Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Theodora Amanda Seidu.
Research into the application of nanocarriers in the delivery of cancer-fighting drugs has been a promising research area for decades. On the other hand, their cytotoxic effects on cells, low uptake efficiency, and therapeutic resistance have limited their therapeutic use. However, the urgency of pressing healthcare needs has resulted in the functionalization of nanoparticles’ (NPs) physicochemical properties to improve clinical outcomes of new, old, and repurposed drugs.
- surface functionalization
- multifunctional nanoparticles (MNPs)
1. Introduction
Cancer could be a comprehensive term that includes a broad run of ailments that can adversely impact any part of the body. Breast, colon, lung, rectum, prostate, skin (nonmelanoma), and stomach cancers are the most common new cases around the world, accounting for nearly 10 million passings in 2020 [1]. It is expected that within the following two decades, new worldwide cancer cases will increase to 22 million [2]. One of the primary determinants is the inability of antitumor drugs to be delivered selectively to cancerous tissue. High systemic antineoplastic agent exposure often results in dose-limiting toxicity. As a result, a targeted delivery system is critical for overcoming current limitations in cancer therapy. The intricate biology of tumor cells with several biological barriers, such as mononuclear scavenger cell uptake; extravasation via the vascular endothelial membrane; and physiological features such as hypoxemia, low pH, and elevated pressure of interstitial fluid, emphasizes the necessity to invent and formulate an effective antitumor drug delivery system [3].
Therefore, an idealized drug delivery system (DDS) must possess two components, spatial placement (the potential to target) and temporal delivery (controllability of the release) of the drug [4]. The ability of a DDS to target and have a controlled release of cargo will enable an increase in the drug’s effectiveness and minimize adverse effects [5,6][5][6]. The most widely used drug delivery platform is nanoparticles (NPs), classified into two types—organic and inorganic NPs—with some popular ones being lipid-based NPs, polymeric NPs, protein NPs, and inorganic NPs. Nanoparticulate drug delivery systems (NPDDSs) demonstrate great potential as DDSs. Due to their nano size, increased surface-to-volume ratio, and advantageous physicochemical properties, they can modulate the pharmacodynamics and pharmacokinetic profiles of drugs by improving their therapeutic index [7]. They also express different but preferable chemical, physical, and biological effects [8]. NPDDSs are continually investigated to solve the deviance in conventional drug delivery systems. Many drugs possess a hydrophobic element; therefore, they have precipitation issues in higher concentrations and, although excipients are designed to prevent drug precipitation, they come with toxicity as a setback [9]. Due to the nature of NPDDS, they provide an amphipathic environment for drugs, thereby enhancing drug solubility, solving these issues of precipitation, and increasing their bioavailability [10]. Liposomes can simultaneously convey both hydrophilic and hydrophobic drugs due to the hydrophilic internal structure of liposomes, which significantly advances drug loading effectiveness [11]. Additionally, liposomes’ cell membrane architecture facilitates effective cell affinity and greatly boosts cellular uptake.
In recent years, inorganic NPs have also been extensively used for theragnostic cancer treatment due to their outstanding chemical and physical features. A typical inorganic nanoparticle is mesoporous silica NP, which has high drug loading instigated by surface pores filled with cargo. However, the body’s inability to metabolize the inorganic materials can lead to severe tissue injury. Functionalization by adding organic moieties to curb this setback (See in Figure 1) is, therefore, necessary [12]. Recently, the focal point in drug delivery technology has been the designs/functionalization and applications of NPDDS; for example, polymer and surface conjugations are being explored [13,14][13][14].
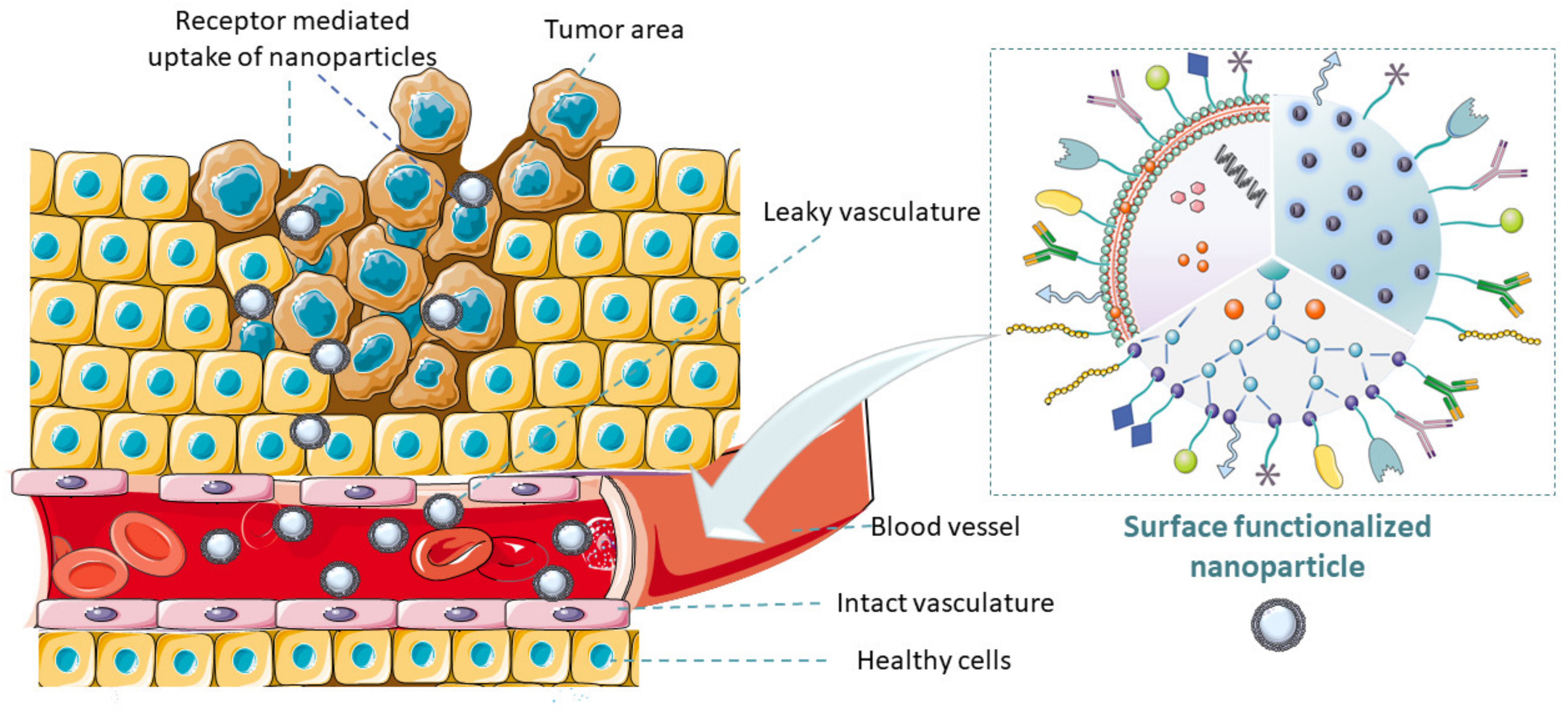
Figure 1. Graphical representation of the abstract. Illustration of tumor microenvironment and how surface-functionalized nanocarriers containing antitumor drugs actively target tumor cells.
