You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 4 by Catherine Yang and Version 3 by Catherine Yang.
The continuously increasing CO2 levels in the atmosphere have mandated the development of innovative strategies and technologies to reduce their emissions. Currently, the use of CO2 as a raw material is becoming promising in the context of sustainable development and environmental protection CO2 is already used in different fields, such as food processing, drinks, medicine, and industry, but these sequestration methods are characterized by temporary operation [98] However, CO2 may also be used in the synthesis of fine chemicals and fuels, representing a promising approach for feedstock substitution in a sustainable way.
- CO2 methanation
- carbon capture and storage
- structured catalysts
1. CO2 to HC through Methanol Pathway
CO2 conversion into methanol has been extensively studied by the scientific community, and among different catalysts, those based on Cu/ZnO are the most widely investigated [1] However, despite the successes that have been reported, the reaction pathway is still unclear. In fact, formate species (HCOO*) and hydrocarboxyl species (COOH*) have each been reported as the first hydrogenation products. The formation of hydrocarbons from methanol is mainly achieved with the aid of a zeolitic catalyst, generally HZSM-5 and/or SAPO-34 [2] More than 20 possible mechanisms have been proposed for the reaction [3] However, the dual HC pool mechanism is of great interest. HC intermediates, specified as (CH2)n, represent the adsorbate and may also contain several poly-condensed aromatic species characterizing coke (containing less H than indicated). Recent studies have shown that the mechanism of methanol-to-HC conversion proceeds via two steps:
The product distribution mainly depends on the topological structure of zeolite, its acidity, and operating conditions [8].
Very recently, tandem catalysts constituted by metal oxides and HZSM-5 zeolites with different morphologies (spherical, hollow, sheet, and chain) were studied [9]. The research demonstrated that the ZSM-5 morphology is important in determining the catalyst performance, with higher CO2 conversion and selectivity to the desired product shown by the sheet and chain configurations, respectively. Zeolites with different topologies in combination with metal oxides have also been investigated, and the results revealed that the pore dimensions of the zeolites are fundamental for directing the products towards the desired ones [10][11][12]. In particular, the larger the pores are, the heavier the obtained hydrocarbons are.
2. CO2 to HC through RWGS
Among all of the available catalysts for CO2 conversion via the RWGS process, Fe-based catalysts have attracted worldwide attention [13]. In the initial stage, CO2 is converted into CO, which is subsequently hydrogenated to olefins and paraffin on active sites of Fe-carbide, as proposed by Xu et al. [14]. By means of a bifunctional system (such as acidic zeolite), the so-obtained olefins and paraffin products are then converted into other hydrocarbons through different reactions of aromatization, hydrocracking, hydro-isomerization, oligomerization, and cyclization plus H-transfer. The reaction chemistry is apparently very complex and therefore produces various reaction intermediates [14][15]. Monomolecular and bimolecular activation mechanisms have been proposed for paraffin conversion into HC. The former mechanism was proposed by Haag et al. [16] who described the protonation of the alkane molecule to form carbonium ions that can undergo C–C or C–H bond cleavage. Subsequently, the carbonium ions produce olefin via the back-donation of a proton to zeolite. The latter mechanism can be achieved by the protonation of paraffin using the Bronsted acid site, which is then used to form a dimer with another olefinic HC [17][18]. Once the paraffin activation is achieved, subsequent conversion into various HCs proceeds. Catalytic cracking can proceed through both bimolecular and monomolecular mechanisms. However, the monomolecular mechanism predominantly occurs at high temperatures, while the bimolecular mechanism usually occurs at a mild temperature (<350 °C). Aromatization can proceed through polymerization reactions to form dienes [19], which is then followed by cyclization in zeolite channels and multi-step H-transfer with olefin, yielding aromatics and paraffin [20]. Subsequently, inter-conversion reactions such as isomerization, disproportionation, and alkylation/dealkylation take place. In the recent literature, Fe-based catalysts doped with either alkali metals or non-metal elements have been proposed, aiming at improving the selectivity of C2+ olefins [21][22]. The addition of Ce may play a key role in the activation and dissociation of adsorbed CO2 due to the mobile oxygen vacancy in ceria oxides, which can accelerate the migration of oxygen. In this sense, FeCeNa catalysts have shown good performance in terms of CO2 conversion to olefins [23].
3. Direct Conversion of CO2 to HC
The hydrogenation of CO2 to generate HC fuels involves two routes (i.e., direct and indirect routes), which are often referred to as the chemical process. The goal of producing methane and subsequent long-chain HC is the basis for the conversion of CO2 into HC fuels. Therefore, in the energy-related scenario, the CO2 methanation reaction has acquired remarkable interest in recent years. Indeed, it constitutes the core of the power-to-methane (PtM) process chain. This recently developed technology involves a stepwise conversion, which aims to efficiently employ the surplus energy derived from renewable power sources. The main issue of generating power from renewables is, in fact, the oscillation of electrical energy production over time [24]. This phenomenon, known as cycling, can be due to meteorological conditions, the time of day, and other aleatoric factors. The uncontrollable intermittency of power generation causes severe problems and imbalances in the power grid, making the integration of renewables harder and even less economically feasible [25]. Plenty of possible solutions to store the surplus energy have been investigated over time, resulting in the consensus that systems such as batteries and pumped-hydro systems have high capacity costs and low energy density compared to hydrocarbon [26]. Therefore, an efficient solution is to convert the surplus electrical energy into chemical energy, which can be achieved via water electrolysis. Nevertheless, the product of this conversion is hydrogen, which is a high-added-value product but presents several drawbacks, such as the difficulty in storage and transportation and the limitation on its distribution through the natural gas grid. To overcome the problem of hydrogen management, its conversion to hydrocarbons is particularly promising. In particular, CO2 hydrogenation to methane, mostly known as CO2 methanation, represents an attractive process to efficiently store the surplus energy from renewables. The methane produced through this process is referred to as synthetic or substitute natural gas (SNG). It has plenty of applications, and, above all, it can be inserted into the natural gas grid without limitations [27]. Together with the advantage of being a solution for power storage, allowing the more efficient integration of renewables into the energy scenario, CO2 methanation also has a remarkable impact on the environment from a different point of view. Indeed, it enables CO2 consumption—for example, by employing CO2 from sequestration systems—and can thus be considered a carbon capture and utilization (CCU) process [28][29]. Overall, the PtM process chain can be represented as in Figure 1.
