1. Antibiotic Resistance: The Role of MDR Pumps
Antimicrobial resistance is a major global problem that could lead to 10 million annual deaths by 2050
[1][49]. Therefore, much attention is now paid to understanding the mechanisms of antibiotic resistance and overcoming them. The mechanisms of protection of bacteria from antibiotics are complex and diverse, and can be caused by both genetic factors and the physiological state of the bacteria themselves. These mechanisms may be natural for a particular microorganism or acquired from other microorganisms.
The main mechanisms of resistance are considered to be: (1) drug uptake restriction, (2) drug target modification, (3) drug inactivation and (4) active drug release, (5) target switching, and (6) target sequestration
[2][3][22,50]. At the same time, the role of some resistance mechanisms is often overestimated at the expense of others (See
Figure 1).
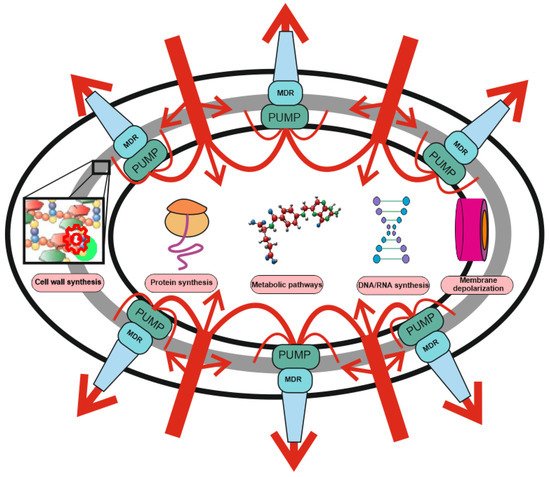
Figure 1. Two-level protection of bacteria from antibiotics: the role of MDR pumps in antibiotic resistance. Protection against antibiotics includes non-specific protection due to the operation of combination of MDR pumps with a wide range of substrate specificity (level 1), and specific protection (level 2), including drug or target modification, drug inactivation, target switching and target sequestration. Antibiotic uptake (red arrows) is significantly weakened or stopped under the action of non-specific protection by means MDR pumps’ composition and only a small part of the drugs reaches the targets (protein synthesis, DNA/RNA synthesis, membrane depolarization, metabolic pathways, and cell wall synthesis). The efflux pump contribution may not be visible, but it can increase the MIC by several orders of magnitude. MDR pumps are involved in resistance to antibiotics with any mechanism of action and protect bacteria even against those antibiotics for which they do not have specific resistance mechanisms.
An illustration of this is the transformation of SkQ1 (10-(6′-Plastoquinonyl)decyltriphenylphosphonium) from the mitochondria-targeted antioxidant and “non-antibiotic”
[4][51] into the most effective antibiotics with one of a deep-studied mechanism of action. SkQ1 is an ideal tool for studying MDR pumps, since it acts on bacterial bioenergetics and, being in the membrane, reduces the membrane potential of bacteria. The only protection against such a substance is the operation of the MDR pumps.
It was believed that the sensitivity of bacteria to SkQ1 is determined by the complexity of the cell wall, and Gram-positive bacteria with a more simply arranged cell wall are sensitive to it, while Gram-negative bacteria with a complex cell wall are resistant. This was explained by the difficulty of SkQ1 penetration through the double membrane of gram-negative bacteria, thus, resistance was reduced only to the ability to hinder the penetration of the substance into the bacterial cell
[5][13]. However, further studies showed
[6][14] that deletion of any of the AcrAB-TolC pump proteins (AcrA, AcrB, or TolC), with the exception of the small protein AcrZ
[7][15], led to a complete loss of resistance to SkQ1 in deletion mutants, which completely changed the concept of resistance. Obviously, in this case, the resistance turned out to be independent of the complexity of the cell wall and the very ability of the substance to penetrate through cell membranes, as previously thought, and was dependent solely on the presence of the AcrAB-TolC pump. It seemed that the resistance of
Escherichia coli bacteria and the sensitivity of other gram-negative bacteria are associated precisely with the presence of this pump however, everything turned out to be even more complicated. All SkQ1-sensitive gram-negative bacteria have an AcrAB-TolC pump in their cell envelope, which seemed to contradict the hypothesis. Only the analysis of the AcrB protein sequences of the AcrAB-TolC pump made it possible to explain everything. The protein sequences of resistant bacteria, for example,
Klebsiella pneumoniae, were close to that of
E. coli, while they differed by 35–60% in sensitive bacteria, which made it possible to formulate a functional criterion for evaluating MDR homologous pumps
[8][52]. Obviously, in the process of research, there was a reassessment of the mechanisms of resistance to SkQ1 from the mechanism of “limitation of drug absorption” to the mechanism of “active release of the drug”, which indicates a previous underestimation of the mechanism of antibiotic efflux which apparently required a reevaluation.