NPDDS are modified using various techniques to serve as diagnostics or therapeutic vehicles for many diseases [15]. Functionalization of NPs includes the modification of NPs with various targeting ligands, diagnostic agents, imaging agents, biomolecules, oligonucleotides, peptides, antibodies, stimuli-sensitive ligands, etc. to improve their properties or introduce new features to enable targeting with accuracy [16]. Most functionalized nanoparticles possess improved physicochemical properties, enhanced targeting ability, bioavailability, biocompatibility, anticorrosiveness, antiagglomerative, and noninvasive characteristics. An increasing number of researches are being undertaken to functionalize nanoparticles to enhance their gross efficiency and modality [14,15,17,18][14][15][17][18].
2. Nanoparticles and Their Classifications
The two main classifications of nanoparticles are organic and inorganic nanoparticles. Liposomes, dendrimers, and polymeric nanoparticles are examples of organic nanoparticles. Inorganic nanoparticles include mesoporous silica, gold NPs, carbon nanotubes, etc. [19,20][19][20]. Figure 2 presents the differences and applications of the two main classes of nanoparticles. The methods used to obtain nanoparticles significantly impact their morphology; size; structure; electrochemical, physicochemical, optical, and electrical properties; and general performance in cancer therapy [19].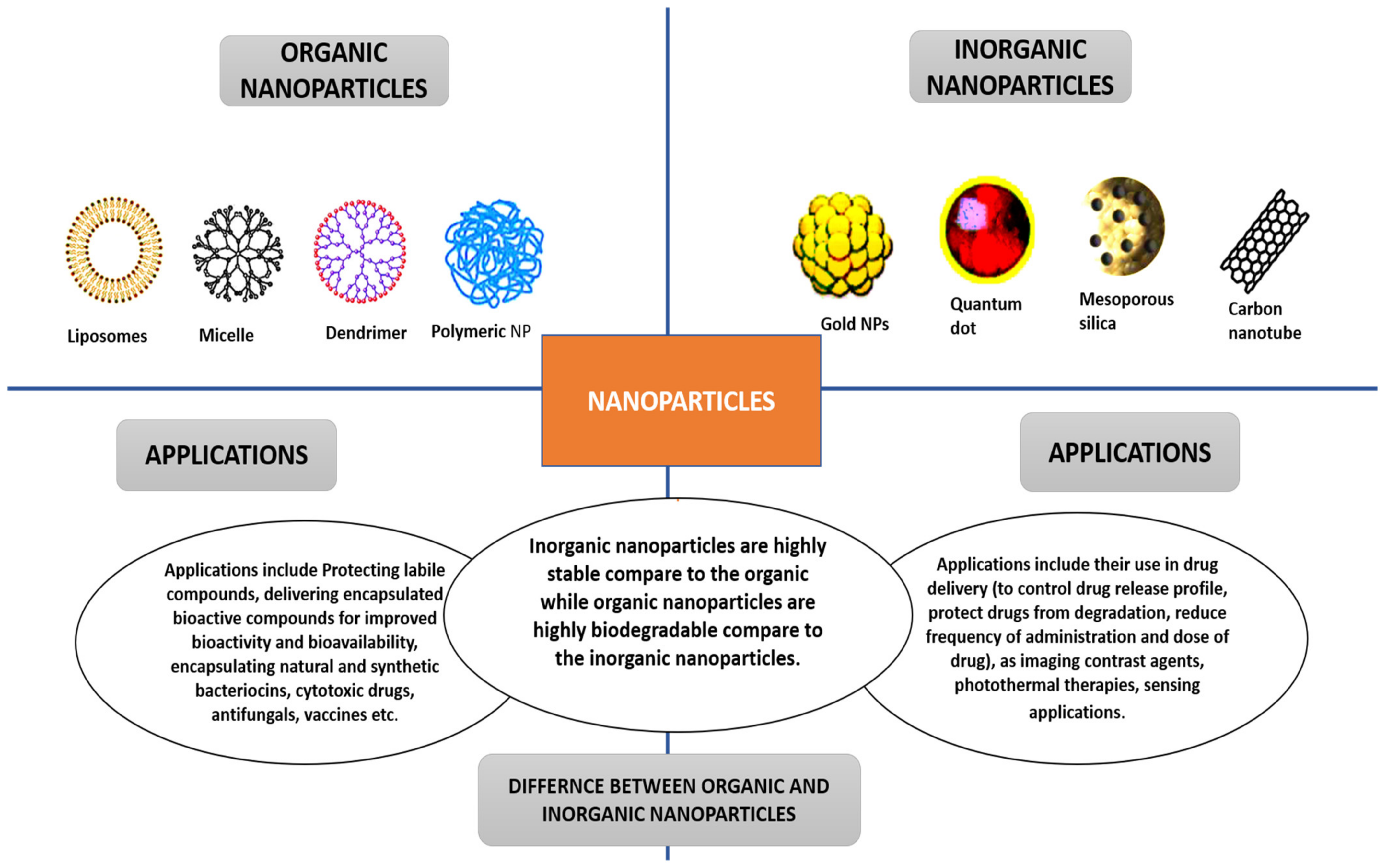
Figure 2. Nanoparticles’ classifications, applications, and differences.
3. Liposomal Nanoparticulate DDS
Conventional liposomes usually have the drawback of being subsumed by the reticuloendothelial system (RES) and lead to short-term circulation [11]. This process (RES) occurs when opsonin (Serum protein) perceives them as foreign substances, and they are disrupted by immune cells. To overcome this setback (short circulation time), the liposome surface is plated with PEG to extend circulation by magnifying repelling forces between liposomes and serum elements; this forms the class of liposomes called PEGylated liposomes [26]. Liposomes can be grafted diversely with targeting ligands (ligand-targeted liposomes); examples include peptides and antibody fragments to implement diverse surface engineering strategies for targeting at requisite tumor sites using overexpressed receptors [27]. Immunoliposomes were also produced by chemically attaching antibodies or fragments of antibodies to the liposomal surface, leading to solid precision for their target antigens. In cancer treatment, the use of targeted liposomes formulated using a range of different engineering techniques has proven minimal off-target effects on healthy tissues [28,29,30][28][29][30]. Multifunctional liposomes are considered the advanced form of the single-functionality liposomes and have the potential to overcome the setbacks faced by the single-functionalized liposomes. A variety of functionalities are incorporated using various functionalization techniques and modifications. Liposomes having two ligands (dual-functional) [31], liposomes having two ligands with two anticancer drugs [32], liposomes having a targeting ligand with an imaging agent [33], theragnostic liposomes containing an imaging agent and therapeutic agent [34], etc. have been reported. Liposomes are used in cancer therapy [35] and other specialties such as mycosis (fungal infection) [36], gene transfer therapy [37], and vaccine nanocarriers [38,39][38][39].3.1. Functionalization of Liposomes Using Targeted Ligands
Liposomes are imbued with varied targeting ligands using one or a combination of surface engineering strategies [40]. Ligands are most often covalently linked to liposomes via interactions between reactive groups on the liposome’s surface and particular groups within the ligand. Targeting ligands could be covalently or noncovalently incorporated to customize a targeted liposomal system for cancer treatment. Liposomes are functionalized with diverse targeting ligands via three reactions: amide bond formation involving carboxyl and amino groups, disulfide bond creation by reacting pyridyldithiols and thiol group, and thioether bond formed by reacting maleimide (MAL) and thiol group [40,41][40][41]. Preformed liposomes with reactive groups on their surfaces, such as MAL, were ligated with PEG derivatives in the postcoating technique [42]. PEG–lipid micelles were used in one technique to incorporate PEG–lipid conjugates into the liposome membrane without disrupting the liposomes. The conjugation reaction between thiol on the surface of ligands and MAL on liposomes is a critical surface engineering approach for liposomes with a suitable targeting ligand [43]. Targeting ligands commonly used are antibodies or antibody fragments, cell-targeting peptides or cell-penetrating peptides, and small molecules such as folate, based on their use in tumor therapy and aptamers. Some are shown in Table 1.Table 1. Liposomes’ surface modifications with various moieties and their applications.
