As the solid waste by-product from the delayed coking process, high-sulfur petroleum coke (HSPC), which is hardly used for green utilization, becomes a promising raw material for Hg
0
removal from coal-fired flue gas. The effects of the physical–chemical evolution of HSPC on Hg
0
removal are discussed. The improved micropores created by pyrolysis and KOH activation could lead to over 50% of Hg
0
removal efficiency with the loss of inherent sulfur. Additional S-containing and Br-containing additives are usually introduced to enhance active surface functional groups for Hg
0
oxidation, where the main product are HgS, HgBr, and HgBr
2
. The chemical–mechanical activation method can make additives well loaded on the surface for Hg
0
removal.
- high-sulfur petroleum coke
- Hg0 removal
1. Introduction
2. The Effect of the Evolution of Pore Structure on Hg0 Removal
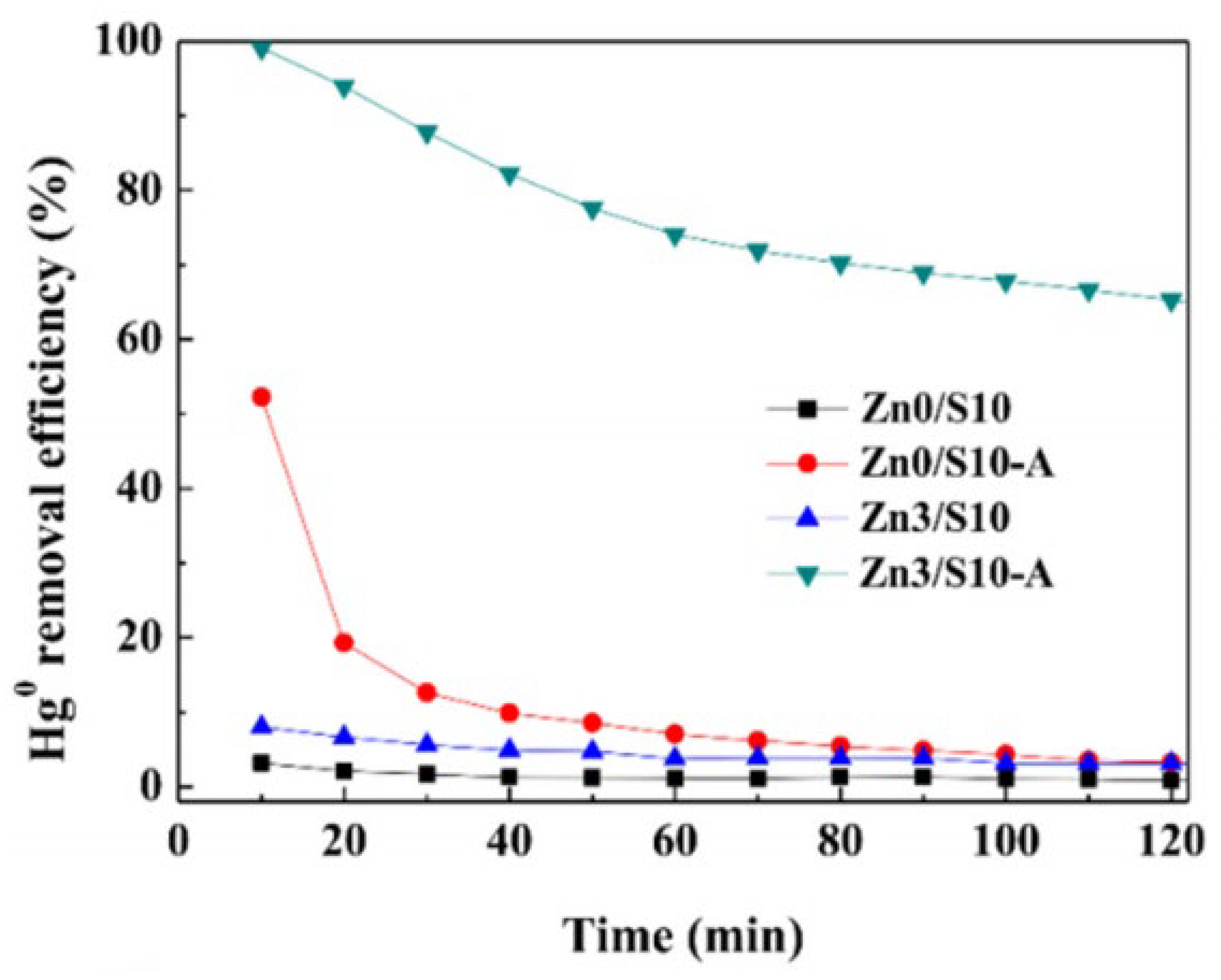
The pore structure of raw HSPC is identified as poor for Hg0 adsorption due to its dense structure. In particular, the Brunauer–Emmett–Teller (BET) surface area of raw HSPC is tested to be less than 1.1 m2/g [36] and the total pore volume is almost zero [37]. It is the reason for the poor performance of pore structure on Hg0 adsorption and also the lack of positions for active sites. The current modification process to active petroleum coke, including only or combined chemical, pyrolysis, mechanochemistry, and KOH activation, almost improved the pore structure of HSPC to a certain extent. For only chemical activation, Xiao et al. [38] used a chemical–mechanical bromination process [39] for brominating the