Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 4 by Rita Xu and Version 3 by Rita Xu.
Actinomycetes are currently one of the major sources of bioactive secondary metabolites used for medicine development. Accumulating evidence has shown that Nocardiopsis, a key class of actinomycetes, has the ability to produce novel bioactive natural products.
- actinomycetes
- Nocardiopsis
- natural products
- bioactivities
- medicinal potentiality
1. Introduction
Actinomycetes belong to Gram-positive bacteria and are one of the biggest bacterial phyla [1][2]. The high G+C DNA content of actinomycetes implied their enormous biosynthetic potential to produce various natural products with diverse structures and important commercial applications [1][2]. Two-thirds of all naturally derived antibiotics have been discovered from actinobacteria [3][14]. Approximately 70% of the pharmaceutically active natural products which are currently used in clinics are isolated from actinobacteria [5][26][27][38], including a series of anticancer, antifungal, antibacterial, antihelminthic, and immunosuppressive drugs [13].
Nocardiopsis is an important genus of actinobacterium for its extensive application in agriculture [39], industry [10], and environmental protection [411], especially for its potential ability to produce new natural products [25][412]. By mid-2021, 3% of marine actinomycetes-derived natural products were produced by Nocardiopsis, and Nocardiopsis are the third-largest actinomycetes in terms of producing marine compounds [13]. In addition, Nocardiopsis are a prolific source of bioactive natural products in both marine and terrestrial environments [1] and are widely distributed in multiple ecosystems [412], including deserts [514], deep ocean [515], coastal wetlands [16], and saline–alkali soil [617]. Nocardiopsis species have the ability to survive under different and hostile environmental conditions, mainly benefitting from their excretion of enzymes, multipurpose genetic constitution, and their ability to produce compuponible solutes and surfactants [412][18][619][720]. Besides these characteristics, the members of this genus have the