| Type of Moiety | Application of Functionalized Liposome | Ref. | |
|---|---|---|---|
| Vitamins | |||
| Biotin | To target EGFR, Quantum dots were coupled to an epidermal growth factor ligand. | [44] | |
| Vitamin A | Skin fibrosis is treated with a siRNA carrier. siRNA carrier to resolve liver cirrhosis |
[45,46] | [45][46] |
| Folic acid | Macrophage targeting with ovarian carcinoma. Oligodeoxynucleotide targeting cancer cells. |
[47,48] | [47][48] |
| Carbohydrates | |||
| Glucose | Drug delivery for capillary endothelial cells in the brain. | [49] | |
| Sucrose | Doxorubicin-loaded liposomes for cancer treatment | [50] | |
| Lectins | Pulmonary drug delivery | [51] | |
| Antibody fragments | |||
| scfv | Trastuzumab–Liposomes for advanced breast cancer | [52] | |
| Anti-CD 133 Mab | Bevacizumab-containing liposomes for glioblastoma | [53] | |
| Anti-transferrin scFv antibody fragment | Plasmid DNA-carrying liposome for prostate cancer cell lines | [54] | |
| Aptamer | |||
| IL-4R⍺ | Tumor growth inhibition through targeting the tumor microenvironment | [55] | |
| xPSM-A9 | To combat the expression of a membrane antigen (prostate specific) on prostate cancer cells. | [56] | |
| Anti-CD44 | Selectively targeting cancer cells | [57] |
3.2. Functionalization of Liposomes Using Antibody
Surface alteration of liposomes with antibodies can be achieved by linking antibodies or their fragments to the surfaces of liposomes to produce immunoliposomes using several methods [58]. One method uses a covalent link between an antibody or fragments of antibody with liposomal lipids. Another method is to chemically modify the liposome to enhance its hydrophobicity by adding an appropriate substituent, typically resulting in a greater affinity for the liposome’s bilayers [59]. A recent strategy is to use the antibody fragment (e.g., fragment antibody-binding (Fab) or single-chain fragment variable (scFv)) in place of the entire antibody to avoid the risk of inactivation of the antibody or initiation of the immune response during surface functionalization and to reduce particle sizes to enhance efficient delivery of anticancer agents. There are several reviews on the techniques for antibody derivatization and the development of reactive groups to be coupled with lipids or preformed stealth liposome [60]. Numerous reagents are also employed in thiolating antibodies (e.g., 2-Iminothiolane (Traut’s reagent) to produce sulfhydryl group). The antibody moieties contain groups modified for active targeting, such as thiol, carboxyl, and amine groups. Usage of a sulfhydryl group in connecting a thiolated antibody with a lipid-containing reactive group, i.e., MAL, is the primary strategy and has been widely studied in the literature. The sulfhydryl group is susceptible to oxidation and can be prevented by substituting oxygen with ethylenediaminetetraacetic acid [60]. Liposome surface functionalization with fragments of antibody has been accomplished using the same methods. Carbonic anhydrase, CA, are zinc metalloenzymes found on the surface of red blood cells [41]. Many tumors exhibit hypoxia. In cancer microenvironments, hypoxia is associated with reduced extracellular pH (around pH 6.5). Due to the lack of oxygen, carbonic anhydrases (Cas IX and Cas XII) are overexpressed in several solid tumors, predominantly lung and brain tumors. CA IX is more active than CA XII [61]. In a study, CA IX targeted immunoliposomes encapsulated with docetaxel was fabricated. The in vitro binding and cell uptake of liposomes to A549 cells (CA-IX positive and CA-IX negative) was explored by means of fluorescence-based flow cytometry. Results showed increased uptake in CA-IX positive A549 cells [62].3.3. Functionalization of Liposomes with Peptides
Liposomes’ surfaces are also functionalized with peptides, primarily through covalent and noncovalent bonding [63]. Peptides are covalently linked to liposomes via various linkages, including the MAL, peptide, sulfanyl, disulfide, and phosphatidylethanolamine-linker. Presently, disulfide and thioester links have been extensively studied. Noncovalent linkage has been used to attach amphipathic peptides to liposomes. Peptides used for the surface modification of nanocarriers can be classified into two types: cell-penetrating peptides (CPP) and cell-targeting peptides (CTP), which are nonspecific and receptor-specific (directly bind and increase internalization) respectively [63]. Paclitaxel-encapsulated T7 targeted liposomes inhibited tumor growth in ovarian-cancer-bearing mice more effectively than nonfunctionalized liposomes and free drug [64]. Human glioma cell growth was inhibited by doxorubicin-containing cyclic RGD peptide-modified liposomes (U87MG cell-line). The cyclic RGD binds to integrins, which are overexpressed in various tumors. Integrins are also highly specific for cyclic RGD [65]. PEGylated liposomes with CPP and an acid-sensitive hydrazone bond was made. It was discovered that a 4 percent CPP-to-lipid ratio resulted in greater liposome internalization efficiency into targeted compartments [66]. Patra et al. developed liposomes comprising carbon dots and CPP (polyarginine) for the delivery of curcumin throughout the skin. The inclusion of carbon dots aided in the imaging of skin fluorescence [67].3.4. Functionalization of Liposomes with Aptamers
