pseudomonas putida strain S34, Pseudomonas fluorescens strain R167 and R. irregularis alleviated the adverse effects of salinity and significantly increased the grain yield of rice.
Table 1. List of major microorganisms interacting with AMF in rice mycorrhizosphere.
| Type of Microorganisms | Microbe Species | AMF Species | Effects | Reference |
|---|
| Type of Microorganisms | Full Name | Abbreviations | |||
|---|---|---|---|---|---|
| Bacteria | A. Lipoferum, B. megaterium |
Glomus sp., Gigasporasp sp. and Acaulospora sp. | Rice growth↑, grain yield↑ |
[15][50] | |
| A. brasilense, B. cepacia |
F. mosseae | ||||
| AMF | Rhizophagus irregularis | R. irregularis | |||
| Rice growth↑, | grain yield↑, P uptake↑ |
[ | |||
| Funneliformis mosseae | 18 | ] | F. mosseae[53] | ||
| H. seropedicae | F. mosseae | Rice growth↑ | [17][52] | ||
| P. fluorescens, P. putida | R. irregularis | Grain yield↑, salinity↓ | [16][51] | ||
| P. jessenii, P. synxantha | A natural AMF consortium (Mnat) and a single-spore AMF strain (Mss2) | No change | [13][48] | ||
| S. thermocarboxydus | |||||
| Gigaspora candida | G. candida | F. mosseae | Rice growth↑ | [19][54] | |
| Fungi | |||||
| Glomus aggregatum | G. aggregatum | G. fulvum, | |||
| Glomus caledonium | G. caledonium | ||||
| Glomus coronatum | G. coronatum | R. solani | R. fasciculatum, F. mosseae, G. aggregatum, G. candida, and G. gigantea |
AMF spore density and infection↑, sheath blight↓ |
|
| Glomus deserticola | [ | 20 | G. deserticola | ][55] | |
| R. irregularis, F. mosseae, G. deserticola, R. fasciculatum, S. dussii, and G. microaggregatum |
Sheath blight↑ | [21][56] | |||
| Glomus fulvum | Soil faunas and other microbes | M. graminicola | R. irregularis, F. mossae |
F. mossae decreased M. graminicola multiplication, while R. irregularis did not |
[22][32] |
| Blue-Green Algae | G. microcarpium | Rice growth↑, grain yield↑ |
[23][57] |
Table 2. List of abbreviations for AMF and other microorganisms.
| G. fulvum | ||
| Gigaspora gigantea | ||
| G. gigantea | ||
| Glomus microaggregatum | ||
| G. microaggregatum | ||
| Glomus viscosum | G. viscosum | |
| Rhizophagus fasciculatum | R. fasciculatum | |
| Sclerocystis dussii | S.dussii | |
| Bacteria | Azospirillum brasilense | Brasilense |
| Azospirillum lipoferum | A. lipoferum | |
| Bacillus megaterium | B. megaterium | |
| Bacillus subtilis | B. subtilis | |
| Burkholderia cepacia | B. cepacia | |
| Herbaspirillum seropedicae | H. seropedicae | |
| Pseudomonas fluorescens | P. fluorescens | |
| Pseudomonas jessenii | P. jessenii | |
| Pseudomonas putida | P. putida | |
| Pseudomonas synxantha | P. synxantha | |
| Streptomyces thermocarboxydus | S. thermocarboxydus | |
| Fungi | Rhizoctonia solani | R. solani |
| Magnaporthe oryzae | M. oryzae | |
| Soil fauna | Meloidogyne graminicola | M. graminicola |
1.2. Endo-Bacteria
Some endo-bacteria inside AMF cytoplasms are also regarded as PGPR, establishing an intimate interaction with AMF. The endobacteria of Glomeromycota are the most thoroughly investigated bacterial endosymbionts [24][58]. Two types of endosymbionts are known in Glomeromycota: (i) a rod-shaped, Gram-negative beta-proteobacterium Candidatus Glomeribacter gigasporarum (CaGg), limited members of the Gigasporaceae family [25][59]; (ii) a coccoid bacterium displaying a homogeneous Gram-positive-like wall structure, Mollicutes-related endobacteria (Mre), widely distributed across Glomeromycota [26][60]. It is also well accepted that some actinobacteria are considered potential PGPR [27][28][61,62], and evidence obtained from Lasudee et al. [19][54] showed that Streptomyces thermocarboxydus isolate S3 isolated from spores of F. mosseae promoted the growth of rice plants grown in low-nutritional soil under drought-induced stress (Table 1). The abbreviations for AMF and endo-bacteria can be seen in Table 2. According to Bonfante et al. [29][63] and Salvioli et al. [30][64], the mechanisms involved in the endo-bacteria affecting fungal performance are as follows: (1) release of substances, e.g., volatiles, which affects fungal gene expression; (2) production of lectins which attach to the fungal surface; (3) degradation of fungal cell wall; and (4) injection of molecules into the fungal spore.