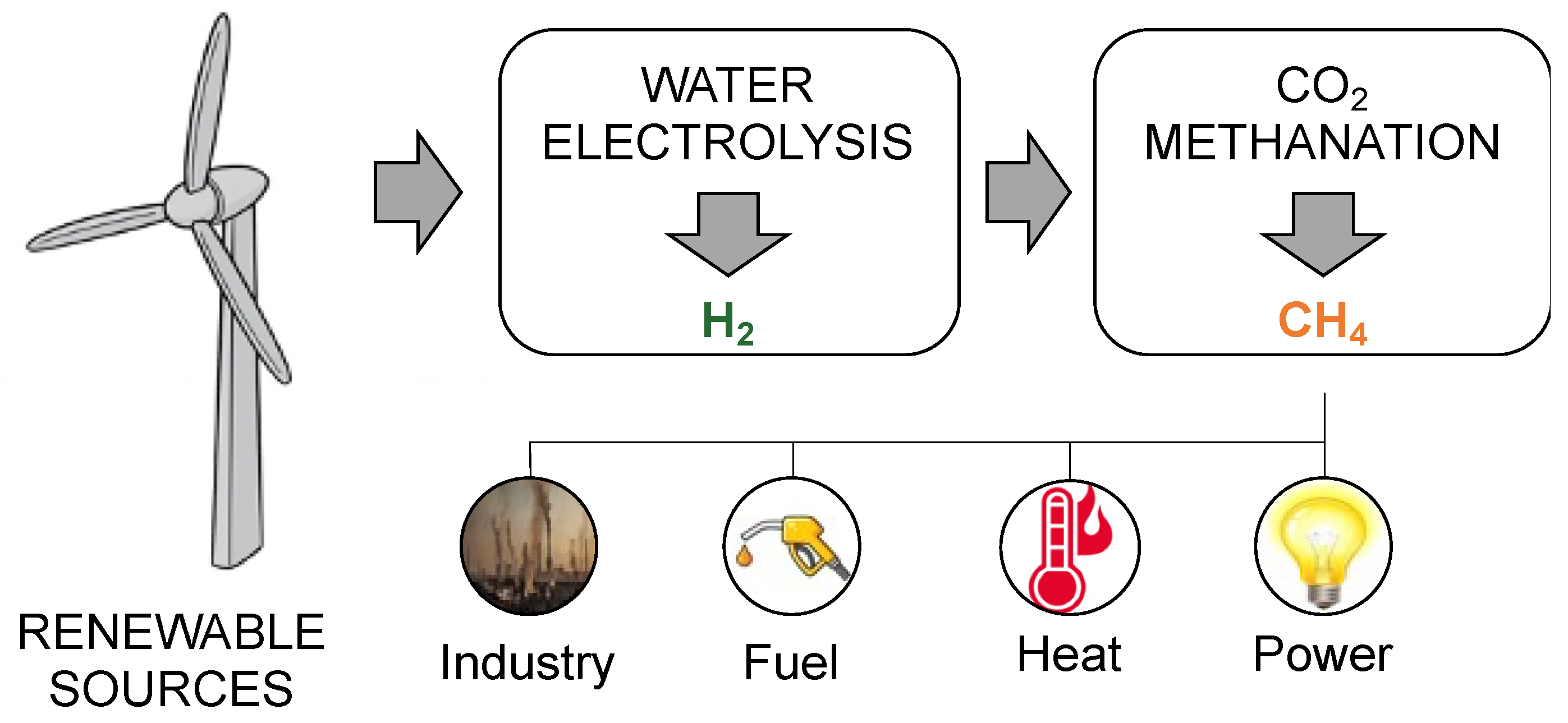
Figure 1. PtM process chain. From source to applications.
CO2 methanation follows Equation (1), and it is an exothermic equilibrium reaction. Therefore, it presents issues related to heat management in the catalytic system, which can be summarized as follows. The heat of reaction induces a thermal gradient across the catalytic bed and occasional hotspot formation, which can be detrimental to catalyst stability, leading to sintering phenomena, reduction in the number of active sites, and loss in activity. Furthermore, the increase in local temperature represents a disadvantage for the thermodynamic equilibrium of the reaction, which is promoted at low temperatures. On the other hand, operating temperatures that are too low are detrimental to the reaction kinetics, leading to lower selectivity of the reacting system and overall lower activity.