Aptamers are oligonucleotide-based single-stranded DNA or RNA sequences that can target receptor sites on the facet of tumor tissues. Systematic ligand development using exponential-enrichment technology resulted in the production of aptamers with greater attraction for the molecules targeted [68]. Liposomes with a targeting aptamer ligand attached to their surface and containing antitumor cisplatin have been reported. Nucleolins (NLC) were the aptamer’s preferred target. Aptamer-controlled liposomes were formed by incorporating the cholesterol-labeled aptamer with the other liposomal components prior to hydration. Parallel to free drug and nontargeted liposomes (loaded with drug), aptamer-targeted liposomes were found to have increased antiproliferative activity in breast cancer cells (MCF-7) overexpressing NCL. This research shows that breast cancer cells that overexpress NCL were deliberately targeted. An aptamer-based liposomal preparation was proposed to combat multidrug resistance (MDR) in breast cancer, and P-gp transporter is overexpressed in MDR metastatic breast cancer cells [69]. This overexpression can be reduced by using siRNA to silence it. Liposomes loaded with siRNA and loaded with aptamer A6 (which has attraction for HER2 receptors on breast cancer cells) were prepared to improve siRNA delivery into breast cancer cells. Aptamer A6 was brooded with prefabricated liposomes that contained a PEG–MAL, MAL group that could be conjugated with the aptamer. A6-directed liposomes were produced during the incubation period. According to the study’s findings, aptamer-directed liposomes could convey siRNA (targeted at P-gp) into cancerous breast cells to counter chemoresistance [70].3.5. Liposome Functionalization with Small Molecules
In cancer therapeutics, small molecules such as folate, affibody, carbohydrate, and others have been used as targeting moieties for surface functionalization of liposomes. Aimed at cervical cancer therapy, folate-targeted liposomes encapsulating imatinib have been formulated. The film hydration approach was used to create liposomes, including a folate–lipid conjugate during the lipid film forming step. Transmembrane pH gradient was used to load imatinib into liposomes. Folate-targeted liposomes decreased the IC50 value of cervical tumors (HeLa-cells) sixfold, from 910 M to 150 M [71]. It has been reported that HER2-targeting affibody (Z00477)2-Cys coupled liposomes are used to treat breast cancer, i.e., TUBO cloned cells and SK-BR-3 cells. Thioether linkage was used to reduce affibody and conjugate it with DSPE-PEG-MAL micelles. Cisplatin was solvated to an aqueous phase during the formulation before being loaded into liposomes. The ethanol injection technique was used to create liposomes. Surface modification of cisplatin-containing liposomes with affibody-conjugated micelles was achieved after a 4-h incubation at 47 °C. Affisomes demonstrated increased cellular uptake and therapeutic effectiveness [72].3.6. Dual-Ligand Functionalization of Liposomes
Surface-functionalized liposomes with two ligands has also been reported with promising outcomes. Kang et al. used chimeric-ligand aimed multifunctional liposomes, such as folate linked-peptide-1 and two ligand-directed liposomes, such as folate and peptide-1-targeted liposomes, using the film hydration technique. The foremost step is to create PEGylated liposomes with a terminal MAL group. The liposomes were then cultured with chimeric-ligands such as folate and peptide-1 ligand to produce chimeric-ligand driven liposomes and a double ligand guided liposome, respectively. The FITC-dextran-loaded multifunctional liposomes were tested on uterine-cervical cell lines (HeLa) and human-keratinocyte cell lines (HaCaT). Double ligand driven liposomes outperformed chimeric-ligand directed liposomes in cell uptake and cytotoxicity in the HeLa cell lines [73]. Zhang et al. also confirmed the development of targeted liposomes having two peptides (TfR and VEGFR2 specific peptides) and two antitumor agents (doxorubicin and vincristine), which enhanced drug delivery and optimized therapeutic efficacy in the brain (see results in Figure 3) [32]. Zong et al. also developed doxorubicin liposomes with two peptides (TAT and T7), which demonstrated improved therapeutic efficiency in glioma therapy in animals contrasted to one ligand dox liposomes and just dox [74].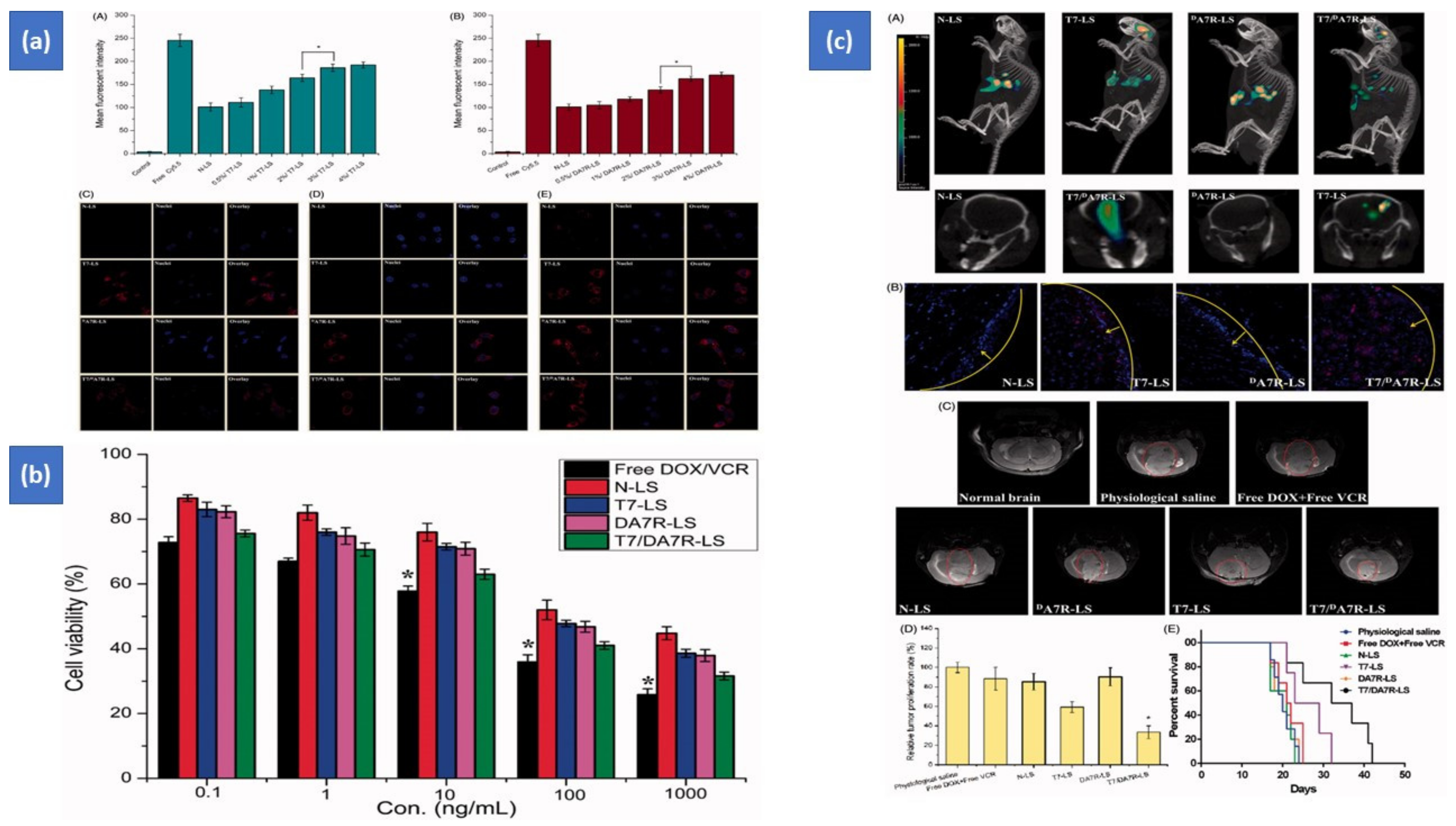
Figure 3. Targeted liposomes having two peptides (TfR- and VEGFR2-specified peptides) and two antitumor agents (doxorubicin and vincristine). (a) Cellular uptake (After 2 h at 37 °C, cellular uptake of Cy5.5-loaded liposomes of varying densities of T7 (A) and DA7R (B) in C6 cells. The cells’ auto-fluorescence was used as the control. Cellular uptake of varied Cy5.5 loaded lyposomes by bEND.3 cells (C), HUVECs (D), and C6 (E) cells); (b) cytotoxicity study (The cytotoxic activity of free DOX + free VCR, as well as some liposomes containing DOX and VCR); (c) biodistribution study (The biodistribution of Cy5.5 in varied liposomes in mice with intracranial C6 glioma was ascertained using an IVIS® Spectrum-CT (A). A CLSM was used to show the allocation of Cy5.5 in the brains of mice with intracranial C6 glioma (B). 16 days after inoculation, MRI of physiologic and pathological brains (C). Glioma tumor cell division rate in the brain (D). Survival curves according to Kaplan–Meier (E). The yellow line represents the intracranial glioma margin, and the arrow represents the glioma cells. The red is Cy5.5, and the nuclei are stained with DAPI (blue). Effectiveness after treatment with different formulations at 1 mg/kg (DOX 0.8 mg/kg + VCR 0.2 mg/kg) on days 8, 10, 12, and 14 after inoculation.) This designed system could go through the blood–brain barrier and blood–tumor barrier, with enhanced cellular uptake and cytotoxicity [32]. Copyright 2017, Taylor & Francis Journals. * p < 0.05.