ability to produce abundant bioactive compounds, which may allow them to prevail in different habitats [412]. Extracts from cultivated Nocardiopsis have exhibited cytotoxic [721][22], antimicrobial [823][824], antifibrotic [25], and anti-inflammatory [25] activities. Comprehensive analyses of Nocardiopsis metabolites by LC-HRES-MS, HRMS, or GC/MS have led to the identification of bioactive and structurally diverse compounds [721][26][927][928]. The bioactive secondary metabolites isolated from Nocardiopsis include antimicrobial agents [1029][30][1031], tumor promoters [1132], cytotoxic compounds [33][1134], kinase and P-glycoprotein inhibitors [1235][1236], immunoregulators [37], and natural products with other multiple bioactivities [1338][39][1340]. The secondary metabolites discovered from Nocardiopsis have shown a great diversity of structural frameworks, including polyketides [1441][42][1443], alkaloids [1544][1545], and terpenoids [46][1647].
Bennur et al. reviewed the bioactive natural products derived from Nocardiopsis prior to 2015 [12], while Ibrahim et al. conducted a literature review regarding all of the secondary metabolites discovered from Nocardiopsis from 2016 to February 2018 [5]. The sources, distribution, bioactivities, biosynthesis, and structural characteristics of the compounds isolated from Nocardiopsis between March 2018 and 2021 are comprehensively summarized.
2. Polyketides
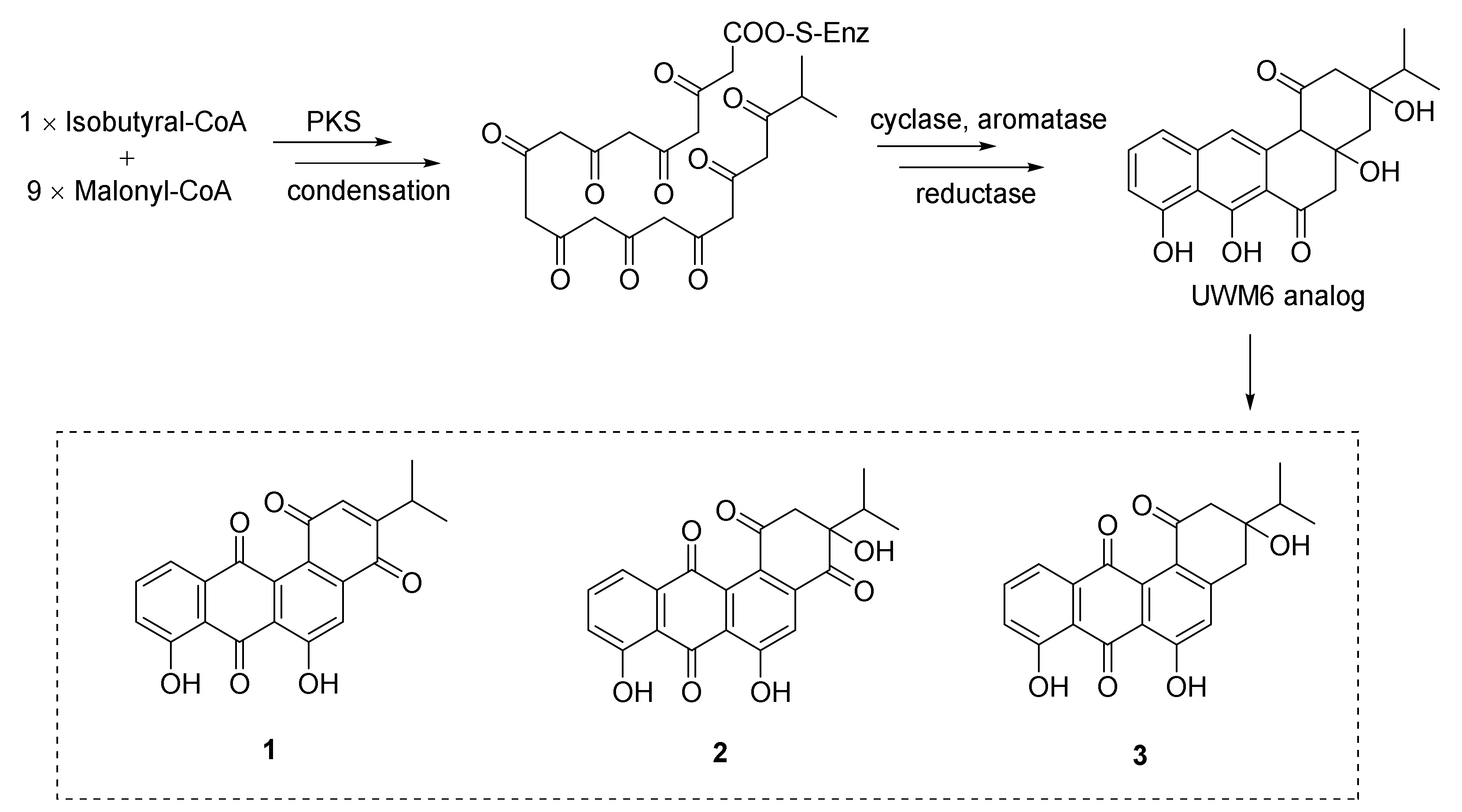
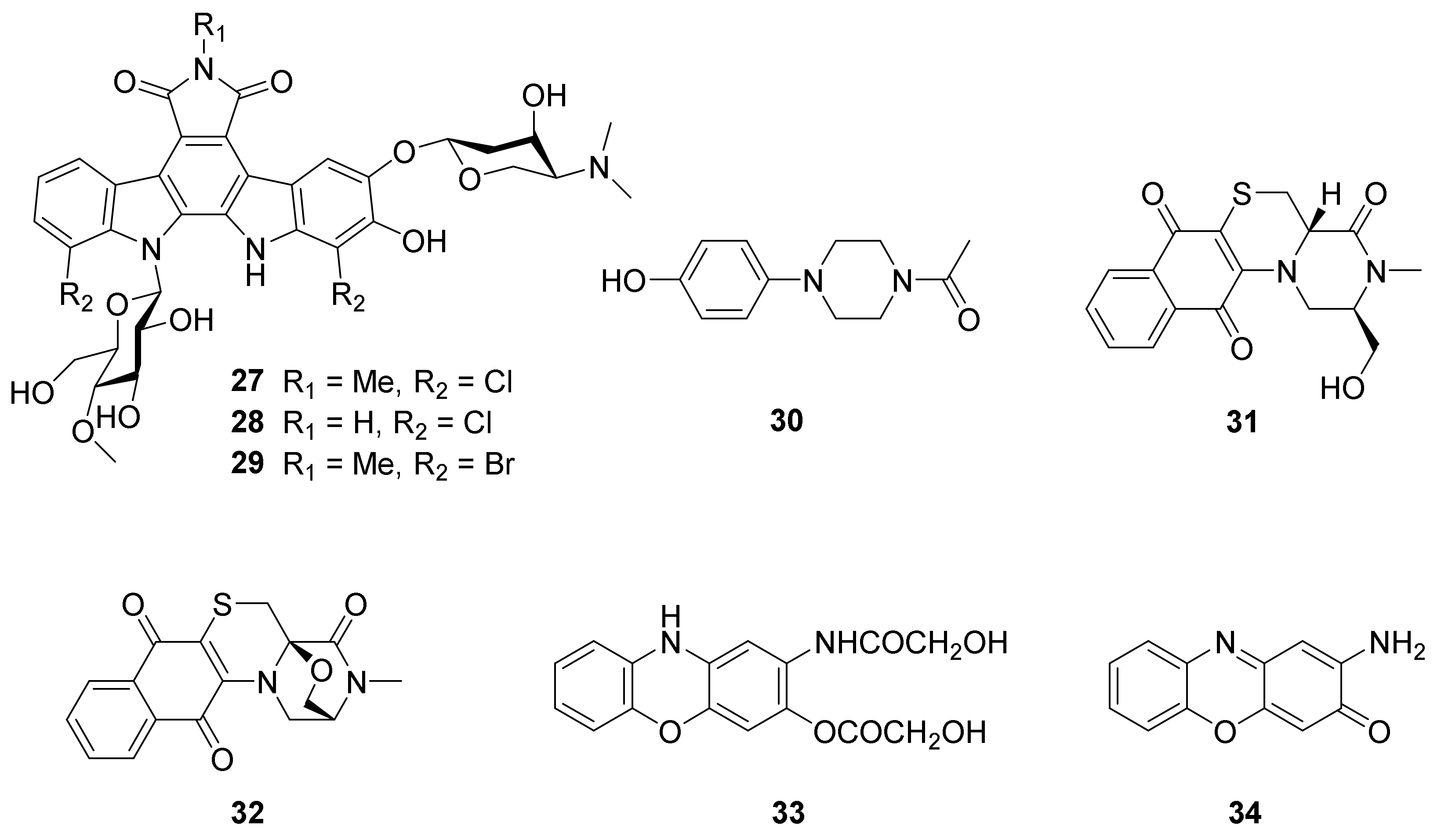
Three new angucyclines, nocardiopsistins A–C (1–3), were obtained from the deep-sea sponge-derived Nocardiopsis sp. strain HB-J378 (Figure 1) [1648]. The antibacterial activities of compounds 1–3 were tested against MRSA (methicillin-resistant Staphylococcus aureus) and 1–3 exhibited antibacterial activity with MIC values of 3.12–12.5 μg/mL. Three core genes were identified through bioinformatic analysis of the sketch genome of the strain HB-J378 in a biosynthetic gene cluster encoding a typical aromatic or type II polyketide synthase (PKS) system, including acyl carrier protein (ACP), ketoacyl: ACP synthase α-subunit (KSα) and β-subunit (KSβ). The brief