CO2 + 4H2 ⇄ CH4 + 2H2O
(
The recent attention towards CO2 methanation drove a remarkable increase in scientific publications related to the topic in the past five years. Different catalytic formulations and catalytic systems have been explored with the aim of addressing the above-discussed issues and obtaining efficiently performing catalysts in order to allow the implementation of this process in the industrial scenario. The research approaches to the enhancement of the CO2 methanation process can be classified as:
-
Catalytic formulation optimization through the investigation of new active metals or supports;
-
Utilization of structured catalysts in different shapes and materials;
-
Evaluation of innovative reactor configurations (membrane reactors);
-
New technologies (cold plasma reactors).
Concerning catalytic formulations, nickel is the most widely employed active species in CO2 methanation. A comprehensive resume of the pros and cons of its application includes: its low cost and natural abundance, together with its high activity towards CO2 activation, as the main advantages; a relatively high activation temperature, low dispersion, and reducibility as the main disadvantages [30]. Few other metals can offer the same positive characteristics as Ni; therefore, it is widely applied, and the drawbacks of its utilization are frequently compensated by support modification or a second metal addition [31]. For bimetallic formulations, Fe and Co (as transition metals) are widely reported in the literature, while, among noble metals, Ru is surely the most effective species for CO2 methanation. In particular, it shows a relative activity higher than Ni [32] and strongly promotes the direct reaction mechanism, leading to a higher selectivity towards methane. Another basic aspect of CO2 methanation is that a bifunctional reaction mechanism is always established: the support is involved in the reactant activation, and therefore, it plays a crucial role [33]. Plenty of studies in the literature report on the optimization of catalytic formulations, which have been recently satisfactorily reviewed [32][34][35][36][37]. Therefore, the researchers focus on the most recent applications of engineered solutions to conduct the reaction.
Methanation over Structured Catalysts
In recent years, most of the interest has been devoted to structured catalysts. These are constituted by a carrier with a complex geometry, upon which the support and the active phases are deposited via several techniques; structured catalysts can offer several advantages for this process. First, they can be made of highly conductive materials, such as aluminum, silicon carbide, or metal alloys. The high thermal conductivity plays a fundamental role, considering the exothermicity of the reaction: indeed, these structures allow the flattening of the temperature profile within the catalytic bed, reducing the formation of hotspots and therefore the sintering phenomena and local thermodynamic limitations [38]. Furthermore, some of these structures can offer enhanced mixing characteristics, which reduce the possibility of diffusion resistance effects [39].
The most common geometrical configuration of structured catalysts is certainly the channeling structure, which is typical of honeycomb or corrugated sheet monoliths: when considering only the most conductive materials, the former are usually made of silicon carbide (SiC), while the latter are generally composed of metallic alloys; on the other hand, ceramic materials such as cordierite are frequently employed because of their ability to anchor metallic species. Indeed, NiFe/cordierite monoliths were tested in CO2 methanation conditions in a detailed study concerning the management of the hotspot phenomena that are characteristic of this system [40]. The catalysts were obtained via in situ growth of the nanoparticles, and they were classified into high-activity and low-activity monoliths. The authors reported that low- and high-activity catalysts can be alternated over the bed, leading to a remarkably smoother increase in temperature due to the exothermic reaction, with a non-significant loss in methane yield.
Considering that Ni/CeO2 was widely reported as one of the most promising formulations for CO2 methanation in terms of both activity and selectivity towards methane, FeCrAlloy sheets decorated with CeO2 nanorods to support Ni particles were recently evaluated [41]. This preparation was optimized in order to increase the stability, considering that in harsh conditions, the structured catalyst undergoes rapid deactivation. As a result, the catalysts were efficient, demonstrating higher catalytic activity compared to the powder form. Micromonoliths in commercial FeCrAlloy stainless steel were tested in CO2 methanation with a coating of a previously optimized catalytic formulation (15 wt.% Ni, 0.5 wt.% Ru, and 10 wt.% Mg over alumina) [42]. The authors observed that the textural properties of the original powder catalysts were unchanged after deposition on the 3D structure, even though the structured catalyst exhibited sintering after the stability test. Furthermore, a noteworthy result was that transport limitations were observed at high weight hourly space velocity (WHSV) conditions, which can be ascribable to the channeling structure. Indeed, the same kind of 3D structure obtained with aluminum sheets was employed with a Ni/CeO2 coating with a variety of configurations (plain, stacked, segment, and multi-stacked), proving that avoiding a channeling flow regime is beneficial for the reaction [43]. This conclusion is further upheld by comparative studies that involve channeling structures (such as honeycomb monoliths) and randomly organized structures (open-cell foams). The comparison between an alumina open-cell foam and a cordierite monolith highlighted that the external mass transfer is maximized in the case of the open-cell foam thanks to its characteristic irregular structure, which ensures a more intimate contact between phases [44]. In particular, the light-off temperature (which is a typical parameter to be taken into account in CO2 methanation and can be considered the temperature at which the reaction starts—i.e., it has a significant conversion) was found to be remarkably lower in the foam catalytic system. The same result in terms of optimized heat and mass transfer was observed through a comparison between an aluminum open-cell foam and a SiC monolith, with the foam-supported catalyst showing the best performance in terms of methane yield and light-off temperature [45]. CO2 methanation was also investigated over high-pore-density (75 ppi—pores per inch) metallic (Ni) foams, which were coated with CeO2 (via electrodeposition) and impregnated with Ru [46]. The high pore density of the carrier and the low thickness of the coating produced outstanding transport properties, suggesting that this catalyst is particularly suitable for the process intensification of highly exothermic catalytic reactions.