3.7. Stimuli-Sensitive Liposomes
Stimuli cause liposomes to become unstable, resulting in the release of trapped payload. The primary stimuli used to enhance the delivery of anticancer drugs to the required site via liposomes include temperature, pH, magnetic field, ultrasound, light, redox, and enzymes [75].3.7.1. Temperature-Responsive Liposomes
Liposomes are constituted of thermosensitive lipids that are stable at 37 °C. Thermoresponsive liposomes were utilized to target cancer cells with an elevated temperature compared with the rest of the body. When the temperature was raised from 37 to 41 °C, the liposomes made of cholesterol and dipalmitoylphosphatidylcholine released approximately 80 percent of the encased methotrexate within 30 min. Temperature-sensitive liposomes are utilized in the commercial antitumor liposomal formula Thermodox® (Celsion, Lawrenceville, NJ, USA). It constitutes 1-myristoyl-2-stearoyl-sn-glycerol-3-phosphocholine, which has a 40 °C transition temperature [76,77][76][77].3.7.2. pH-Sensitive Liposomes
pH-sensitive liposomes are stable at pH 7.5; however, changes in pH, such as those found in cancer tissues—that is, low pH—cause the encapsulated cargo to be released due to bilayer instability [78,79][78][79]. At the tumor site, the pH can drop to 5.7 [80]. 18:1 or DODAP DAP is a pH-sensitive lipid that is frequently employed to create pH-responsive liposomes [41]. In one study, antigenic peptides originating from ovalbumin and pH-responsive fusogenic polymer were loaded into liposomes to develop peptide vaccine-based cancer therapy, which resulted in decreased tumor volume [81].3.7.3. Magnetic-Field-Sensitive Liposomes
Magnetic-field-sensitive liposomes have iron oxide cores (magnetite and Fe3O4) that magnetize when exposed to an extrinsic magnetic field [79]. Magneto-liposomes containing 5-fluorouracil were created. The film hydration technique was exploited to produce liposomes. Evaporation under vacuum was utilized to create a lipid film of PC solution in chloroform, which was then hydrated with Fe3O4 suspension in water. Due to the hyperthermia effect, a magnetic field triggered the drug to be released in human colon cancer cells and tumor growth suppression was noticed [82]. In a study, liposomes comprising iron-oxide and methotrexate accumulated extra into target tissue in a model mouse when an extrinsic magnetic field was applied versus the same liposomes when no extrinsic magnetic field was applied [83].3.7.4. Ultrasound-Sensitive Liposomes
When tiny gas bubbles in liposomes are exposed to ultrasound waves, they generate echo sound, which allows for ultrasound imaging. Ultrasound waves can also break up liposome systems, allowing the drug to be released at the desired location [84]. Breast tumor progression was significantly inhibited in MDA-MB-231 tumor-bearing mice when doxorubicin liposomes with a CO2 bubble creating thermosensitive system were used instead of just thermosensitive doxorubicin liposomes without gas. Thermoresponsive liposomes proficient in damaging lipid bilayers are formed by rehydrating dried lipid films with citrate buffer and generating CO2 bubbles (300 mM, pH 4). Due to a synergy between burst drug release and hyperthermia-induced CO2 production, doxorubicin’s antitumor activity was increased. An ultrasound imaging technique was used to track the CO2 production caused by hyperthermia. In this report, the drug is discharged from liposomes as a side effect of hyperthermia, which induces CO2 production in the liposomes [85].3.7.5. Multiple Stimuli-Sensitive Liposomes
A recent phenomenon is the evolution of liposomal formulas that respond to multiple stimuli simultaneously. Many researchers are interested in customized nanocarriers cotriggered by multiple stimuli in various organisms (e.g., extracorporeal, tumor tissue, cell, subcellular organelles) as they can overcome sequential physiologic and pathologic change barriers to deliver diverse therapeutic “payloads” to the desired targets. Furthermore, DDSs that are sensitive to several stimuli provide an excellent platform for agent codelivery and reversing multidrug resistance [86]. Liposomes comprising a pH-responsive monomer (2-propyl acrylic acid) and a temperature-sensitive monomer (N-Isopropylacryamide (NIPAAm)) were developed utilizing p(NIPAAm-co-PAA) copolymer. Variations in pH and temperature impacted the release of doxorubicin. The film hydration approach was used to make liposomes, and doxorubicin was encapsulated using a pH gradient. MR-directed ultrasound was utilized on heat-defined tissues and activated local release of drug. The administration of these liposomes resulted in increased cytotoxicity in breast tumors. The goal of developing duple-sensitive liposomes was to reduce the side effects on healthy tissues [87]. A double stimuli-sensitive liposomal structure was established to tackle the issue of CPP deterioration in CPP-siRNA conjugates. A suitable strategy and a current surge for increasing the potential of cancer treatment is the production of liposomes that react to stimuli and have surfaces designed to aim at receptors overexpressed on the surface of tumor cells and in the tumor microenvironment. Antibody-targeted thermoresponsive liposomes were developed, according to a previous article. As per the research, after surface modification with hCTMO1-antibody, the thermal and physicochemical characteristics of conventional thermoresponsive liposomes (TTSL) were retained. TTSL was made using the film hydration technique. By conjugating thiolated antibodies with the MAL group, DSPE-PEG-MAL-hCTMO1 micelles were produced. By incubating DSPE-PEG-MAL-hCTMO1 micelles with preformulated TTSL for one hour at 60 °C, the thermoresponsive, targeted liposomal structure was developed. Compared with conventional thermosensitive liposomes, the implementation of produced liposomal structure with heating at 42 °C for one hour led to elevated cellular uptake and cytotoxicity in breast tumors (MDA-MB-435 cells) overexpressing the MUC1 gene [88]. It is worth noting that developing stimuli-responsive liposomes with surfaces designed to target receptors primarily expressed on the surface of tumor cells or in the tumor microenvironment is a promising option and provides a new surge for maximizing the potential of cancer therapy [89].References
- Global Cancer Observatory. Cancer Today; International Agency for Research on Cancer: Lyon, France; Available online: https://gco.iarc.fr/today (accessed on 10 February 2021).
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157.
- Sriraman, S.K.; Aryasomayajula, B.; Torchilin, V.P. Barriers to drug delivery in solid tumors. Tissue Barriers 2014, 2, e29528.