petroleum coke sample, as shown in Figure 1c. Although the mechanical impregnation method presented well bromine loading, both specific surface area and average pore size were slightly reduced to 1.66 m2/g and 0.012 cm3/g, which was caused by the blockage of pores during the bromination process. It is believed that external mechanical force caused by grinding could not promote the development of rich pores. While this bromination method made an excellent performance of Hg0 removal, it was almost owing to the chemically loaded bromine of C-Br rather than the contribution of pore structure.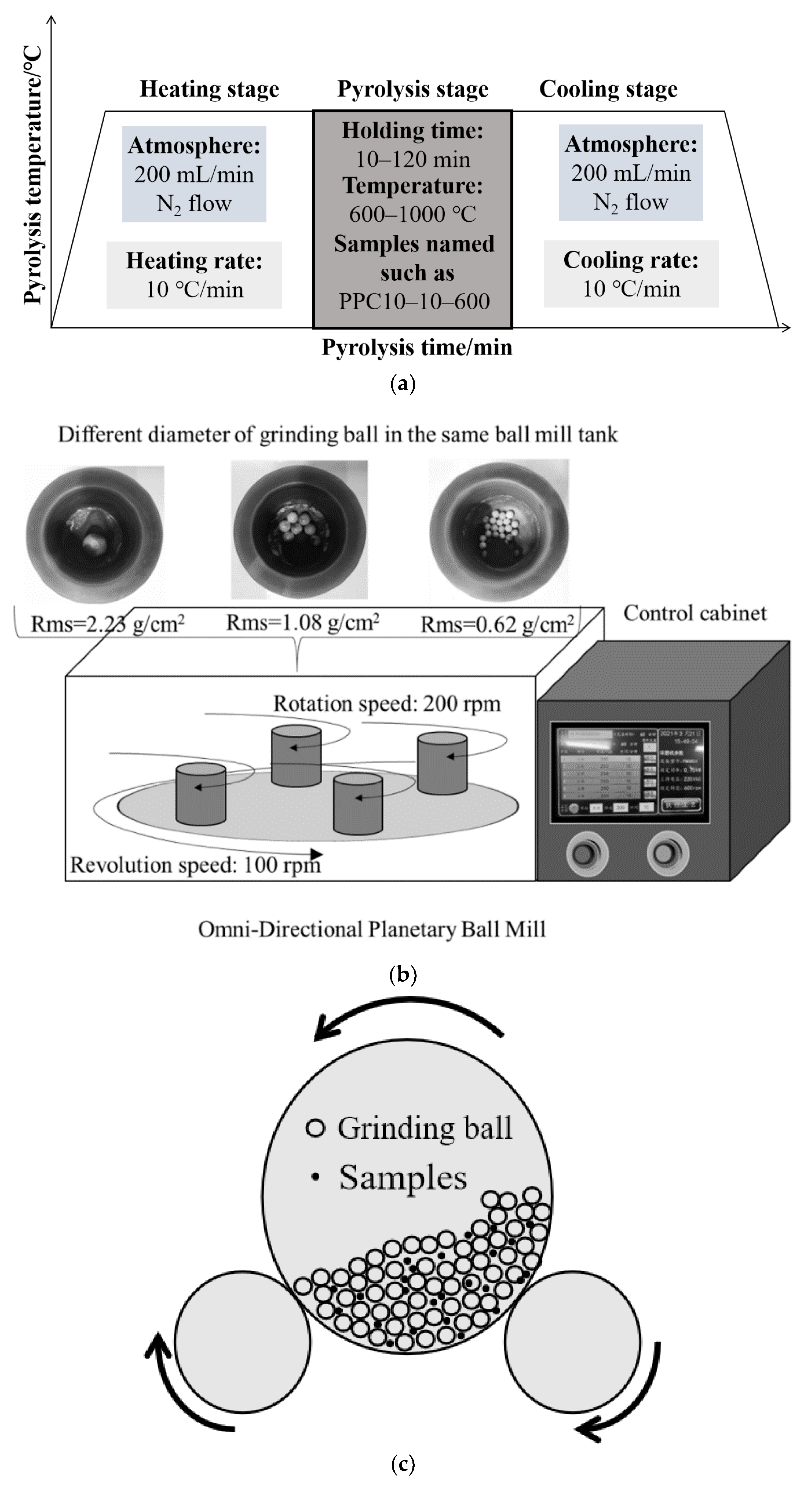
References
- Wang, M.; Wan, Y.; Guo, Q.; Bai, Y.; Yu, G.; Liu, Y.; Zhang, H.; Zhang, S.; Wei, J. Brief review on petroleum coke and biomass/coal co-gasification: Syngas production, reactivity characteristics, and synergy behavior. Fuel 2021, 304, 121517.
- Cai, W.; Li, K.; Jiang, K.; Lv, D.; Liu, Y.-Q.; Wang, D.; Wang, X.; Lai, C. Utilization of high-sulfur-containing petroleum coke for making sulfur-doped porous carbon composite material and its application in supercapacitors. Diam. Relat. Mater. 2021, 116, 108380.
- Tyutrin, A.A.; Burdonov, A.E.; Bushuev, K.S. Expanding the Application Scope of Fine Dust from Petroleum Coke Calcining Furnaces in Aluminum Production. Mater. Sci. Forum 2022, 1052, 482–487.
- Veluri, P.S.; Katchala, N.; Anandan, S.; Pramanik, M.; NarayanSrinivasan, K.; Ravi, B.; Rao, N.T. Petroleum Coke as an Efficient Single Carbon Source for High-Energy and High-Power Lithium-Ion Capacitors. Energy Fuels 2021, 35, 9010–9016.