The importance of the thermal conductivity of the carrier was evaluated with a comparison between two 40 PPI open-cell foams, one made of alumina (low conductivity) and one made of SiC (high conductivity) [47]. Activity tests were performed in a bench-scale reactor with an integrated cooling system in order to evaluate the effect of co-current or counter-current refrigeration of the system. The use of carriers having similar geometrical and morphological properties is particularly significant to the aim of discerning the thermal conductivity effect; therefore, the outcomes of this evaluation offer a noteworthy perspective in the application of highly conductive structured catalysts. The results showed that, as expected, the SiC foam offered a flatter thermal profile, which is beneficial from a thermodynamic perspective. Furthermore, with the Al2O3-based catalyst, the difficulty of removing heat with the refrigerating medium was ascribed to the radial heat transfer resistance.
According to this result, a simulation study of CO2 methanation over ceramic paper catalysts revealed that the most relevant contribution to the effective heat transfer is the radial heat dispersion through conduction; in contrast, the dynamic contributions are not significant, considering the low linear gas velocity [48].
Due to the remarkable enhancement of reaction performance in the presence of random 3D structures, research has been devoted to the reproduction of ordered geometries with the same properties. This result was achieved through additive manufacturing (AM). Regular periodic 3D structures with two different layer stacking configurations were tested for CO2 methanation applications [49]. The best results were obtained with the “zig-zag” layer organization, which allowed improved heat and mass transfer. Furthermore, with the optimized configuration, it was also observed that stainless steel is a beneficial material for ensuring a better coating adhesion compared to copper [50].
Innovative Reactor Configurations
The most consolidated solution to conduct CO2 methanation is surely the adiabatic fixed-bed reactor or cooled fixed-bed reactors. The former can be operated with inter-stage cooling, even though this solution requires many auxiliary units (heat exchanger). The latter, instead, is a more compact solution; however, the temperature and pressure drop control is more complicated. Other solutions, such as fluidized bed reactors and microchannels reactors, are still in the early stage of research. The interest towards microchannel reactors is mainly related to the possibility of having a mass transfer completely dominated by diffusion, as the normal velocity with respect to the surface is almost null. Fuentes et al. [51] simulated a microchannel system and showed that with a large number of channels having a small cross-sectional area, the prevalence of diffusive forces could be increased. These enhance methane production, and therefore, the resulting product stream is more suitable for insertion in the natural gas infrastructure.
The integration of membrane technology with a catalytic reaction always draws remarkable attention in terms of process intensification, as it can enhance the catalytic performance over the thermodynamic equilibrium. Their application is frequently intended to remove a product from the catalytic zone in order to force the reaction equilibrium towards the products. In methanation systems, membrane reactors could be applied for water removal but also for a local supply of hydrogen, and vice versa.
In situ water removal in CO2 methanation systems theoretically provides 100% conversion of CO2 when water is completely removed (R = 0.99) at any temperature, pressure, or CH4/CO2 ratio [52]. For water separation, hydrophilic membranes are applied. The main drawback is that non-condensable gases pass through the membrane via the Knudsen diffusion mechanism, even though their permeability is reduced by progressively increasing water capillary condensation. The first attempt to employ a membrane reactor for the Sabatier process was performed in 1997 with a water-permeable membrane: as a result, an increase in CO2 conversion of 18% was obtained with the integrated system compared to the conventional fixed-bed reactor [53]. Experimental data of integrated systems available in the literature were successfully approximated by a simulation in which a water-permeable hydroxy sodalite (H-SOD) membrane and H2 as the sweep gas were considered [54]. Hydrogen was employed because it minimizes the reactant loss and maximizes the product permeation. The simulations indicated that enhanced conversion could be obtained in mild temperature and pressure conditions, which potentially represent a lower operating expense (OPEX). Furthermore, a more in-depth evaluation in a 2D simulation study demonstrated that the highest permeation flux was established at the inlet, while the outlet was characterized by a balance between H2O permeation and the production rate [55]. Nevertheless, the simulation results showed a 90% water removal and an 8.3% CO2 conversion increase. As an outcome, since the reaction rate was found to be much higher than water permeation, the authors concluded that for reactors with high GHSVs, the utilization of membranes with high water permeance is mandatory.
The introduction of a hydrogen-permeable membrane in CO2 methanation systems is an attractive alternative for the coupling of different processes. An example was provided by Miyamoto et al. [56], who integrated NH3 decomposition, which provides hydrogen, with CO2 methanation. Therefore, hydrogen was removed from the first reaction system and supplied to the second by a Pd membrane, with the obtainment of a significant increase in both NH3 decomposition and H2 separation. Nevertheless, the local supply of hydrogen was not effective in improving the methanation rate, as it was found to be comparable to the value obtained in the packed-bed reactor; on the other hand, CH4 selectivity was increased, resulting in an enhancement of reaction performance. A coupled system of dehydrogenation/hydrogenation reactions was also proposed by Bian et al. [57] in their CFD simulation. Cyclohexane dehydrogenation was considered at the retentate side, while CO2 methanation was set at the permeate side. This study supports the previously reported result that a high-permeance membrane is mandatory in order to enhance the performance of both reactions.