biosynthetic route for 1–3 was proposed according to the discovered oviedomycin pathway [49]. Compounds 1–3 were supposed to be biosynthesized by taking advantage of one molecule of isobutyral-CoA and nine molecules of malonyl-CoA, through complex enzymatic reactions to obtain the key angucycline biosynthetic intermediate UWM6, an analog of 1–3 [1750], to acquire 1–3 (see Figure 1) [1648].
The new strain
Nocardiopsis
sp. CG3 (DSM 106572), collected from the saltpan of Kenadsa (22 km west of Bechar, located in southwest Algeria), was discovered to have the ability to produce new bioactive natural products in a screening program
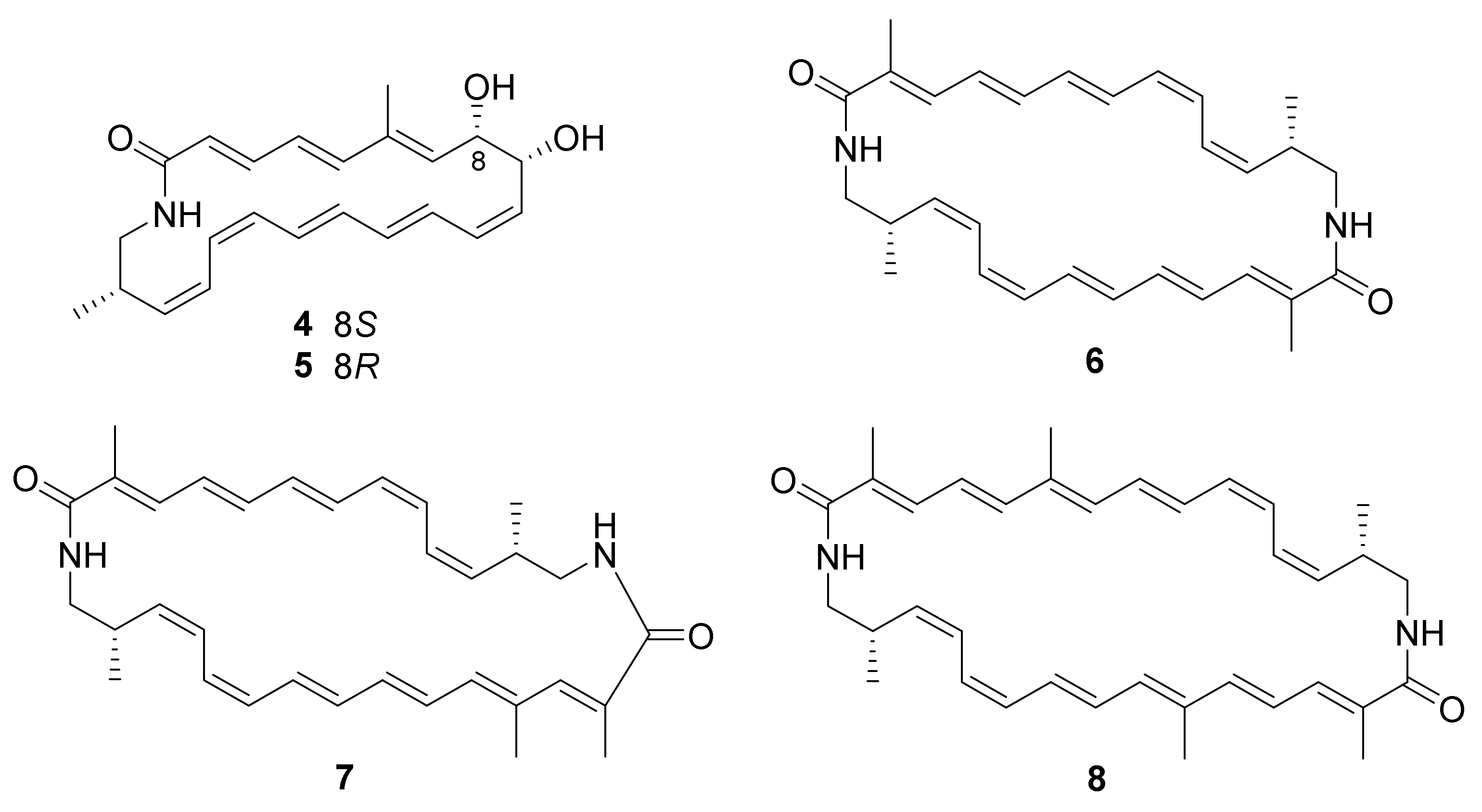
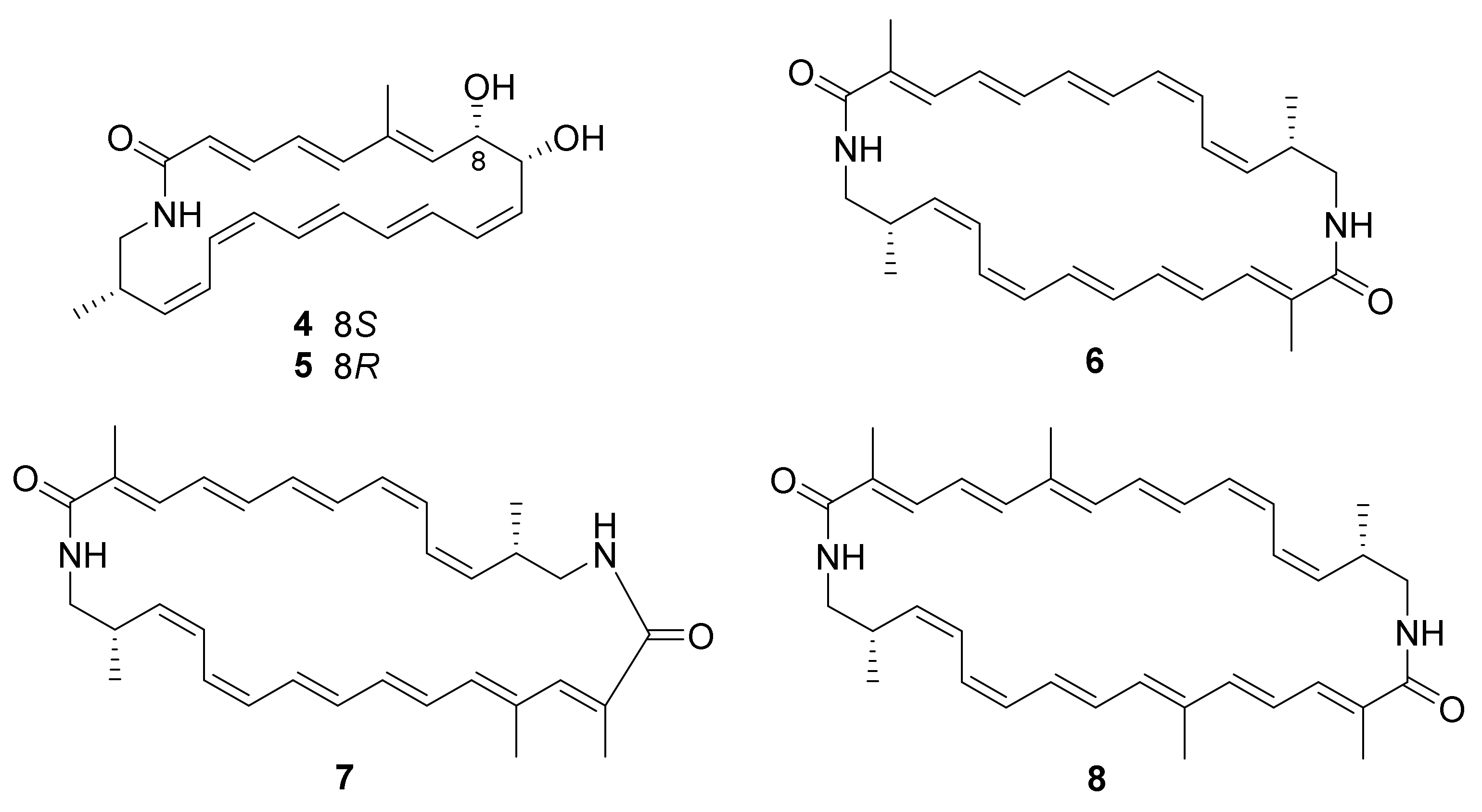
. Chemical investigation of the strain led to the isolation of five new polyene macrolactams, kenalactams A−E (
4
−
8
) (
Figure 2
)
. The biosynthetic pathway of polyketide kenalactam A (
4
) was studied by feeding experiments and was found to used L-alanine as the nitrogen-bearing starter unit. Compounds
6
−
8
exhibited cytotoxic activity against HeLa (cervical cancer cells KB3.1) and PC-3 (human prostate cancer) cell lines, with IC
50
values ranging from 2.1 to 6.8 μM
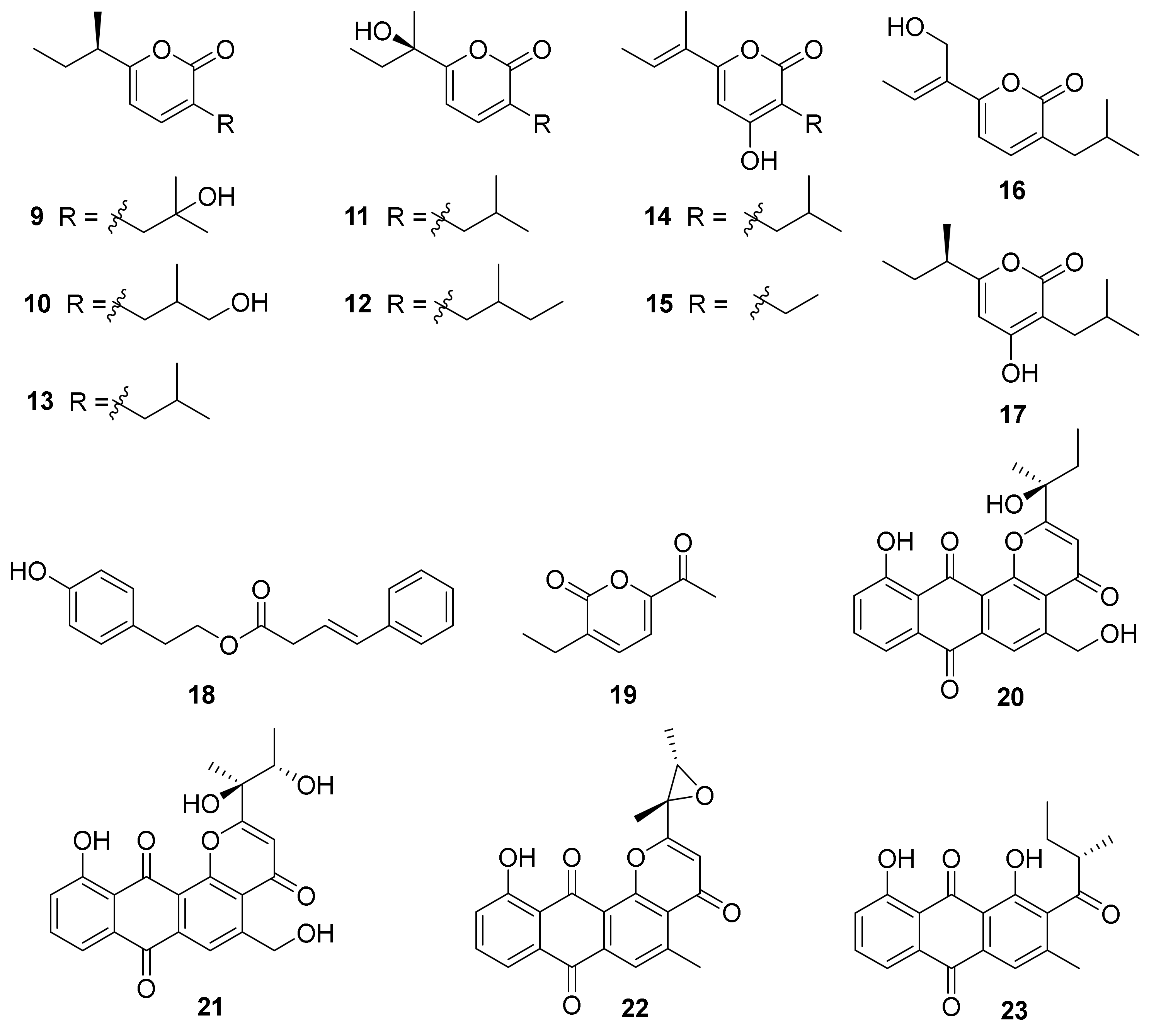
Eight new α-pyrone derivatives, nocahypyrones A–H (9–16), along with one known analog, germicidin G (17) (Figure 3) were isolated from the strains Nocardiopsis sp. HDN154-146 and HDN154-168, which were collected from soil samples derived from the Takla Makan desert area in Xinjiang Province, China. This was the first time it was reported that compounds 13 and 16 showed cytoprotective activity by inducing the expression of phase II detoxifying enzymes [52]. Aldo-keto reductase family1 member C1 (AKR1C1), human NAD(P)H: quinone oxidoreductase 1 (NQO1), superoxide dismutase 2 (SOD2), and heme oxygenase 1(HO-1), belonging to phase II detoxifying enzymes, have been illustrated to possess significant roles in defending mammalian cells against oxidative damage and excessive inflammatory reaction [1853][1854]. Compounds 13 and 16 displayed cytoprotective activity by inducing the expression level of SOD2 and HO-1 in HaCaT cells [52].