1.3. MHB
MHB were first identified by Garbaye (1994), and mostly include Proteobacteria such as Pseudomonas, Oxalobacteraceae, Actinomycetes such as Streptomyces, and Firmicutes such as Bacillus [2][31][32][37,65,66]. A number of studies have stated the synergistic effect of AMF and MHB, including some which focused on rice crops. MHB may play an important role in enhancing the activity and development of AMF, providing nutrients to plants and AMF, as well as promoting their defenses [33][34][67,68]. For example, the co-inoculation of Azospirillum lipoferum, Bacillus megaterium and AMF increased the growth and grain yield of rice, at the nursery and in the main field, under the System of Rice Intensification (SRI) [15][50]. There have also been studies that showed the opposite effects of AMF, where the use of two fluorescent Pseudomonas strains (Pseudomonas jessenii R62; Pseudomonas synxantha R81) on rice yields was far less pronounced over two cropping seasons [13][48] (Table 1). The abbreviations for AMF and MHB can be seen in Table 2. It is reported that MHB are usually fungal-specific but not plant-specific [35][69], which means that MHB can promote the growth of specific AMF, even if in symbiosis with their non-specific host plant. Therefore, the opposite conclusions above were possibly found because of the use of inappropriate bacterial types and AMF species. Moreover, since there are relatively few studies of AMF and MHB in the rice rhizosphere, the common unresolved issues (such as whether increased AMF growth and survival by MHB are due to the production of growth factors, the detoxification of soil allelochemicals, or the antagonism of competitors and/or parasites) remain unresolved in rice [36][70].
1.4. DB
DB, which adversely affects plant growth, are also found to be interactive with AMF. Their unfavorable effects on plant growth are probably due to the production of harmful substances such as phytotoxins, competition for food resources with other soil microorganisms, and their inhibitory effects on AM fungal activities [3][38]. Various mechanisms have been demonstrated to explain biocontrol activity by AMF, including: (1) parasitizing pathogen, (2) higher competition for colonization sites and host photosynthates, (3) secondary metabolites production, (4) the modification of the microbial community, (5) promoting plant nutrient uptake and anatomical changes in the root system, and (6) inducting plant systemic resistance [37][38][71,72]. Although some deleterious bacteria reside in the rice rhizosphere, few studies have reported the effect of AMF on the DB control.
Interestingly, PGPR may be detrimental for mycorrhizal colonization, implying the probable existence of ‘functional competition’ between beneficial microorganisms [39][40][73,74]. This depends on the mycorrhizosphere conditions, such as AM growth and development, microbial growth stage, and environmental conditions [41][75]. Such characteristics indicate the importance of the choice of AMF and rhizobacteria used for inoculum production to achieve sustainable agriculture [42][43][76,77].