- Liu, L.; Li, Z.; Wu, S.; Li, D.; Cai, N. Conversion characteristics of lignite and petroleum coke in chemical looping combustion coupled with an annular carbon stripper. Fuel Process. Technol. 2021, 213, 106711.
- Shan, Y.; Guan, D.; Meng, J.; Liu, Z.; Schroeder, H.; Liu, J.; Mi, Z. Rapid growth of petroleum coke consumption and its related emissions in China. Appl. Energy 2018, 226, 494–502.
- Andrews, A.; Lattanzio, R.K. Petroleum Coke: Industry and Environmental Issues; Congressional Research Service: Washington DC, USA, 2013.
- Mackey, T.K.; Contreras, J.T.; Liang, B.A. The Minamata Convention on Mercury: Attempting to address the global controversy of dental amalgam use and mercury waste disposal. Sci. Total Environ. 2014, 472, 125–129.
- Wang, L.; Hou, D.; Cao, Y.; Ok, Y.S.; Tack, F.M.; Rinklebe, J.; O’Connor, D. Remediation of mercury contaminated soil, water, and air: A review of emerging materials and innovative technologies. Environ. Int. 2020, 134, 105281.
- Streets, D.G.; Devane, M.K.; Lu, Z.; Bond, T.C.; Sunderland, E.M.; Jacob, D.J. All-time releases of mercury to the atmosphere from human activities. Environ. Sci. Technol. 2011, 45, 10485–10491.
- Liu, K.; Wang, S.; Wu, Q.; Wang, L.; Ma, Q.; Zhang, L.; Li, G.; Tian, H.; Duan, L.; Hao, J. A highly resolved mercury emission inventory of Chinese coal-fired power plants. Environ. Sci. Technol. 2018, 52, 2400–2408.
- Zhao, S.; Pudasainee, D.; Duan, Y.; Gupta, R.; Liu, M.; Lu, J. A review on mercury in coal combustion process: Content and occurrence forms in coal, transformation, sampling methods, emission and control technologies. Prog. Energy Combust. Sci. 2019, 73, 26–64.