Chemical investigation of the actinobacterium Nocardiopsis sp. HDN 17-237 led to the isolation of one new β,γ-butenoate derivative, phenylbutenote (18), and one new α-pyrone, nocapyrone T (19) (Figure 3). The strain HDN 17-237 was collected from deep-sea water from the Mariana Trench (depth 4448 m, 10°21.100′ N, 142°17.574′ E, gathered in September 2016). Compounds 18 and 19 were evaluated for antibacterial and antioxidant activities, while neither of them exhibited obvious activity [55].
Comprehensive research of the secondary metabolites of an Antarctic marine animal sample-derived actinomycete, N. aegyptia HDN19-252, combined with a molecular networking approach in the Global Natural Products Social (GNPS) platform, obtained the isolation of four new anthraquinone derivatives, saliniquinones G−I (20–22) and heraclemycin E (23) (Figure 3). Compounds 20 and 21 showed potent antibacterial activities against six evaluated bacterial strains—Bacillus subtilis, methicillin-resistant coagulase-negative staphylococci (MRCNS), B. cereus, Proteus sp., Mycobacterium phlei, and Escherichia coli—with MIC values of 3.1−12.5 µM [1956].
Three new macrolides, borrelidins C−E, along with one known analog, borrelidin, which showed antibacterial and anticancer activities, were isolated from a saltern-derived halophilic Nocardiopsis sp. in 2017 [43]. These borrelidins were found to have the ability to relieve the amyloid-β induced toxicity in the HT22 cell line in 2021, indicating the potential of borrelidins to be developed as Alzheimer’s disease drugs [1957].
3. Alkaloids
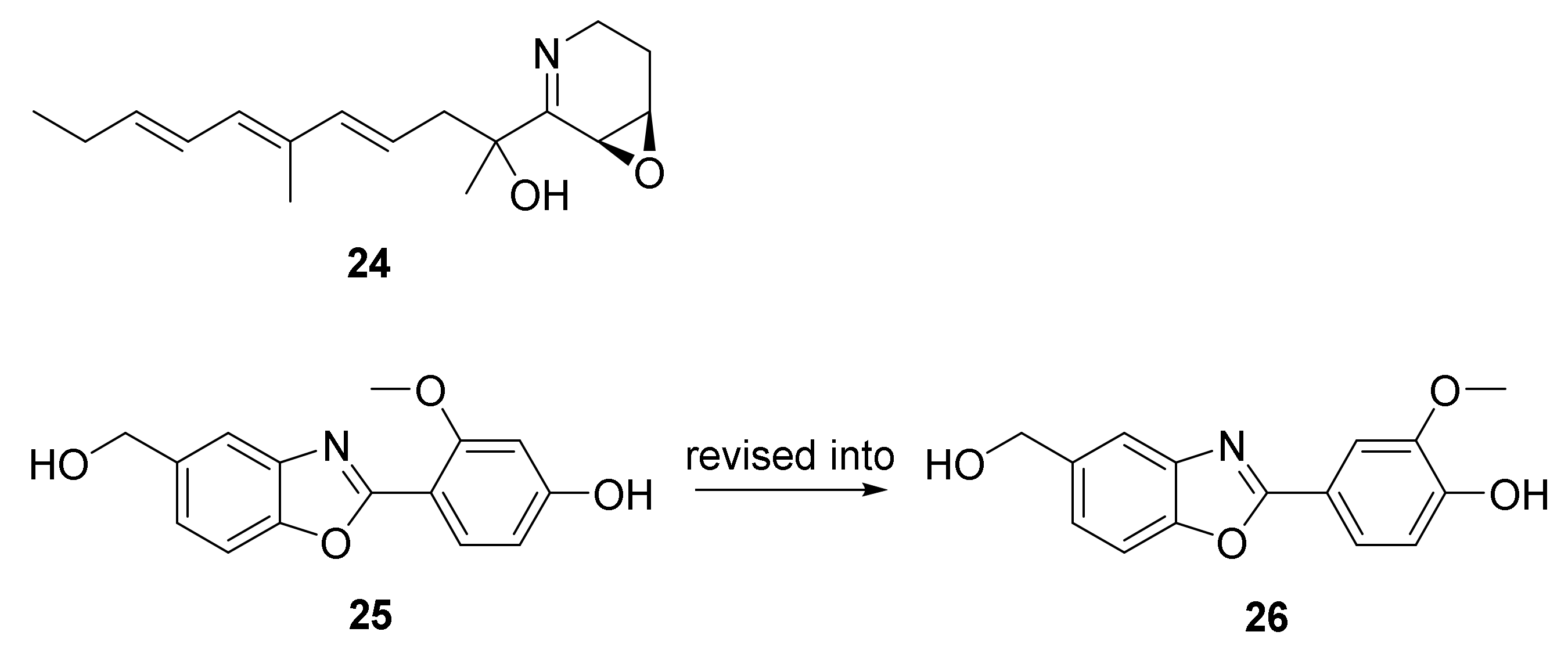
The approach of an optimized nitroso-based probe (dienophile probe), promoting the detection of compounds containing conjugated alkenes in crude broth extracts, was employed in the chemical investigation of a marine-derived Nocardiopsis sp. CNY-503 and gained the isolation of one new polyketide alkaloid, named nocarditriene (24), which contained an unprecedented epoxy-2,3,4,5-tetrahydropyridine structure (Figure 4) [58].
The structure of nocarbenzoxazole G (25), isolated from the marine-derived actinomycete N. lucentensis DSM 44048 in 2015 [2060], was revised into the nocarbenzoxazole G (26) molecule by total synthesis in 2019. The benzoxazole skeleton was constructed with microwave assistance and continued by carbon–carbon bond formation with relevant aryl bromides [2059]. Compound 26 was found to display moderate cytotoxicity against HepG2 and HeLa cell lines with IC50 values of 16 and 14 μM, respectively [2060].