CO2 Methanation under NTP Process
The interest towards non-thermal plasma (NTP) applications in methanation processes is primarily related to the main reactant activation. Indeed, CO2 is a highly stable molecule, and therefore, in conventional catalytic systems, C=O bond breakage is promoted with the use of suitable active species (Ni or Ru, for example); furthermore, each catalytic formulation has its own light-off temperature, which is just below 200 °C. When plasma is applied, high-energy species such as free radicals and ions are involved, and these species can activate CO2 molecules without the necessity of external heating. In NTP technology, several configurations can be realized, although the most employed solution is represented by the dielectric barrier discharge (DBD) reactor [58]. The application of NTP to CO2 methanation is a remarkably recent topic; nevertheless, several catalytic systems have been evaluated under plasma conditions. Plasma is particularly useful for the removal of water molecules from the catalytic surface: this was specifically observed over Si/Al zeolite-supported catalysts in a DBD reactor [59]. Furthermore, as NTP is expected to reduce the reaction barrier thanks to its non-equilibrium nature, it potentially allows performing the reaction without a catalyst. The study conducted by Ahmad et al. [60] was indeed focused on a plasma-Ni system. The authors considered the NTP + Ni catalyst hybrid system and compared it with a Ni catalyst in a thermal (Ni-T) system and an NTP-driven reaction. As outcomes, the plasma-assisted system allowed consistent methane selectivity at a remarkably low temperature (150 °C). The hybrid plasma–catalytic system compared to the thermal–catalytic system resulted in a CO2 conversion 20 times higher and a CH4 selectivity 5 times higher than the Ni–T configuration. Non-thermal plasma was also evaluated in the presence of more complex catalytic formulations (hydrotalcite-derived catalysts with Ni–Fe active species) with satisfactory results, suggesting its suitability in dynamic systems working with excess energy [61]. Even though the NTP is a low-temperature technology, the exothermicity of CO2 methanation cannot be neglected. The study of Bosét-Peiró et al. [62] showed that a pseudo-adiabatic plasma-assisted configuration could reach an energy efficiency of 73%, and therefore, the overall process is undeniably less energetically demanding. A combination of non-thermal plasma technology and a structured catalyst was evaluated by Gao et al. [63], who performed a complex study related to the mechanisms in plasma-assisted CO2 methanation. The authors observed that the system is structure-dependent, as different structures led to different CH4 selectivities due to the dissimilar discharges within the catalytic bed. Furthermore, the mechanistic study highlighted that, due to the plasma presence, several radicals were formed in the system (CO, CO(ν), H, and H(ν)). In particular, CO(ν1) was individuated as the main vibrational state, resulting in a reduced energy barrier and, therefore, in a lower activation temperature. The short-term stability under NTP conditions was recently evaluated by the same authors over a Ni–Y/CeO2 formulation, showing excellent stability for 12 h and remarkable catalytic activity with a CO2 conversion of 84% and selectivity to methane of 83% [64].
Industrial Applications and Outlook
CO2 hydrogenation to methane is a captivating topic in several fields, ranging from CO2 utilization to methane production and renewable inclusion in power generation technologies. This new concept has been widely studied in recent years, leading to the obtainment of a mature technology, at least for thermo-catalytic CO2 methanation. Germany can be regarded as the leader in PtM technology: in Stuttgard and in Wertle, two power-to-methane plants (with capacities of 250 kW and 6300 kW power input, respectively) produce methane for Audi. Furthermore, Germany is also at the cutting edge of biological methanation technologies, with the first commercial-scale project with a 1 MW capacity [34]. Innovative solutions such as structured catalysts, membrane reactors, and plasma-assisted systems are, therefore, highly promising for the industrial scenario, as they represent possible solutions for the intensification of this newborn process, aiming to further enhance energy efficiency.
References
- Alvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the greener production of Formates/Formic acid, methanol, and DME by heterogeneously catalyzed CO2 hydrogenation processes. Chem. Rev. 2017, 117, 9804–9838.
- Yarulina, I.; De Wispelaere, K.; Bailleul, S.; Goetze, J.; Radersma, M.; Abou-Hamad, E.; Vollmer, I.; Goesten, M.; Mezari, B.; Hensen, E.J.M.; et al. Structure–performance descriptors and the role of Lewis acidity in the methanol-to-propylene process. Nat. Chem. 2018, 10, 804–812.
- Ojelade, O.A.; Zaman, S.F. A review on CO2 hydrogenation to lower olefins: Understanding the structure-property relationships in heterogeneous catalytic systems. J. CO2 Util. 2021, 47, 101506.
- Chowdhury, A.D.; Houben, K.; Whiting, G.T.; Mokhtar, M.; Asiri, A.M.; Al-Thabaiti, S.A.; Basahel, S.N.; Baldus, M.; Weckhuysen, B.M. Initial carbon–Carbon bond formation during the early stages of the methanol-to-Olefin process proven by zeolite-trapped acetate and methyl acetate. Angew. Chem. Int. 2016, 55, 15840–15845.
- Cai, D.; Wang, Q.; Jia, Z.; Ma, Y.; Cui, Y.; Muhammad, U.; Wang, Y.; Qian, W.; Wei, F. Equilibrium analysis of methylbenzene intermediates for a methanol-to-olefins process. Catal. Sci. Technol. 2016, 6, 1297–1301.