The significant contribution of the “drug absorption limitation” mechanism is apparently determined in large part by insufficient investigation of a large number of antibiotics and the pleiotropy of the action of pumps, when many pumps can pump out one antibiotic and the removal of even a few pumps has little effect on the overall effect of antibiotic outflow and conventional microbiological methods are not capable of detecting them adequately. Looking at the antibiotic efflux sensitivity profile of
E. coli [9][53], one can see that the “active drug release” mechanism leads to a 50–100-fold increase in the minimum inhibitory concentration for antibiotics, and
thwe
researchers observe the same order of magnitude in the case of SkQ1
[6][14]. Thus, MDR pumps appear to play a key but underestimated role in antibiotic resistance.
To understand how great this influence is, it is necessary to pay attention to the main mechanisms of action of antibiotics.
Table 1 presents the main types of antibiotics and the effect of MDR pumps on bacterial resistance to them. Not surprisingly, antibiotics that affect the biosynthesis of proteins, DNA, RNA or disrupt metabolic processes, such as folate synthesis, for which penetration is a necessary condition for antibacterial action, demonstrate a dependence of resistance on pump operation
[9][10][11][12][13][14][15][16][17][18][19][20][53,54,55,56,57,58,59,60,61,62,63,64]. However, pumps having any effect on antibiotics that do not penetrate into the cell cytoplasm, but are localized on the membrane, or even beyond it
[21][22][23][24][25][65,66,67,68,69], would cause sincere surprise if it were not for the fact that some pumps expel their substrates from the periplasmatic space
[26][70]. Thus, MDR pumps are a universal tool that protects the bacterial cell itself and its microenvironment from the negative effects of xenobiotics.
Table 1. The role of MDR pumps in antibiotic resistance. Antibiotics groups by mechanism of action.
2. Alternative Resistance Mechanisms Using MDR Pumps
Bacteria use their MDR pump system to effectively defend against various antibiotics and toxins. Moreover, they use various approaches to improve its effectiveness. Resistant phenotypes can arise as a result of increased pump activity due to their overexpression, as is observed with the addition of sublethal concentrations of antibiotics, which, through a cascade of feedback-driven interactions, causes an increase in the expression of MDR pump genes that evacuate these antibiotics
[27][71].
ThWe
researchers observe a similar approach in the case of unicellular eukaryotes
[28][72], which indicates a universal defense mechanism.
In addition, pumps may contribute to different ways of expelling antibiotics, but cooperate to work together, efflux antibiotics and provide an even higher level of protection than necessary
[29][30][31][73,74,75]. This fact indicates that a large concentration of antibiotics is required to overcome the resistance from MDR pumps, and even without special protection mechanisms, bacteria are well protected from negative effects only due to the action of MDR pumps.
Another interesting aspect is the asymmetric accumulation of MDR pumps during cell division, when the pumps are mainly located at the old poles, and new poles are newly created and MDR pumps are synthesized de novo
[32][76]. This creates a variable resistance profile during the cell cycle, which makes it possible for a population to contain bacteria with different expression status of MDR pumps. When encountering an antibiotic, the least resistant ones die and are used in two mechanisms: (1) adsorption of antimicrobials on the surface of dead cells, thereby protecting the remaining bacteria
[33][34][77,78]; and (2) secretion of a “necrosignal” that causes the activation of protective pathways in surviving bacteria
[35][79]. As a result, the bacterial population acquires increased resistance to antibiotics.
Undoubtedly, an interesting mechanism is the increased rate of gene mutation in bacteria under the action of antibiotics
[36][37][80,81], which can lead to a protective effect, including mutations in MDR pumps and heterogeneity of their expression
[38][82]. Given that gene copy number change is a common event in all genomes
[39][83], doubling the MDR pump genes results in an increased chance of survival when antibiotics are added. Thus, gene duplication leads to an increase in resistance, and may be an alternative to changing the level of pump expression.