- Li, Y.; Yu, J.; Liu, Y.; Huang, R.; Wang, Z.; Zhao, Y. A Review on Removal of Mercury from Flue Gas Utilizing Existing Air Pollutant Control Devices (APCDs). J. Hazard. Mater. 2022, 427, 128132.
- Li, G.; Wu, Q.; Xu, L.; Wen, M.; Liu, K.; Tang, Y.; Zou, J.; Wang, F.; Wang, Y.; Wang, S. A review on adsorption technologies for mercury emission control. Bull. Environ. Contam. Toxicol. 2019, 103, 155–162.
- Beckers, F.; Rinklebe, J. Cycling of mercury in the environment: Sources, fate, and human health implications: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 693–794.
- Sasmaz, E.; Kirchofer, A.; Jew, A.D.; Saha, A.; Abram, D.; Jaramillo, T.F.; Wilcox, J. Mercury chemistry on brominated activated carbon. Fuel 2012, 99, 188–196.
- Hu, J.; Geng, X.; Duan, Y.; Zhao, W.; Zhu, M.; Ren, S. Effect of mechanical–chemical modification process on mercury removal of bromine modified fly ash. Energy Fuels 2020, 34, 9829–9839.
- Ochedi, F.O.; Liu, Y.; Hussain, A. A review on coal fly ash-based adsorbents for mercury and arsenic removal. J. Clean. Prod. 2020, 267, 122143.
- Zhu, C.; Duan, Y.; Wu, C.-Y.; Zhou, Q.; She, M.; Yao, T.; Zhang, J. Mercury removal and synergistic capture of SO2/NO by ammonium halides modified rice husk char. Fuel 2016, 172, 160–169.
- Zafari, R. Synthesis and Study of Modified-Nanocrystalline Cellulose Effective for SO2 Capture; University of Ottawa: Ottawa, ON, Canada, 2021.
- Liu, Z.; Adewuyi, Y.G.; Shi, S.; Chen, H.; Li, Y.; Liu, D.; Liu, Y. Removal of gaseous Hg0 using novel seaweed biomass-based activated carbon. Chem. Eng. J. 2019, 366, 41–49.
- Yang, J.; Zhao, Y.; Zhang, J.; Zheng, C. Regenerable cobalt oxide loaded magnetosphere catalyst from fly ash for mercury removal in coal combustion flue gas. Env. Sci. Technol. 2014, 48, 14837–14843.
- Liu, Z.; Liu, D.; Zhao, B.; Feng, L.; Ni, M.; Jin, J. Mercury Removal Based on Adsorption and Oxidation by Fly Ash: A Review. Energy Fuels 2020, 34, 11840–11866.
- Wang, S.; Zhang, Y.; Gu, Y.; Wang, J.; Yu, X.; Wang, T.; Sun, Z.; Romero, C.E.; Pan, W.-p. Coupling of bromide and on-line mechanical modified fly ash for mercury removal at a 1000 MW coal-fired power plant. Fuel 2019, 247, 179–186.
- Graydon, J.W.; Zhang, X.; Kirk, D.W.; Jia, C.Q. Sorption and stability of mercury on activated carbon for emission control. J. Hazard. Mater. 2009, 168, 978–982.
- Yang, W.; Li, Y.; Shi, S.; Chen, H.; Shan, Y.; Liu, Y. Mercury removal from flue gas by magnetic iron-copper oxide modified porous char derived from biomass materials. Fuel 2019, 256, 115977.
- Shen, F.; Liu, J.; Dong, Y.; Wu, D. Mercury removal by biomass-derived porous carbon: Experimental and theoretical insights into the effect of H2S. Chem. Eng. J. 2018, 348, 409–415.
- Spessato, L.; Bedin, K.C.; Cazetta, A.L.; Souza, I.P.; Duarte, V.A.; Crespo, L.H.; Silva, M.C.; Pontes, R.M.; Almeida, V.C. KOH-super activated carbon from biomass waste: Insights into the paracetamol adsorption mechanism and thermal regeneration cycles. J. Hazard. Mater. 2019, 371, 499–505.