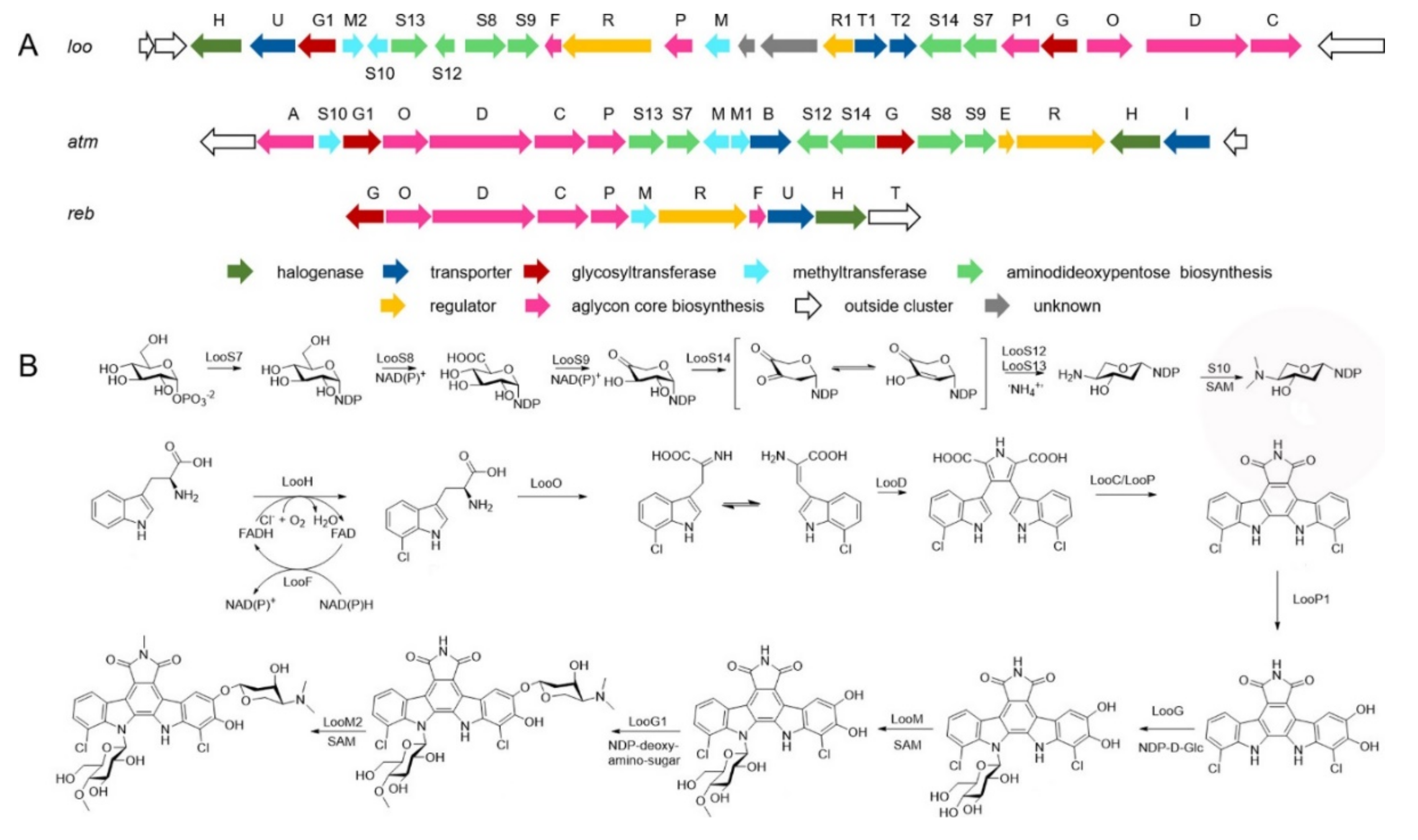
Chemical investigation of the strain N. flavescens NA01583, which was gained from marine sediment gathered at the coast near Hainan Island in 2016 through the genome mining of an indolocarbazole-type gene cluster, led to the isolation of three new indolocarbazole alkaloids, named loonamycins A−C (27−29) (Figure 5) [61].
Compound 29 was produced successfully by the allogenetic expression of the complete loo gene cluster in a vicarious host, Streptomyces lividans K4−114. The indolocarbazole skeleton of 27−29, belonging to the family of indolocarbazole alkaloids, is structurally similar to that of rebeccamycin and staurosporine with an additional rare modified tryptophan ring. The molecular bases of these modifications were investigated by carefully analyzing loo BGC for further genome mining and combinational biosynthetic research. The loo gene cluster sustains a ∼36 kb continuous DNA sequence, including 25 open reading frames which take charge of biosynthesis, regulation, and resistance (Figure 6A). A possible biosynthetic pathway for loonamycin was detected based on this bioinformatic analysis (Figure 6B). In particular, compound 27 showed potent cytotoxic activities toward eight cancer cell lines, including Sum1315 (breast cancer), SH-SY5Y (neuroblastoma), HCT116 (colorectal cancer), HT29 (colorectal cancer), HCC78 (lung cancer), HeLa (cervical cancer), SW620 (colorectal cancer), and SW872 (liposarcoma), with IC50 values of 41−283 nM [61].
The broth culture crude extracts of actinobacterium Nocardiopsis sp. SCA30, derived from marine sediments collected from Havelock Island, Andaman, and the Nicobar Islands, India (11.96° N, 93.00° E), displayed cytotoxic activities against a series of cell lines, including HT 29, HCT 15, MDA-MB 468, and MCF 7 at concentrations ranging from 62.5 to 1000 µg/mL. The strain extracts also showed antibacterial activities against MRSA ATCC NR-46171 and NR-46071 with MIC values of 7.81 and 15.62 µg/mL, respectively. Compound 1-acetyl-4-4(hydroxyphenyl)piperazine (30) (Figure 5) was isolated from the crude extracts of Nocardiopsis sp. SCA30 through LC-MS analysis and NMR chemical structural identification approved to be an antibacterial and cytotoxic compound [22].