- Yarulina, I.; Chowdhury, A.D.; Meirer, F.; Weckhuysen, B.M.; Gascon, J. Recent trends and fundamental insights in the methanol-to-hydrocarbons process. Nat. Catal. 2018, 1, 398–411.
- Zhu, J.; Li, Y.; Muhammad, U.; Wang, D.; Wang, Y. Effect of alkene co-feed on the MTO reactions over SAPO-34. Chem. Eng. J. 2017, 316, 187–195.
- Usman, M.; Zhu, J.; Chuiyang, K.; Arslan, M.T.; Khan, A.; Galadima, A.; Muraza, O.; Khan, I.; Helal, A.; Al-Maythalony, B.A.; et al. Propene adsorption-chemisorption behaviors on H-SAPO-34 zeolite catalysts at different temperatures. Catalysts 2019, 9, 919.
- Tian, H.; He, H.; Jiao, J.; Zha, F.; Guo, X.; Tang, X.; Chang, Y. Tandem catalysts composed of different morphology HZSM-5 and metal oxides for CO2 hydrogenation to aromatics. Fuel 2022, 314, 123119.
- Tada, S.; Kinoshita, H.; Ochiai, N.; Chokkalingam, A.; Hu, P.; Yamauchi, N.; Kobayashi, Y.; Iyoki, K. Search for solid acid catalysts aiming at the development of bifunctional tandem catalysts for the one-pass synthesis of lower olefins via CO2 hydrogenation. Int. J. Hydrog. Energy 2021, 46, 36721–36730.
- Tong, M.; Chizema, L.G.; Chang, X.; Hondo, E.; Dai, L.; Zeng, Y.; Zeng, C.; Ahmad, H.; Yang, R.; Lu, P. Tandem catalysis over tailored ZnO-ZrO2/MnSAPO-34 composite catalyst for enhanced light olefins selectivity in CO2 hydrogenation. Microporous Mesoporous Mater. 2021, 320, 111105.
- Mou, J.; Fan, X.; Liu, F.; Wang, X.; Zhao, T.; Chen, P.; Li, Z.; Yang, C.; Cao, J. CO2 hydrogenation to lower olefins over Mn2O3-ZnO/SAPO-34 tandem catalysts. Chem. Eng. J. 2021, 421, 129978.
- Dorner, R.W.; Hardy, D.R.; Williams, F.W.; Willauer, H.D. Heterogeneous catalytic CO2 conversion to value-added hydrocarbons. Energy Environ. Sci. 2010, 3, 884–890.
- Smith, Z.P.; Bachman, J.E.; Li, T.; Gludovatz, B.; Kusuma, V.A.; Xu, T.; Hopkinson, D.P.; Ritchie, R.O.; Long, J.R. Increasing M2(dobdc) loading in selective mixed-matrix membranes: A rubber toughening approach. Chem. Mater. 2018, 30, 1484–1495.
- Batchu, R.; Galvita, V.V.; Alexopoulos, K.; Van der Borght, K.; Poelman, H.; Reyniers, M.-F.; Marin, G.B. Role of intermediates in reaction pathways from ethene to hydrocarbons over H-ZSM-5. Appl. Catal. A Gen. 2017, 538, 207–220.
- Kotrel, S.; Knozinger, H.; Gates, B.C. The Haag–Dessau mechanism of protolytic cracking of alkanes. Microporous Mesoporous Mater. 2000, 35–36, 11–20.
- Sartipi, S.; Makkee, M.; Kapteijn, F.; Gascon, J. Catalysis engineering of bifunctional solids for the one-step synthesis of liquid fuels from syngas: A review. Catal. Sci. Technol. 2014, 4, 893–907.
- Hagen, A.; Roessner, F. Ethane to aromatic hydrocarbons: Past, present, Future. Catal. Rev. 2000, 42, 403–437.
- Joshi, Y.V.; Thomson, K.T. Brønsted Acid Catalyzed Cyclization of C7 and C8 Dienes in HZSM-5: A Hybrid QM/MM Study and Comparison with C6 Diene Cyclization. J. Phys. Chem. C 2008, 112, 12825–12833.
- Tabor, E.; Bernauer, M.; Wichterlova, B.; Dedecek, J. Enhancement of propene oligomerization and aromatization by proximate protons in zeolites; FTIR study of the reaction pathway in ZSM-5. Catal. Sci. Technol. 2019, 9, 4262–4275.
- Zhang, Z.; Huang, G.; Tang, X.; Yin, H.; Kang, J.; Zhang, Q.; Wang, Y. Zn and Na promoted Fe catalysts for sustainable production of high-valued olefins by CO2 hydrogenation. Fuel 2022, 309, 122105.
- Sun, Z.; Chen, X.; Lu, F.; Zhou, L.; Zhang, Y. Effect of Rb promoter on Fe3O4 microsphere catalyst for CO2 hydrogenation to light olefins. Catal. Commun. 2022, 162, 106387.
- Zhang, Z.; Liu, Y.; Jia, L.; Sun, C.; Chen, B.; Liu, R.; Tan, Y.; Tu, W. Effects of the reducing gas atmosphere on performance of FeCeNa catalyst for the hydrogenation of CO2 to olefins. Chem. Eng. J. 2022, 428, 131388.