25. Interactions between AMF and Other Fungi
Fungi are another important group in the mycorrhizosphere, playing significant roles in ecosystem function, such as phosphorus (P) solubilization, nitrogen fixation, and the synthesis of indole acetic acid (IAA) for plant growth promotion [44][78]. In the rice mycorrhizosphere, fungi communities are commonly composed of Penicillium, Aspergillus, Talaromyces, and Trichoderma, etc [45][2]. Pathogenic fungi can also reside in the rice mycorrhizosphere, interacting with AMF such as Rhizoctonia solani, a phytopathogenic fungus that causes rice sheath blight. AMF could establish direct interaction with other fungi, which could in turn affect rice growth (Table 1, Figure 1). The abbreviations for AMF and other fungi can be seen in Table 2. Baby et al. [20][55] showed that an increase in AMF spore density and infection decreased the sheath blight disease in rice; however, Bernaola et al. [21][56] indicated that lesion lengths and susceptibility to sheath blight infection were higher in rice plants colonized by AMF. Different types of fungi can affect the level of symbiotic efficiency between AMF and rice performance. Furthermore, parameters such as microbial growth stage, environmental factors, and AMF growth and development can also affect their interaction. Studies have also shown that pathogenic fungi can interact with AMF through their effects on plant growth [3][38]. Although there are examples of interaction between AMF and other fungi in other kinds of plants, such as soybean, tomato, Brassica juncea L, Oil Palm, etc., there are relatively few studies concerning AMF and other fungi interaction in the rice mycorrhizosphere.
36. Interactions between AMF and Other Microorganisms
In addition to the rhizobacteria and fungi, AMF also interact with other soil fauna in paddy fields, e.g., protozoa and small nematodes [46][47][79,80] (Table 1, Figure 1). The abbreviations for AMF and soil fauna can be seen in Table 2. Although protozoa (18–250 lb/ac live weight) and soil nematodes (10–260 lb/ac live weight) comprise a relatively small part of the soil biomass, they perform essential functions in the soil environment [48][81]. Protozoa and small nematodes feeding on bacteria and fungi, collectively known as microbial grazers, release plant-available nutrients and suppress disease [49][82]. Soil protozoa are reported to interact with AMF species, and collembola are among the soil protozoa which interact with soil fungi [50][83], however this interaction is not detected in paddy fields. In fact there were few studies concerning AMF and protozoa in the paddy fields.
Not all nematodes are beneficial in agricultural soils. About 10% of soil nematodes feed on plant roots, causing root rot or wilting problems [51][84]. Therefore, AMF not only synergistically interact with soil nematodes, but also play a role in the biocontrol against plant-parasitic nematodes. Studies have reported that F. mossae exhibited suppression of the Meloidogyne graminicola multiplication in rice, while R. irregularis did not [22][32]. The direct effects of AMF species on the pathogens include competition for limited space or nutrients, and the indirect effects include alteration of plant root morphology and exudation, as well as induced plant systemic resistance. The detailed mechanisms involved in AMF against pathogens will be described below, either directly or indirectly. However, although AMF are one of the most important components of the soil ecosystem and large numbers of soil fauna are found in the paddy fields, little is known about the interactions between AMF species, and thus further research should pay more attention to this aspect.
Interestingly, AMF could also cooperate with Cyanobacteria (blue-green algae) (Figure 1). The abbreviations for AMF and BGA can be seen in Table 2. BGA are the main components of the microbial community in rice paddy fields, contributing to the fertility of agricultural ecosystems [52][85]. Their beneficial effects on the crop growth, yield, and nitrogen fixation of such ecosystems have been reported [53][86]. Rice may respond favorably to the combined application of BGA, AMF, and N fertilizer, such as through improvements in grain yield, straw yield, nutrient availability, soil structure, and alkali soil reclamation. The combined effect of biological N2 fixation by BGA and the plant growth regulators produced by AMF resulted in increased grain and straw yield [38][72].