- Dunn, A.; Dunn, D.; Macmillan, A.; Whan, R.; Stait-Gardner, T.; Price, W.; Lim, M.; Boyer, C. Spatial and temporal control of drug release through pH and alternating magnetic field induced breakage of Schiff base bonds. Polym Chem. 2014, 5, 3311–3315.
- Shah, A.; Aftab, S.; Nisar, J.; Ashiq, M.N.; Iftikhar, F.J. Nanocarriers for targeted drug delivery. J. Drug Deliv. Sci. Technol. 2021, 62, 1024–1026.
- Dutta, B.; Barick, K.C.; Hassan, P.A. Recent advances in active targeting of nanomaterials for anticancer drug delivery. Adv. Colloid Interface Sci. 2021, 296, 102509.
- Aminu, N.; Bello, I.; Umar, N.M.; Tanko, N.; Aminu, A.; Audu, M.M. The influence of nanoparticulate drug delivery systems in drug therapy. J. Drug Deliv. Sci. Technol. 2020, 60, 101961.
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1477–3155.
- Carissimi, G.; Montalbán, G.M.; Fuster, G.M.; Víllora, G. Nanoparticles as Drug Delivery Systems. In 21st Century Nanostructured Materials-Physics, Chemistry, Classification, and Emerging Applications in Industry, Biomedicine, and Agriculture, 1st ed.; Pham Phuong, V., Ed.; IntechOpen: London, UK, 2021; Volume 10, p. 5772.
- Thassu, D.; Deleers, M.; Pathak, Y.V. (Eds.) Nanoparticulate Drug Delivery System, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019; Volume 166, pp. 1–376.
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth Liposomes: Review of the basic science, rational and clinical application existing and potential. Int. J. Nanomed. 2006, 1, 297–315.
- Blume, G.; Cevc, G. Liposomes for the sustained drug release in vivo. Biochim. Biophys. Acta Biomembr. 1990, 1029, 91–97.
- Grigoletto, A.; Tedeschini, T.; Canato, E.; Pasut, G. The evolution of polymer conjugation and drug targeting for the delivery of proteins and bioactive molecules. WIRES Nanomed. Nanobiotechnol. 2021, 13, e1689.
- Svenson, S.; Case, R.I.; Cole, R.O.; Hwang, J.; Kabir, S.R.; Lazarus, D.; Lim Soo, P.; Ng, P.S.; Peters, C.; Shum, P.; et al. Tumor Selective Silencing Using an RNAi-Conjugated Polymeric Nanopharmaceutical. Mol. Pharm. 2016, 13, 737–747.
- Thiruppathi, R.; Mishra, S.; Ganapathy, M.; Padmanabhan, P.; Gulyás, B. Nanoparticle functionalization and its potentials for molecular imaging. Adv. Sci. 2016, 4, 1600279.
- Yu, M.K.; Park, J.; Jon, S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2012, 2, 3–44.
- Montes-Fonseca, S.L.; Orrantia-Borunda, E.; Aguilar-Elguezabal, A.; González Horta, C.; Talamás-Rohana, P.; Sánchez-Ramírez, B. Cytotoxicity of functionalized carbon nanotubes in J774A macrophages. Nanomed. Nanotechnol. Biol. Med. 2012, 6, 853–859.
- Qu, H.; Tong, S.; Song, K.; Ma, H.; Bao, G.; Pincus, S.; Zhou, W.; O’Connor, C. Controllable in situ synthesis of magnetite coated silica-core water-dispersible hybrid nanomaterials. Langmuir 2013, 29, 10573–10578.
- Chinecherem Nkele, A.I.; Ezema, F. Diverse Synthesis and Characterization Techniques of Nanoparticles. In Thin Films; Alicia, A., Ed.; IntechOpen: London, UK, 2020.
- Poon, C.; Patel, A.A. Organic and inorganic nanoparticle vaccines for prevention of infectious diseases. Nano Express 2020, 1, 12001.
- Mazayen, Z.M.; Ghoneim, A.M.; Elbatanony, R.S.; Basalious, E.B.; Bendas, E.R. Pharmaceutical nanotechnology: From the bench to the market. Future J. Pharm. Sci. 2022, 8, 12.
- Lobatto, M.E.; Fuster, V.; Fayad, Z.A.; Mulder, W.J.M. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat. Rev. Drug Discov. 2011, 10, 835–852.
- Wagner, V.; Dullaart, A.; Bock, A.K.; Zweck, A. The emerging nanomedicine landscape. Nat. Biotechnol. 2006, 24, 1211–1217.
- Mohanraj, V.J.; Chen, Y. Nanoparticles—A review. Trop. J. Pharm. Res. 2007, 5, 561–573.
- Tiwari, G.; Tiwari, R.; Bannerjee, S.; Bhati, L.; Pandey, S.; Pandey, P.; Sriwastawa, B. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2–11.
- Hatakeyama, H.; Akita, H.; Harashima, H. The Polyethyleneglycol Dilemma: Advantage and Disadvantage of PEGylation of Liposomes for Systemic Genes and Nucleic Acids Delivery to Tumors. Biol. Pharm. Bull. 2013, 36, 892–899.
- Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Almatroudi, A.; Rahmani, A.H. Recent strategies towards the surface modification of liposomes: An innovative approach for different clinical applications. 3 Biotech 2020, 10, 163.
- Aronson, M.R.; Medina, S.H.; Mitchell, M.J. Peptide functionalized liposomes for receptor targeted cancer therapy. APL Bioeng. 2021, 5, 011501.
- Durymanov, M.O.; Rosenkranz, A.A.; Sobolev, A.S. Current approaches for improving intratumoral accumulation and distribution of nanomedicines. Theranostics 2015, 5, 1007–1020.
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. Int. J. Mol. Sci. 2018, 19, 195.
- Yuan, D.; Zong, T.; Gao, H.; He, Q. Cell penetrating peptide TAT and brain tumor targeting peptide T7 dual modified liposome preparation and in vitro targeting evaluation. Yao Xue Xue Bao 2015, 50, 104–110.
- Zhang, Y.; Zhai, M.; Chen, Z.; Han, X.; Yu, F.; Li, Z.; Xie, X.Y.; Han, C.; Yu, L.; Yang, Y.; et al. Dual-modified liposome codelivery of doxorubicin and vincristine improve targeting and therapeutic efficacy of glioma. Drug Deliv. 2017, 24, 1045–1055.
- Portnoy, E.; Lecht, S.; Lazarovici, P.; Danino, D.; Magdassi, S. Cetuximab-labeled liposomes containing near-infrared probe for in vivo imaging. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 480–488.
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286.
- Fenske, D.B.; Cullis, P.R. Liposomal nanomedicines. Expert Opin. Drug Deliv. 2008, 5, 25–44.
- Klingspor, L. Liposomal amphotericin B (AmBisome) for fungal infections in immunocompromised adults and children. Clin. Microbiol. Infect. 2001, 7, 68–79.
- Zylberberg, C.; Gaskill, K.; Pasley, S.; Matosevic, S. Engineering liposomal nanoparticles for targeted gene therapy. Gene Ther. 2017, 24, 441–452.
- Wassef, N.M.; Alving, C.R.; Richards, R.L. Liposomes as Carriers for Vaccines. Immunomethods 1994, 4, 217–222.