3. MDR Pumps in Bacteria
Despite the wide variety of xenobiotics, MDR bacterial pump systems belong to six large groups: (1) the ATP-binding cassette (ABC) family; (2) the proteobacterial antimicrobial compound efflux (PACE) family; (3) the major facilitator superfamily (MFS); (4) the multidrug and toxin extrusion (MATE) family; (5) the small multidrug resistance (SMR) family; (6) the resistance-nodulation-cell division (RND) family
[40][84]. Most of the families have an early origin and have been preserved in the course of evolution, as evidenced, for example, by the ubiquitous distribution of the MFS and RND families both among prokaryotes and eukaryotes
[41][42][85,86]. Currently, the recently discovered proteobacterial antimicrobial compound efflux (PACE) family remains the least studied family
[43][87]. Among other well-studied transporter families, only the ATP-binding cassette (ABC) family uses energy in the form of ATP to do the job of substrate efflux. For all other families, the energy of the proton (or H+/Na+) gradient is used to perform work (See
Figure 2).
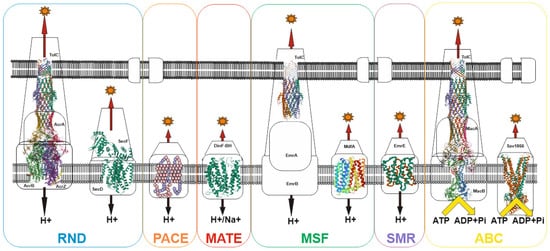
Figure 2. Schematic of representative major superfamilies of bacterial multidrug transporters and tripartite assemblies. Structures of representatives of each of the transporter families (except PACE) are presented. Protein Databank (PDB) identifiers from left to right: AcrABZ-TolC (5O66), SecDF (3AQP), DinF-BH (4LZ6), TolC (2XMN), MdfA (4ZOW), EmrE (3B62), MacAB-TolC (5NIL), and Sav1866 (2HYD). The structures of the proteobacterial antimicrobial compound efflux (PACE) class of transporters have not yet been experimentally resolved and are therefore represented here as a basic outline.
This apparently indicates that two main states can exist for a bacterial cell: (1) absence of potential on the membrane but presence of ATP, when only the transporters of the ATP-binding cassette (ABC) family work; (2) the presence of a potential on the membrane and, accordingly, ATP, in which case all transporters work. Since the membrane potential is associated with ATP generation, the absence of ATP in the presence of the potential can be excluded. Thus, voltage-dependent pumps predominate in prokaryotes, which is explained by the high potential on the membrane (~140–220 mV for
E. coli [44][88]) and the absence of the need to convert the potential into ATP. This provides an advantage in the pumping rate. In eukaryotes, the situation is slightly different: the main pumps are ATP-dependent, since ATP generation and a high membrane potential (~180 mV
[45][89]) occur in mitochondria, and thus the energy of the membrane potential cannot be directly used to perform work. Hence, the combination of voltage-dependent and ATP-dependent pumps determine the resistance profile of bacteria.
Gram-negative bacteria differ from gram-positive bacteria by the presence of an outer membrane
[46][90], which serves as an additional barrier preventing the penetration of substances into the bacterial cell. The main features of the permeability of gram-negative bacteria are well described in
[47][91], therefore, in summary,
we would like to note that there are three main ways of penetration of substances through the outer membrane: (1) penetration due to porins (see
Figure 3), for example, OmpF, OmpC; (2) transfer by specific channels (e.g., FadL); and (3) transfer of lipophilic compounds by flip-flop across the lipid component of the membrane.
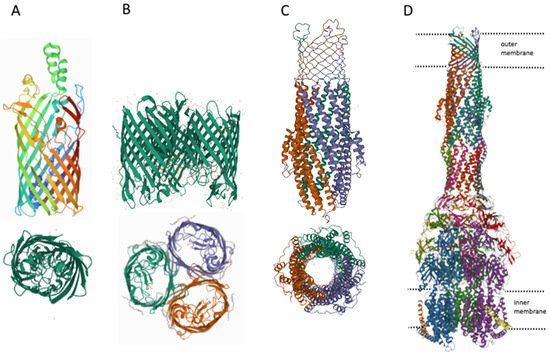
Figure 3. Structures of outer membrane proteins FadL (A), OmpF (B), TolC (C), and transporter AcrABZ-TolC (D). PDB identifiers for FadL (2R88), and OmpF (6ZHP).