- Chen, W.; Gong, M.; Li, K.; Xia, M.; Chen, Z.; Xiao, H.; Fang, Y.; Chen, Y.; Yang, H.; Chen, H. Insight into KOH activation mechanism during biomass pyrolysis: Chemical reactions between O-containing groups and KOH. Appl. Energy 2020, 278, 115730.
- Wu, M.B.; Zha, Q.F.; Qiu, J.S.; Han, X.; Guo, Y.S.; Li, Z.F.; Yuan, A.J.; Sun, X. Preparation of porous carbons from petroleum coke by different activation methods. Fuel 2005, 84, 1992–1997.
- Zhu, M.; Yan, Q.; Duan, Y.; Li, J.; Zhang, X.; Han, Z.; Meng, J.; Wang, S.; Chen, C.; Wei, H. Study on Preparation and Mercury Adsorption Characteristics of Columnar Sulfur-Impregnated Activated Petroleum Coke. Energy Fuels 2020, 34, 10740–10751.
- She, M.; Jia, C.Q.; Duan, Y.; Zhu, C. Influence of Different Sulfur Forms on Gas-Phase Mercury Removal by SO2-Impregnated Porous Carbons. Energy Fuels 2020, 34, 2064–2073.
- Shen, C.; Wang, H.; Shen, H.; Wu, J.; Zhu, Y.; Shi, W.; Zhang, X.; Ying, Z. NH4Br-Modified Biomass Char for Mercury Removal in a Simulated Oxy-fuel Atmosphere: Mechanism Analysis by X-ray Photoelectron Spectroscopy. Energy Fuels 2020, 34, 9872–9884.
- Ma, A.; Zhao, S.; Luo, H.; Sun, Z.; Xie, X.; Liao, Y.; Liang, X.; Li, H. Mercury removal from coal-fired flue gas of high-sulfur petroleum coke activated by pyrolysis and mechanochemical method. Chem. Eng. J. 2022, 429, 132154.
- Sun, Z.; Ma, A.; Zhao, S.; Luo, H.; Xie, X.; Liao, Y.; Liang, X. Research progress on petroleum coke for mercury removal from coal-fired flue gas. Fuel 2022, 309, 122084.
- Lee, S.H.; Rhim, Y.J.; Cho, S.P.; Baek, J.I. Carbon-based novel sorbent for removing gas-phase mercury. Fuel 2006, 85, 219–226.
- Huo, Q.; Wang, Y.; Chen, H.; Han, L.; Wang, J.; Bao, W.; Chang, L.; Xie, K. ZnS/AC sorbent derived from the high sulfur petroleum coke for mercury removal. Fuel Process. Technol. 2019, 191, 36–43.
- Xiao, Y.; Pudasainee, D.; Gupta, R.; Xu, Z.; Diao, Y. Bromination of petroleum coke for elemental mercury capture. J. Hazard. Mater. 2017, 336, 232–239.
- Fuente-Cuesta, A.; Diaz-Somoano, M.; Lopez-Anton, M.; Cieplik, M.; Fierro, J.; Martínez-Tarazona, M. Biomass gasification chars for mercury capture from a simulated flue gas of coal combustion. J. Environ. Manag. 2012, 98, 23–28.
- Li, C.; Liu, X.; Zhou, Z.; Dai, Z.; Yang, J.; Wang, F. Effect of heat treatment on structure and gasification reactivity of petroleum coke. Int. J. Coal Sci. Technol. 2016, 3, 53–61.
- Zhao, S.; Luo, H.; Ma, A.; Xie, W.; Sun, K.; Sun, Z. Influence of pyrolysis conditions on the mercury removal characteristics and physicochemical properties of biomass coke. Fuel 2022, 313, 122979.
- Hong, D.; Zhou, J.; Hu, C.; Zhou, Q.; Mao, J.; Qin, Q. Mercury removal mechanism of AC prepared by one-step activation with ZnCl2. Fuel 2019, 235, 326–335.
- Zhao, J.; Dai, Y.; Xu, J.; Chen, S.; Xie, J. Synthesis and electrochemical characterization of mesoporous carbons prepared by chemical activation. J. Electrochem. Soc. 2008, 155, A475.
- Adinata, D.; Daud, W.M.A.W.; Aroua, M.K. Preparation and characterization of activated carbon from palm shell by chemical activation with K2CO3. Bioresour. Technol. 2007, 98, 145–149.