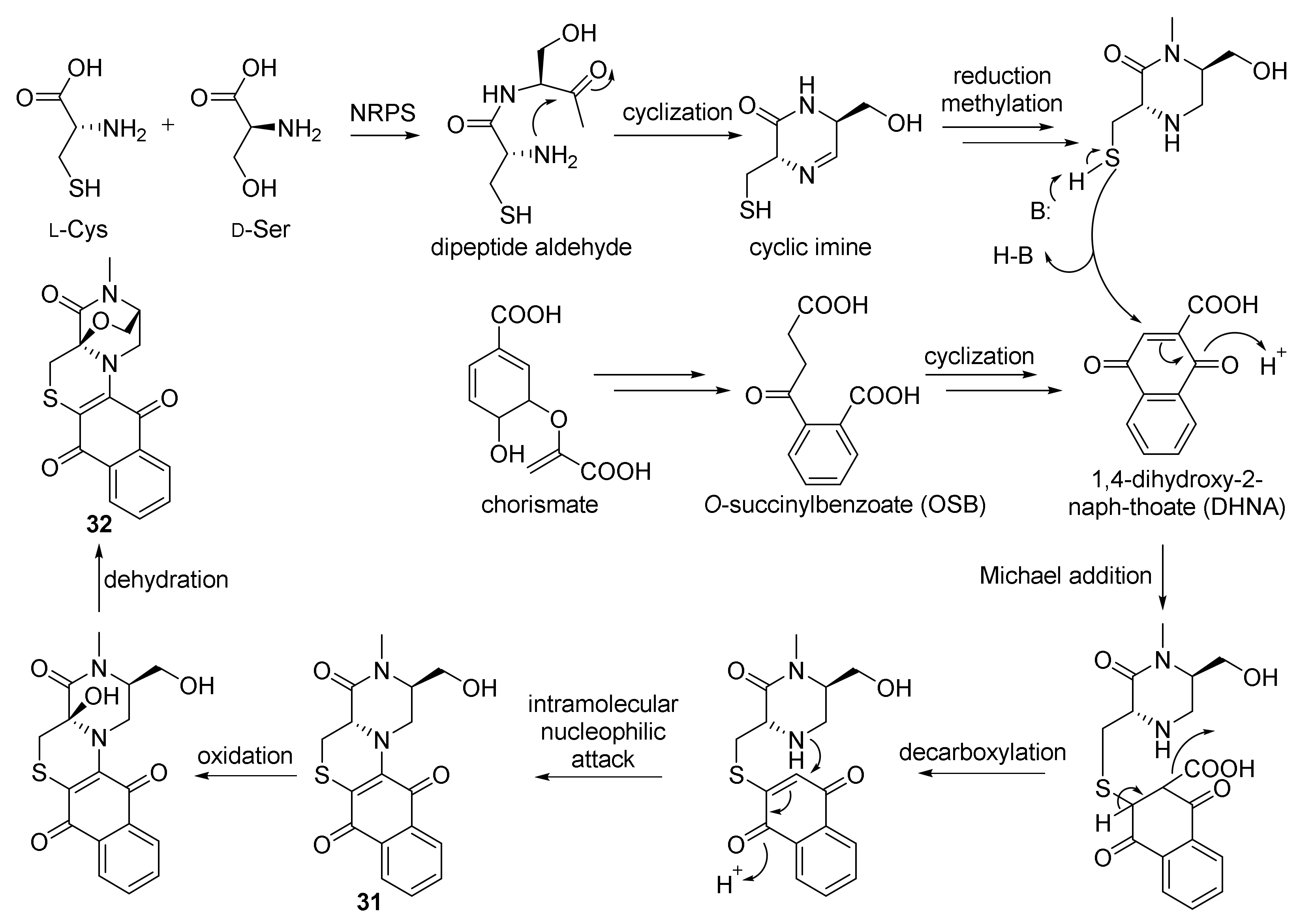
Chemical investigation of actinomycete N. dassonvillei SCSIO 40065 derived from the marine sponge Petrosia sp., which was collected on the seabed near Yongxing Island in the South China Sea at a depth of 20 m, led to the isolation of two polycyclic thioalkaloides, dassonmycins A (31) and B (32) (Figure 5). The new isolated compounds 31 and 32 contained the skeleton of a 6/6/6/6-fused tetracyclic ring featuring a naphthoquinone [2,3-e] piperazine-[1,2-c] thiomorpholine. Both compounds exhibited antibacterial activities against Micrococcus luteus SCSIO ML01, B. subtilis 1064, MRSA shhsA1, and S. aureus ATCC 29213 with MICs of 8−64 μg/mL. Compound 31 was found to display weak inhibited growth of Vibrio alginolyticus ATCC 13214 and Enterococcus faecalis ATCC 29212, with MIC values of 32 μg/mL. Compounds 31 and 32 exhibited moderate cytotoxicity against four human cancer cell lines—HepG-2, SF-268, MCF-7, and A549—with IC50 values of 12−34 μM [2162]. Biosynthetically, 31 and 32 are proposed to be biosynthesized by a non-ribosomal peptide synthetase (NRPS) route combined with a chorismate pathway (Figure 7) [2162].
A marine sediment-derived actinobacterium N. dassonvillei JS106 showed potent antiquorum sensing activities against S. aureus and Pseudomonas aeruginosa [2163]. The marine sediment sample was gathered from Lianyungang, China. Secondary metabolites research of the strain JS106 led to the isolation of one new compound, 2-hydroxyacetate-3-hydroxyacetamido-phenoxazine (HHP, 33), and one known analog questiomycin A (34) (Figure 5). Both of these two compounds (33 and 34) exhibited antibiofilm activity against Chromobacterium violaceum 12472 with IC50 values of 23.59 and 6.82 μg/mL, respectively [2163].
References
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Clement, C.; Meier-Kolthoff, J.P.; Klenk, H.-P.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. Jose, P.A.; Maharshi, A.; Jha, B. Actinobacteria in natural products research: Progress and prospects. Microbiol. Res. 2021, 246, 126708.
- Mahajan, G.B.; Balachandran, L. Antibacterial agents from actinomycetes—A review. Front. Biosci. Elite Ed. 2012, E4, 240–253. Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Clement, C.; Meier-Kolthoff, J.P.; Klenk, H.-P.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43.
- Li, J.W.H.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. Demain, A.L.; Sanchez, S. Microbial drug discovery: 80 years of progress. J. Antibiot. 2009, 62, 5–16.
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.-K. Pharmaceutically active secondary metabolites of marine actinobacteria. Microbiol. Res. 2014, 169, 262–278. Mahajan, G.B.; Balachandran, L. Antibacterial agents from actinomycetes—A review. Front. Biosci. Elite Ed. 2012, E4, 240–253.
- Adenan, N.H.; Lim, Y.Y.; Ting, A.S.Y. Nocardiopsis sp. for the removal of triphenylmethane dyes: Decolorization and optimization studies. Water Air Soil Pollut. 2021, 232, 414. Ibrahim, A.H.; Desoukey, S.Y.; Fouad, M.A.; Abdelmohsen, U.R.; Kamel, M.S.; Gulder, T.A.M. Natural product potential of the genus Nocardiopsis. Mar. Drugs 2018, 16, 147.
- Bennur, T.; Ravi Kumar, A.; Zinjarde, S.S.; Javdekar, V. Nocardiopsis species: A potential source of bioactive compounds. J. Appl. Microbiol. 2016, 120, 1–16. Li, J.W.H.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165.
- Kumar, S.; Solanki, D.S.; Parihar, K.; Tak, A.; Gehlot, P.; Pathak, R.; Singh, S.K. Actinomycetes isolates of arid zone of Indian Thar Desert and efficacy of their bioactive compounds against human pathogenic bacteria. Biol. Futur. 2021, 72, 431–440. Manivasagan, P.; Kang, K.-H.; Sivakumar, K.; Li-Chan, E.C.Y.; Oh, H.-M.; Kim, S.-K. Marine actinobacteria: An important source of bioactive natural products. Environ. Toxicol. Pharmacol. 2014, 38, 172–188.
- Chen, L.; Wang, Z.; Du, S.; Wang, G. Antimicrobial activity and functional genes of actinobacteria from coastal wetland. Curr. Microbiol. 2021, 78, 3058–3067. Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.-K. Pharmaceutically active secondary metabolites of marine actinobacteria. Microbiol. Res. 2014, 169, 262–278.
- Li, H.-W.; Zhi, X.-Y.; Yao, J.-C.; Zhou, Y.; Tang, S.-K.; Klenk, H.-P.; Zhao, J.; Li, W.-J. Comparative genomic analysis of the genus Nocardiopsis provides new insights into its genetic mechanisms of environmental adaptability. PLoS ONE 2013, 8, e61528. AbdElgawad, H.; Zinta, G.; Abuelsoud, W.; Hassan, Y.M.; Alkhalifah, D.H.M.; Hozzein, W.N.; Zrieq, R.; Beemster, G.T.; Schoenaers, S. An actinomycete strain of Nocardiopsis lucentensis reduces arsenic toxicity in barley and maize. J. Hazard. Mater. 2021, 417, 126055.
- Bennur, T.; Kumar, A.R.; Zinjarde, S.; Javdekar, V. Nocardiopsis species: Incidence, ecological roles and adaptations. Microbiol. Res. 2015, 174, 33–47. Adenan, N.H.; Lim, Y.Y.; Ting, A.S.Y. Nocardiopsis sp. for the removal of triphenylmethane dyes: Decolorization and optimization studies. Water Air Soil Pollut. 2021, 232, 414.