However, it can be imagined that there is at least one other transport channel, the outer membrane protein TolC. The TolC protein is a component of eight MDR pumps: the most well-studied main pump, AcrAB-TolC, and other pumps, AcrAD-TolC, AcrEF-TolC, MdtABC-TolC, MdtEF-TolC, EmrAB-TolC, EmrKY-TolC, and MacAB-TolC. These pumps belong to three classes of transporters: RND, MFS and ABC, which again indicates the need for the simultaneous presence of both ATP-dependent and voltage-dependent pumps. A TolC-containing transporter, such as AcrAB-TolC, consists of the outer membrane channel TolC (common to all pumps), the multidrug efflux pump membrane fusion lipoprotein AcrA (common to AcrAB-TolC and AcrAD-TolC pumps), and the inner membrane multidrug efflux pump RND permease AcrB. The AcrA adapter protein binds the AcrB and TolC proteins to each other and prevents substrates from entering the intermembrane space. Since the TolC protein is the same for all pumps, the possibility of temporary connection through adapter proteins of TolC alternately with one or the other pump, depending on cellular needs, is not ruled out. It is during these possible pump changes that the TolC channel opens into the periplasmic space. Thus, hypothetically it could be possible to assume the existence of four ways of penetration of substances into the periplasmic space.
However, even non-specific protection can be disabled or its impact reduced. There are both specific and natural compounds that reduce the efficiency of pumps
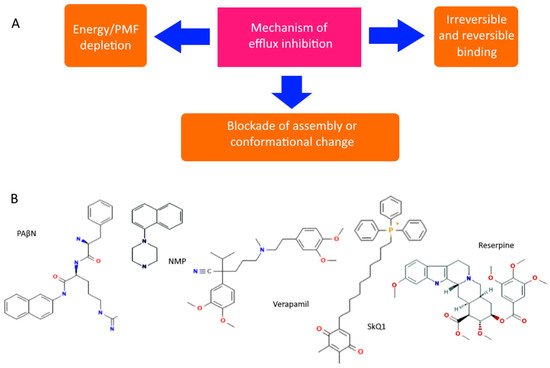
[48][49][92,93]. Although there are quite a few hypothetical mechanisms for reducing pump performance, the most realistic seem to be: (1) competitive or irreversible binding to the active site, preventing the formation of active transporters on the membrane, or blocking conformational changes (open/closed state), and (2) a decrease in membrane potential and ATP level. All known pump inhibitors interfere with MDR pumps by one of these mechanisms, some of which are presented in
Figure 4. PAβN (Phenylalanine-Arginine-β-napthylamide) is a competitive inhibitor and affects membrane permeability
[50][51][94,95]. NMP (1-(1-napthylmethyl)-piperazine) and SkQ1 (10-(plastoquinonyl)decyltriphenylphosphonium) are inhibitors of AcrAB-TolC efflux pumps. The mechanism of action of NMP remains unknown, while the mechanism of action of SkQ1 is well known. SkQ1 is a competitive inhibitor, and also reduces the membrane potential, thus affecting the bioenergetics of bacteria
[6][7][14,15]. Verapamil is a well-known channel blocker that directly binds with the efflux pumps and inhibits the efflux of antibiotics
[52][96]. If the substances discussed above are synthetic, then reserpine refers to indole alkaloids of plant origin. The main targets of reserpine are the MFS and RND pumps, with which it directly binds and inhibits their work
[49][93].
Figure 4. Diagram showing the main mechanisms of overcoming non-specific protection due to inhibition of MDR pumps (A) and some MDR pump inhibitors (B).
It should be noted that although under the action of antibiotics there may be upregulation of pump expression, increasing the number of individual pumps responsible for their efflux, but it is unlikely that bacteria have mechanisms to reduce nonspecific protection. Therefore, the mechanism of suppression of the genes encoding the pumps is rather hypothetical, and the decrease in the expression level can be explained either by a general decrease in bacterial bioenergetics and biosynthesis, or by nonspecific binding to mRNA.