- Xue, H.; Guo, P.; Wen, W.C.; Wong, H. Lipid-Based Nanocarriers for RNA Delivery. Curr. Pharm. Des. 2015, 21, 3140–3147.
- Khidir, A.M.; Saeed, A.A. Ligand-targeted liposomes. Health Prim. Care 2020, 4, 118.
- Goncalves, D.L.; Libardi, A.K.M.; Penteado, D.; Vieira, J.P. Fixed points on trivial surface bundles over a connected CW-complex. Publicationes Mathematicae 2015, 87, 371–384.
- Maruyama, K.; Takizawa, T.; Yuda, T.; Kennel, S.J.; Huang, L.; Iwatsuru, M. Targetability of novel immunoliposomes modified with amphipathic poly(ethylene glycol) s conjugated at their distal terminals to monoclonal antibodies. Biochim. Biophys. Acta Biomembr. 1995, 1234, 74–80.
- Sou, K.; Endo, T.; Takeoka, S.; Tsuchida, E. Poly(ethylene glycol)-Modification of the Phospholipid Vesicles by Using the Spontaneous Incorporation of Poly(ethylene glycol)-Lipid into the Vesicles. Bioconjugate Chem. 2000, 11, 372–379.
- Sigot, V.; Arndt-Jovin, D.J.; Jovin, T.M. Targeted Cellular Delivery of Quantum Dots Loaded on and in Biotinylated Liposomes. Bioconjugate Chem. 2010, 21, 1465–1472.
- Yamakawa, T.; Ohigashi, H.; Hashimoto, D.; Hayase, E.; Takahashi, S.; Miyazaki, M.; Minomi, K.; Onozawa, M.; Niitsu, Y.; Teshima, T. Vitamin A–coupled liposomes containing siRNA against HSP47 ameliorate skin fibrosis in chronic graft-versus-host disease. Blood 2018, 131, 1476–1485.
- Sato, Y.; Murase, K.; Kato, J.; Kobune, M.; Sato, T.; Kawano, Y.; Takimoto, R.; Takada, K.; Miyanishi, K.; Matsunaga, T.; et al. Resolution of liver cirrhosis using vitamin A–coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat. Biotechnol. 2008, 26, 431–442.
- Turk, M.J.; Waters, D.J.; Low, P.S. Folate-conjugated liposomes preferentially target macrophages associated with ovarian carcinoma. Cancer Lett. 2004, 213, 165–172.
- Leamon, C.P.; Cooper, S.R.; Hardee, G.E. Folate-Liposome-Mediated Antisense Oligodeoxynucleotide Targeting to Cancer Cells: Evaluation in Vitro and in Vivo. Bioconjugate Chem. 2003, 14, 738–747.
- Xie, F.; Yao, N.; Qin, Y.; Zhang, Q.; Chen, H.; Yuan, M.; Tang, J.; Li, X.; Fan, W.; Zhang, Q. Investigation of glucose-modified liposomes using polyethylene glycols with different chain lengths as the linkers for brain targeting. Int. J. Nanomed. 2012, 7, 163–175.
- Song, C.K.; Jung, S.H.; Kim, D.D.; Jeong, K.S.; Shin, B.C.; Seong, H. Disaccharide-modified liposomes and their in vitro intracellular uptake. Int. J. Pharm. 2009, 380, 161–169.
- Abu-Dahab, R.; Schäfer, U.F.; Lehr, C.M. Lectin-functionalized liposomes for pulmonary drug delivery: Effect of nebulization on stability and bioadhesion. Eur. J. Pharm. Sci. 2001, 14, 37–46.
- Miller, K.; Cortes, J.; Hurvitz, S.A.; Krop, I.E.; Tripathy, D.; Verma, S.; Riahi, K.; Reynolds, J.G.; Wickham, T.J.; Molnar, I.; et al. HERMIONE: A randomized Phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician’s choice plus trastuzumab in patients with previously treated, anthracycline-naïve, HER2-positive, locally advanced/metastatic breast cancer. BMC Cancer 2016, 16, 352.
- Shin, D.H.; Lee, S.J.; Kim, J.S.; Ryu, J.H.; Kim, J.S. Synergistic Effect of Immunoliposomal Gemcitabine and Bevacizumab in Glioblastoma Stem Cell-Targeted Therapy. J. Biomed. Nanotechnol. 2015, 11, 1989–2002.
- Xu, L.; Huang, C.C.; Huang, W.; Tang, W.H.; Rait, A.; Yin, Y.Z.; Cruz, I.; Xiang, L.M.; Pirollo, K.F.; Chang, E.H. Systemic Tumor-targeted Gene Delivery by Anti-Transferrin Receptor scFv-Immunoliposomes. Mol. Cancer Ther. 2002, 1, 337–346.
- Liu, Y.J.; Dou, X.Q.; Wang, F.; Zhang, J.; Wang, X.L.; Xu, G.L.; Xiang, S.S.; Gao, X.; Fu, J.; Song, H.F. IL-4Rα aptamer-liposome-CpG oligodeoxynucleotides suppress tumour growth by targeting the tumour microenvironment. J. Drug Target. 2017, 25, 275–283.
- Baek, S.E.; Lee, K.H.; Park, Y.S.; Oh, D.K.; Oh, S.; Kim, K.S.; Kim, D.E. RNA aptamer-conjugated liposome as an efficient anticancer drug delivery vehicle targeting cancer cells in vivo. J. Control. Release 2014, 196, 234–242.
- Alshaer, W.; Hillaireau, H.; Vergnaud, J.; Ismail, S.; Fattal, E. Functionalizing Liposomes with anti-CD44 Aptamer for Selective Targeting of Cancer Cells. Bioconjugate Chem. 2015, 26, 1307–1313.
- Riaz, M.K.; Tyagi, D.; Yang, Z. Surface Engineering: Incorporation of Bioactive Compound. In Bioactivity of Engineered Nanoparticles. Nanomedicine and Nanotoxicology; Springer: Singapore, 2017; pp. 111–143.
- Feng, L.; Mumper, R.J. A critical review of lipid-based nanoparticles for taxane delivery. Cancer Lett. 2013, 334, 157–175.
- Manjappa, A.S.; Chaudhari, K.R.; Venkataraju, M.P.; Dantuluri, P.; Nanda, B.; Sidda, C.; Sawant, K.K.; Ramachandra Murthy, R.S. Antibody derivatization and conjugation strategies: Application in preparation of stealth immunoliposome to target chemotherapeutics to tumor. J. Control. Release 2011, 150, 2–22.
- Mahon, B.; Pinard, M.; McKenna, R. Targeting Carbonic Anhydrase IX Activity and Expression. Molecules 2015, 20, 2323–2348.
- Yang, Z.; Wong, B.C.K.; Zhang, H.; Qin, L.; Chen, H.; Lu, A.; Chen, F. Carbonic anhydrase IX-directed immunoliposomes for targeted drug delivery to human lung cancer cells in vitro. Drug Des. Dev. Ther. 2014, 8, 993.
- Koren, E.; Torchilin, V.P. Cell-penetrating peptides: Breaking through to the other side. Trends Mol. Med. 2012, 18, 385–393.
- Wu, H.; Yao, L.; Mei, J.; Li, F. Development of synthetic of peptide-functionalized liposome for enhanced targeted ovarian carcinoma therapy. Int. J. Clin. Exp. Med. 2014, 7, 4809–4818.