- Siddharth, S.; Aswathanarayan, J.B.; Kuruburu, M.G.; Madhunapantula, S.R.V.; Vittal, R.R. Diketopiperazine derivative from marine actinomycetes Nocardiopsis sp. SCA30 with antimicrobial activity against MRSA. Arch. Microbiol. 2021, 203, 6173–6181. Patel, G.B.; Rakholiya, P.; Shindhal, T.; Varjani, S.; Tabhani, N.; Shah, K.R. Lipolytic Nocardiopsis for reduction of pollution load in textile industry effluent and SWISS model for structural study of lipase. Bioresour. Technol. 2021, 341, 125673.
- Goel, N.; Fatima, S.W.; Kumar, S.; Sinha, R.; Khare, S.K. Antimicrobial resistance in biofilms: Exploring marine actinobacteria as a potential source of antibiotics and biofilm inhibitors. Biotechnol. Rep. 2021, 30, e00613. Bennur, T.; Ravi Kumar, A.; Zinjarde, S.S.; Javdekar, V. Nocardiopsis species: A potential source of bioactive compounds. J. Appl. Microbiol. 2016, 120, 1–16.
- Sarmiento-Vizcaíno, A.; Martín, J.; Reyes, F.; García, L.A.; Blanco, G. Bioactive natural products in actinobacteria isolated in rainwater from storm clouds transported by western winds in Spain. Front. Microbiol. 2021, 12, 773095. Chen, J.; Xu, L.; Zhou, Y.; Han, B. Natural products from actinomycetes associated with marine organisms. Mar. Drugs 2021, 19, 629.
- Widada, J.; Damayanti, E.; Alhakim, M.R.; Yuwono, T.; Mustofa, M. Two strains of airborne Nocardiopsis alba producing different volatile organic compounds (VOCs) as biofungicide for Ganoderma boninense. FEMS Microbiol. Lett. 2021, 368, fnab138. Kumar, S.; Solanki, D.S.; Parihar, K.; Tak, A.; Gehlot, P.; Pathak, R.; Singh, S.K. Actinomycetes isolates of arid zone of Indian Thar Desert and efficacy of their bioactive compounds against human pathogenic bacteria. Biol. Futur. 2021, 72, 431–440.
- Lu, C.; Li, Y.; Wang, H.; Wang, B.; Shen, Y. A new phenoxazine derivative isolated from marine sediment actinomycetes, Nocardiopsis sp. 236. Drug Discov. Ther. 2013, 7, 101–104. Pinto-Almeida, A.; Bauermeister, A.; Luppino, L.; Grilo, I.R.; Oliveira, J.; Sousa, J.R.; Petras, D.; Rodrigues, C.F.; Prieto-Davo, A.; Tasdemir, D.; et al. The diversity, metabolomics profiling, and the pharmacological potential of actinomycetes isolated from the Estremadura Spur Pockmarks (Portugal). Mar. Drugs 2022, 20, 21.
- Yamashita, T.; Imoto, M.; Isshiki, K.; Sawa, T.; Naganawa, H.; Kurasawa, S.; Zhu, B.; Umezawa, K. Isolation of a new indole alkaloid, pendolmycin, from Nocardiopsis. J. Nat. Prod. 1988, 51, 1184–1187. Chen, L.; Wang, Z.; Du, S.; Wang, G. Antimicrobial activity and functional genes of actinobacteria from coastal wetland. Curr. Microbiol. 2021, 78, 3058–3067.
- Gao, X.; Lu, Y.; Xing, Y.; Ma, Y.; Lu, J.; Bao, W.; Wang, Y.; Xi, T. A novel anticancer and antifungus phenazine derivative from a marine actinomycete BM-17. Microbiol. Res. 2012, 167, 616–622. Tatar, D. Isolation, phylogenetic analysis and antimicrobial activity of halophilic actinomycetes from different saline environments located near Corum province. Biologia 2021, 76, 773–780.
- Raju, R.; Piggott, A.M.; Huang, X.-C.; Capon, R.J. Nocardioazines: A novel bridged diketopiperazine scaffold from a marine-derived bacterium inhibits P-glycoprotein. Org. Lett. 2011, 13, 2770–2773. Li, H.-W.; Zhi, X.-Y.; Yao, J.-C.; Zhou, Y.; Tang, S.-K.; Klenk, H.-P.; Zhao, J.; Li, W.-J. Comparative genomic analysis of the genus Nocardiopsis provides new insights into its genetic mechanisms of environmental adaptability. PLoS ONE 2013, 8, e61528.
- Wu, Z.; Xie, L.; Xia, G.; Zhang, J.; Nie, Y.; Hu, J.; Wang, S.; Zhang, R. A new tetrodotoxin-producing actinomycete, Nocardiopsis dassonvillei, isolated from the ovaries of puffer fish Fugu rubripes. Toxicon 2005, 45, 851–859. Bennur, T.; Kumar, A.R.; Zinjarde, S.; Javdekar, V. Nocardiopsis species as potential sources of diverse and novel extracellular enzymes. Appl. Microbiol. Biotechnol. 2014, 98, 9173–9185.
- Kim, Y.; Ogura, H.; Akasaka, K.; Oikawa, T.; Matsuura, N.; Imada, C.; Yasuda, H.; Igarashi, Y. Nocapyrones: α- and γ-pyrones from a marine-derived Nocardiopsis sp. Mar. Drugs 2014, 12, 4110–4125. Bennur, T.; Kumar, A.R.; Zinjarde, S.; Javdekar, V. Nocardiopsis species: Incidence, ecological roles and adaptations. Microbiol. Res. 2015, 174, 33–47.
- Zhang, H.; Saurav, K.; Yu, Z.; Mandi, A.; Kurtan, T.; Li, J.; Tian, X.; Zhang, Q.; Zhang, W.; Zhang, C. α-Pyrones with diverse hydroxy substitutions from three marine-derived Nocardiopsis strains. J. Nat. Prod. 2016, 79, 1610–1618. Shady, N.H.; Tawfike, A.F.; Yahia, R.; Fouad, M.A.; Brachmann, A.O.; Piel, J.; Abdelmohsen, U.R.; Kamel, M.S. Cytotoxic activity of actinomycetes Nocardia sp. and Nocardiopsis sp. associated with marine sponge Amphimedon sp. Nat. Prod. Res. 2021, 1–6.
- Siddharth, S.; Aswathanarayan, J.B.; Kuruburu, M.G.; Madhunapantula, S.R.V.; Vittal, R.R. Diketopiperazine derivative from marine actinomycetes Nocardiopsis sp. SCA30 with antimicrobial activity against MRSA. Arch. Microbiol. 2021, 203, 6173–6181.
- Trivedi, N.; Thumar, J. Chemical profiling of antimicrobial metabolites from halophilic actinomycete Nocardiopsis sp. Al-H10-1 (KF384482) isolated from Alang, Gulf of Khambhat, India. bioRxiv 2021.
- Goel, N.; Fatima, S.W.; Kumar, S.; Sinha, R.; Khare, S.K. Antimicrobial resistance in biofilms: Exploring marine actinobacteria as a potential source of antibiotics and biofilm inhibitors. Biotechnol. Rep. 2021, 30, e00613.
- Choi, G.; Kim, G.J.; Choi, H.; Choi, I.-W.; Lee, D.-S. Anti-inflammatory and anti-fibrotic activities of Nocardiopsis sp. 13G027 in lipopolysaccharides-induced RAW 264.7 macrophages and transforming growth factor beta-1-stimulated nasal polyp-derived fibroblasts. Microbiol. Biotechnol. Lett. 2021, 49, 543–551.