- Al-Zakwani, S.S.; Maroufmashat, A.; Mazouz, A.; Fowler, M.; Elkamel, A. Allocation of Ontario’s Surplus Electricity to Different Power-to-Gas Applications. Energies 2019, 12, 2675.
- Castellani, B.; Gambelli, A.M.; Morini, E.; Nastasi, B.; Presciutti, A.; Filipponi, M.; Nicolini, A.; Rossi, F. Experimental Investigation on CO2 Methanation Process for Solar Energy Storage Compared to CO2-Based Methanol Synthesis. Energies 2017, 10, 855.
- Sterner, M.; Specht, M. Power-to-Gas and Power-to-X–The Hystory and Results of Developing a New Storage Concept. Energies 2021, 14, 6594.
- Garbarino, G.; Pugliese, F.; Cavattoni, T.; Busca, G.; Costamagna, P. A Study on CO2 Methanation and Steam Methane Reforming over Commercial Ni/Calcium Aluminate Catalysts. Energies 2020, 13, 2792.
- Rozzi, E.; Minuto, F.D.; Lanzini, A.; Leone, P. Green Synthetic Fuels: Renewable Routes for the Conversion of Non-Fossil Feedstocks into Gaseous Fuels and Their End Uses. Energies 2020, 13, 420.
- Cao, H.; Wang, W.; Cui, T.; Wang, H.; Zhu, G.; Ren, X. Enhancing CO2 Hydrogenation to Methane by Ni-Based Catalyst with V Species Using 3D-mesoporous KIT-6 as Support. Energies 2020, 13, 2235.
- Tsiotsias, A.I.; Charisiou, N.D.; Yentekakis, I.V.; Goula, M.A. Bimetallic Ni-based catalysts for CO2 methanation: A review. Nanomaterials 2021, 11, 28.
- Renda, S.; Ricca, A.; Palma, V. Study of the effect of noble metal promotion in Ni-based catalyst for the Sabatier reaction. Int. J. Hydrog. Energy 2020, 46, 12117–12127.
- Ghaib, K.; Nitz, K.; Ben-Fares, F.Z. Chemical methanation of CO2: A review. ChemBioEng Rev. 2016, 3, 266–275.
- Di Stasi, C.; Renda, S.; Greco, G.; González, B.; Palma, V.; Manyà, J.J. Wheat-straw-derived activated biochar as a renewable support of Ni-CeO2 catalysts for CO2 methanation. Sustainability 2021, 13, 8939.
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P.L. Supported catalysts for CO2 methanation: A review. Catalysts 2017, 7, 59.
- Lee, W.J.; Li, C.; Prajitno, H.; Yoo, J.; Patel, J.; Yang, Y.; Lim, S. Recent trend in thermal catalytic low temperature CO2 methanation: A critical review. Catal. Today 2021, 368, 2–19.
- Ashok, J.; Pati, S.; Hongmanorom, P.; Tianxi, Z.; Junmei, C.; Kawi, S. A review of recent catalyst advances in CO2 methanation processes. Catal. Today 2020, 356, 471–489.
- Fan, W.K.; Tahir, M. Recent trends in developments of active metals and heterogenous materials for catalytic CO2 hydrogenation to renewable methane: A review. J. Environ. Chem. Eng. 2021, 9, 105460.
- Huynh, H.L.; Yu, Z. CO2 Methanation on Hydrotalcite-Derived Catalysts and Structured Reactors: A Review. Energy Technol. 2020, 8, 1901475.
- Palma, V.; Goodall, R.; Thompson, A.; Ruocco, C.; Renda, S.; Leach, R.; Martino, M. Ceria-coated replicated aluminium sponges as catalysts for the CO-water gas shift process. Int. J. Hydrog. Energy 2021, 46, 12158–12168.
- Huynh, H.L.; Tucho, W.M.; Shen, Q.; Yu, Z. Bed packing configuration and hot-spot utilization for low-temperature CO2 methanation on monolithic reactor. Chem. Eng. J. 2022, 428, 131106.
- García-Moncada, N.; Navarro, J.C.; Odriozola, J.A.; Lefferts, L.; Faria, J.A. Enhanced catalytic activity and stability of nanoshaped Ni/CeO2 for CO2 methanation in micro-monoliths. Catal. Today 2022, 383, 205–215.
- Navarro, J.C.; Centeno, M.A.; Laguna, O.H.; Odriozola, J.A. Ru–Ni/MgAl2O4 structured catalyst for CO2 methanation. Renew. Energy 2020, 161, 120–132.
- Ratchahat, S.; Sudoh, M.; Suzuki, Y.; Kawasaki, W.; Watanabe, R.; Fukuhara, C. Development of a powerful CO2 methanation process using a structured Ni/CeO2 catalyst. J. CO2 Util. 2018, 24, 210–219.
- Vita, A.; Italiano, C.; Pino, L.; Laganà, M.; Ferraro, M.; Antonucci, V. High-temperature CO2 methanation over structured Ni/GDC catalysts: Performance and scale-up for Power-to-Gas application. Fuel Process. Technol. 2020, 202, 106365.
- Ricca, A.; Truda, L.; Palma, V. Study of the role of chemical support and structured carrier on the CO2 methanation reaction. Chem. Eng. J. 2019, 377, 120461.
- Cimino, S.; Cepollaro, E.M.; Lisi, L.; Fasolin, S.; Musiani, M.; Vázquez-Gómez, L. Ru/ce/ni metal foams as structured catalysts for the methanation of CO2. Catalysts 2021, 11, 13.