- Chen, Z.; Deng, J.; Zhao, Y.; Tao, T.; Cyclic, R.G.D. Peptide-modified liposomal drug delivery system: Enhanced cellular uptake in vitro and improved pharmacokinetics in rats. Int. J. Nanomed. 2012, 7, 3803–3811.
- Ding, Y.; Sun, D.; Wang, G.L.; Yang, H.G.; Xu, H.F.; Chen, J.H.; Xie, Y.; Wang, Z.Q. An efficient PEGylated liposomal nanocarrier containing cell-penetrating peptide and pH-sensitive hydrazone bond for enhancing tumor-targeted drug delivery. Int. J. Nanomed. 2015, 10, 6199–6214.
- Hammond, N. Retraction: The next generation cell-penetrating peptide and carbon dot conjugated nano-liposome for transdermal delivery of curcumin. Biomater. Sci. 2019, 7, 442.
- Catuogno, S.; Esposito, C.; de Franciscis, V. Aptamer-Mediated Targeted Delivery of Therapeutics: An Update. Pharmaceuticals 2016, 9, 69.
- Cao, Z.; Tong, R.; Mishra, A.; Xu, W.; Wong, G.C.L.; Cheng, J.; Lu, Y. Reversible Cell-Specific Drug Delivery with Aptamer-Functionalized Liposomes. Angewandte Chemie Int. Ed. 2009, 48, 6494–6498.
- Powell, D.; Chandra, S.; Dodson, K.; Shaheen, F.; Wiltz, K.; Ireland, S.; Syed, M.; Dash, S.; Wiese, T.; Mandal, T.; et al. Aptamer-functionalized hybrid nanoparticle for the treatment of breast cancer. Eur. J. Pharm. Biopharm. 2017, 114, 108–118.
- Sriraman, S.K.; Salzano, G.; Sarisozen, C.; Torchilin, V. Anti-cancer activity of doxorubicin-loaded liposomes co-modified with transferrin and folic acid. Eur. J. Pharm. Biopharm. 2016, 105, 40–49.
- Alavizadeh, S.H.; Akhtari, J.; Badiee, A.; Golmohammadzadeh, S.; Jaafari, M.R. Improved therapeutic activity of HER2 Affibody-targeted cisplatin liposomes in HER2-expressing breast tumor models. Expert Opin. Drug Deliv. 2016, 13, 325–336.
- Kang, M.H.; Yoo, H.J.; Kwon, Y.H.; Yoon, H.Y.; Lee, S.G.; Kim, S.R.; Yeom, D.W.; Kang, M.J.; Choi, Y.W. Design of Multifunctional Liposomal Nanocarriers for Folate Receptor-Specific Intracellular Drug Delivery. Mol. Pharm. 2015, 12, 4200–4213.
- Zong, T.; Mei, L.; Gao, H.; Cai, W.; Zhu, P.; Shi, K.; Chen, J.; Wang, Y.; Gao, F.; He, Q. Synergistic Dual-Ligand Doxorubicin Liposomes Improve Targeting and Therapeutic Efficacy of Brain Glioma in Animals. Mol. Pharm. 2014, 11, 2346–2357.
- Movahedi, F.; Hu, R.G.; Becker, D.L.; Xu, C. Stimuli-responsive liposomes for the delivery of nucleic acid therapeutics. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1575–1584.
- Needham, D.; Park, J.Y.; Wright, A.M.; Tong, J. Materials characterization of the low temperature sensitive liposome (LTSL): Effects of the lipid composition (lysolipid and DSPE–PEG2000) on the thermal transition and release of doxorubicin. Faraday Discuss. 2013, 161, 515–534.
- Needham, D.; Anyarambhatla, G.; Kong, G.; Dewhirst, M.W. A New Temperature-Sensitive Liposome for Use with Mildhyperthermia: Characterization and Testing in Human Tumor Xenograft Model. Cancer Res. 2000, 60, 1197–1201.
- Simões, S. On the formulation of pH-sensitive liposomes with long circulation times. Adv. Drug Deliv. Rev. 2004, 56, 947–965.
- Madni, A.; Sarfraz, M.; Rehman, M.; Ahmad, M.; Akhtar, N.; Ahmad, S.; Tahir, N.; Ijaz, S.; Al-Kassas, R.; Löbenberg, R. Liposomal Drug Delivery: A Versatile Platform for Challenging Clinical Applications. J. Pharm. Pharm. Sci. 2014, 17, 401.
- Felber, A.E.; Dufresne, M.H.; Leroux, J.C. pH-sensitive vesicles, polymeric micelles, and nanospheres prepared with polycarboxylates. Adv. Drug Deliv. Rev. 2012, 64, 979–992.
- Yoshizaki, Y.; Yuba, E.; Komatsu, T.; Udaka, K.; Harada, A.; Kono, K. Improvement of Peptide-Based Tumor Immunotherapy Using pH-Sensitive Fusogenic Polymer-Modified Liposomes. Molecules 2016, 21, 1284.
- Clares, B.; Biedma-Ortiz, R.A.; Sáez-Fernández, E.; Prados, J.C.; Melguizo, C.; Cabeza, L.; Ortiz, R.; Arias, J.L. Nano-engineering of 5-fluorouracil-loaded magnetoliposomes for combined hyperthermia and chemotherapy against colon cancer. Eur. J. Pharm. Biopharm. 2013, 85, 329–338.
- Zhu, L.; Torchilin, V.P. Stimulus-responsive nanopreparations for tumor targeting. Integrative Biol. 2013, 5, 96–107.
- Huang, S.L. Ultrasound-Responsive Liposomes. Methods Mol. Biol. 2010, 605, 113–128.
- Han, H.D.; Jeon, Y.W.; Kwon, H.J.; Jeon, H.N.; Byeon, Y.; Lee, C.O.; Cho, S.H.; Shin, B.C. Therapeutic efficacy of doxorubicin delivery by a CO2 generating liposomal platform in breast carcinoma. Acta Biomater. 2015, 24, 279–285.
- Jia, R.; Teng, L.; Gao, L.; Su, T.; Fu, L.; Qiu, Z.; Bi, Y. Advances in Multiple Stimuli-Responsive Drug-Delivery Systems for Cancer Therapy. Int. J. Nanomed. 2021, 16, 1525–1551.
- Ta, T.; Bartolak-Suki, E.; Park, E.J.; Karrobi, K.; McDannold, N.J.; Porter, T.M. Localized delivery of doxorubicin in vivo from polymer-modified thermosensitive liposomes with MR-guided focused ultrasound-mediated heating. J. Control. Release 2014, 194, 71–81.
- Yang, Y.; Yang, Y.; Xie, X.; Xu, X.; Xia, X.; Wang, H.; Li, L.; Dong, W.; Ma, P.; Liu, Y. Dual stimulus of hyperthermia and intracellular redox environment triggered release of siRNA for tumor-specific therapy. Int. J. Pharm. 2016, 506, 158–173.
- Al-Ahmady, Z.S.; Chaloin, O.; Kostarelos, K. Monoclonal antibody-targeted, temperature-sensitive liposomes: In vivo tumor chemotherapeutics in combination with mild hyperthermia. J. Control. Release 2014, 196, 332–343.
More