- Italiano, C.; Ferrante, G.D.; Pino, L.; Laganà, M.; Ferrato, M.; Antonucci, V.; Vita, A. Silicon carbide and alumina open-cell foams activated by Ni/CeO2-ZrO2 catalyst for CO2 methanation in a heat-exchanger reactor. Chem. Eng. J. 2022, 434, 134685.
- Sánchez, A.; Milt, V.G.; Miró, E.E.; Güttel, R. Impact of heat transport properties and configuration of ceramic fibrous catalyst structures for CO2 methanation: A simulation study. J. Environ. Chem. Eng. 2022, 10, 107148.
- Danaci, S.; Protasova, L.; Lefevere, J.; Bedel, L.; Guilet, R.; Marty, P. Efficient CO2 methanation over Ni/Al2O3 coated structured catalysts. Catal. Today 2016, 273, 234–243.
- Danaci, S.; Protasova, L.; Snijkers, F.; Bouwen, W.; Bengaouer, A.; Marty, P. Innovative 3D-manufacture of structured copper supports post-coated with catalytic material for CO2 methanation. Chem. Eng. Process.—Process Intensif. 2018, 127, 168–177.
- Fuentes, I.; Gracia, F. Fluid dynamic analytical model of CO2 methanation in a microreactor with potential application in Power-to-Gas technology. Chem. Eng. Sci. 2022, 251, 117465, in press.
- Catarina Faria, A.; Miguel, C.V.; Madeira, L.M. Thermodynamic analysis of the CO2 methanation reaction with in situ water removal for biogas upgrading. J. CO2 Util. 2018, 26, 271–280.
- Ohya, H.; Fun, J.; Kawamura, H.; Itoh, K.; Ohashi, H.; Aihara, M.; Tanisho, S.; Negishi, Y. Methanation of carbon dioxide by using membrane reactor integrated with water vapor permselective membrane and its analysis. J. Memb. Sci. 1997, 131, 237–247.
- Catarina Faria, A.; Miguel, C.V.; Rodrigues, A.E.; Madeira, L.M. Modeling and Simulation of a Steam-Selective Membrane Reactor for Enhanced CO2 Methanation. Ind. Eng. Chem. Res. 2020, 59, 16170–16184.
- Liu, Z.; Bian, Z.; Wang, Z.; Jiang, B. A CFD study on the performance of CO2 methanation in a water-permeable membrane reactor system. React. Chem. Eng. 2022, 7, 450–459.
- Miyamoto, M.; Hayakawa, R.; Makino, Y.; Oumi, Y.; Uemiya, S.; Asanuma, M. CO2 methanation combined with NH3 decomposition by in situ H2 separation using a Pd membrane reactor. Int. J. Hydrog. Energy 2014, 39, 10154–10160.
- Bian, Z.; Xia, H.; Zhong, W.; Jiang, B.; Yu, Y.; Wang, Z.; Yu, K. CFD simulation on hydrogen-membrane reactor integrating cyclohexane dehydrogenation and CO2 methanation reactions: A conceptual study. Energy Convers. Manag. 2021, 235, 113989.
- Palma, V.; Cortese, M.; Renda, S.; Ruocco, C.; Martino, M.; Meloni, E. A review about the recent advances in selected nonthermal plasma assisted solid–gas phase chemical processes. Nanomaterials 2020, 10, 1596.
- Bacariza, M.C.; Biset-Peiró, M.; Graça, I.; Guilera, J.; Morante, J.; Lopes, J.M.; Andreu, T.; Henriques, C. DBD plasma-assisted CO2 methanation using zeolite-based catalysts: Structure composition-reactivity approach and effect of Ce as promoter. J. CO2 Util. 2018, 26, 202–211.
- Ahmad, F.; Lovell, E.C.; Masood, H.; Cullen, P.J.; Ostrikov, K.K.; Scott, J.A.; Amal, R. Low-Temperature CO2 Methanation: Synergistic Effects in Plasma-Ni Hybrid Catalytic System. ACS Sustain. Chem. Eng. 2020, 8, 1888–1898.
- Wierzbicki, D.; Moreno, M.V.; Ognier, S.; Motak, M.; Grzybek, T.; Da Costa, P.; Gálvez, M.E. Ni-Fe layered double hydroxide derived catalysts for non-plasma and DBD plasma-assisted CO2 methanation. Int. J. Hydrog. Energy 2020, 45, 10423–10432.
- Biset-Peiró, M.; Mey, R.; Guilera, J.; Andreu, T. Adiabatic plasma-catalytic reactor configuration: Energy efficiency enhancement by plasma and thermal synergies on CO2 methanation. Chem. Eng. J. 2020, 393, 124786.
- Gao, Y.; Dou, L.; Zhang, S.; Zong, L.; Pan, J.; Hu, X.; Sun, H.; Ostrikov, K.; Shao, T. Coupling bimetallic Ni-Fe catalysts and nanosecond pulsed plasma for synergistic low-temperature CO2 methanation. Chem. Eng. J. 2021, 420, 127693.
- Gao, W.; Chen, H. Mechanochemical Synthesis of Ni−Y/CeO2 Catalyst for Nonthermal Plasma Catalytic CO2 Methanation. Ind. Eng. Chem. Res. 2022, 61, 1666–1674.
More