- Sarmiento-Vizcaíno, A.; Martín, J.; Reyes, F.; García, L.A.; Blanco, G. Bioactive natural products in actinobacteria isolated in rainwater from storm clouds transported by western winds in Spain. Front. Microbiol. 2021, 12, 773095.
- Gamaleldin, N.M.; Bakeer, W.; El-Gendy, A.O.; Sayed, A.M.; Shamikh, Y.I.; Hassan, H.M.; Horn, H.; Abdelmohsen, U.R.; Hozzein, W.N. Exploration of chemical diversity and antitrypanosomal activity of some Red Sea-derived actinomycetes using the OSMAC approach supported by LC-MS-based metabolomics and molecular modelling. Antibiotics 2020, 9, 629.
- Widada, J.; Damayanti, E.; Alhakim, M.R.; Yuwono, T.; Mustofa, M. Two strains of airborne Nocardiopsis alba producing different volatile organic compounds (VOCs) as biofungicide for Ganoderma boninense. FEMS Microbiol. Lett. 2021, 368, fnab138.
- Wang, Z.; Fu, P.; Liu, P.; Wang, P.; Hou, J.; Li, W.; Zhu, W. New pyran-2-ones from alkalophilic actinomycete, Nocardiopsis alkaliphila sp. nov. YIM-80379. Chem. Biodivers. 2013, 10, 281–287.
- Lu, C.; Li, Y.; Wang, H.; Wang, B.; Shen, Y. A new phenoxazine derivative isolated from marine sediment actinomycetes, Nocardiopsis sp. 236. Drug Discov. Ther. 2013, 7, 101–104.
- Tian, S.; Yang, Y.; Liu, K.; Xiong, Z.; Xu, L.; Zhao, L. Antimicrobial metabolites from a novel halophilic actinomycete Nocardiopsis terrae YIM 90022. Nat. Prod. Res. 2014, 28, 344–346.
- Yamashita, T.; Imoto, M.; Isshiki, K.; Sawa, T.; Naganawa, H.; Kurasawa, S.; Zhu, B.; Umezawa, K. Isolation of a new indole alkaloid, pendolmycin, from Nocardiopsis. J. Nat. Prod. 1988, 51, 1184–1187.
- Lin, Z.; Torres, J.P.; Ammon, M.A.; Marett, L.; Teichert, R.W.; Reilly, C.A.; Kwan, J.C.; Hughen, R.W.; Flores, M.; Tianero, M.D.; et al. A Bacterial source for mollusk pyrone polyketides. Chem. Biol. 2013, 20, 73–81.
- Gao, X.; Lu, Y.; Xing, Y.; Ma, Y.; Lu, J.; Bao, W.; Wang, Y.; Xi, T. A novel anticancer and antifungus phenazine derivative from a marine actinomycete BM-17. Microbiol. Res. 2012, 167, 616–622.
- Kase, H.; Iwahashi, K.; Matsuda, Y. K-252a, a potent inhibitor of protein kinase C from microbial origin. J. Antibiot. 1986, 39, 1059–1065.
- Raju, R.; Piggott, A.M.; Huang, X.-C.; Capon, R.J. Nocardioazines: A novel bridged diketopiperazine scaffold from a marine-derived bacterium inhibits P-glycoprotein. Org. Lett. 2011, 13, 2770–2773.
- Kim, M.C.; Kwon, O.-W.; Park, J.-S.; Kim, S.Y.; Kwon, H.C. Nocapyrones H–J, 3,6-disubstituted α-pyrones from the marine actinomycete Nocardiopsis sp. KMF-001. Chem. Pharm. Bull. 2013, 61, 511–515.
- Wu, Z.; Xie, L.; Xia, G.; Zhang, J.; Nie, Y.; Hu, J.; Wang, S.; Zhang, R. A new tetrodotoxin-producing actinomycete, Nocardiopsis dassonvillei, isolated from the ovaries of puffer fish Fugu rubripes. Toxicon 2005, 45, 851–859.
- Peltola, J.S.P.; Andersson, M.A.; Kampfer, P.; Auling, G.; Kroppenstedt, R.M.; Busse, H.-J.; Salkinoja-Salonen, M.S.; Rainey, F.A. Isolation of toxigenic Nocardiopsis strains from indoor environments and description of two new Nocardiopsis species, N. exhalans sp. nov. and N. umidischolae sp. nov. Appl. Environ. Microbiol. 2001, 67, 4293–4304.
- Kim, Y.; Ogura, H.; Akasaka, K.; Oikawa, T.; Matsuura, N.; Imada, C.; Yasuda, H.; Igarashi, Y. Nocapyrones: α- and γ-pyrones from a marine-derived Nocardiopsis sp. Mar. Drugs 2014, 12, 4110–4125.
- Sun, M.-W.; Guo, Z.-X.; Lu, C.-H. Two new polyketides from Nocardiopsis lucentensis DSM 44048. Nat. Prod. Res. 2016, 30, 1036–1041.
- Zhang, H.; Saurav, K.; Yu, Z.; Mandi, A.; Kurtan, T.; Li, J.; Tian, X.; Zhang, Q.; Zhang, W.; Zhang, C. α-Pyrones with diverse hydroxy substitutions from three marine-derived Nocardiopsis strains. J. Nat. Prod. 2016, 79, 1610–1618.
- Kim, J.; Shin, D.; Kim, S.-H.; Park, W.; Shin, Y.; Kim, W.K.; Lee, S.K.; Oh, K.-B.; Shin, J.; Oh, D.-C. Borrelidins C.–E: New antibacterial macrolides from a saltern-derived halophilic Nocardiopsis sp. Mar. Drugs 2017, 15, 166.
- Zhang, X.; He, H.; Ma, R.; Ji, Z.; Wei, Q.; Dai, H.; Zhang, L.; Song, F. Madurastatin B3, a rare aziridine derivative from actinomycete Nocardiopsis sp. LS150010 with potent anti-tuberculosis activity. J. Ind. Microbiol. Biotechnol. 2017, 44, 589–594.
- Eliwa, E.M.; Abdel-Razek, A.S.; Frese, M.; Wibberg, D.; Halawa, A.H.; El-Agrody, A.M.; Bedair, A.H.; Kalinowski, J.; Sewald, N.; Shaaban, M. New bioactive compounds from the marine-derived actinomycete Nocardiopsis lucentensis sp. ASMR2. Z. Naturforsch. B J. Chem. Sci. 2017, 72, 351–360.
- Sun, M.-W.; Zhang, X.-M.; Bi, H.-L.; Li, W.-J.; Lu, C.-H. Two new sesquiterpenoids produced by halophilic Nocardiopsis chromatogenes YIM 90109. Nat. Prod. Res. 2017, 31, 77–83.
- Hamed, A.; Abdel-Razek, A.S.; Frese, M.; Stammler, H.G.; El-Haddad, A.F.; Ibrahim, T.M.A.; Sewald, N.; Shaaban, M. Terretonin N: A new meroterpenoid from Nocardiopsis sp. Molecules 2018, 23, 299.
- Xu, D.; Nepal, K.K.; Harmody, D.; McCarthy, P.J.; Wright, A.E.; Wang, G.; Chen, J.; Zhu, H. Nocardiopsistins A–C: New angucyclines with anti-MRSA activity isolated from a marine sponge-derived Nocardiopsis sp. HB-J378. Synth. Syst. Biotechnol. 2018, 3, 246–251.
- Lombo, F.; Brana, A.F.; Salas, J.A.; Mendez, C. Genetic organization of the biosynthetic gene cluster for the antitumor angucycline oviedomycin in Streptomyces antibioticus ATCC 11891. ChemBioChem 2004, 5, 1181–1187.
- Kharel, M.K.; Pahari, P.; Shepherd, M.D.; Tibrewal, N.; Nybo, S.E.; Shaaban, K.A.; Rohr, J. Angucyclines: Biosynthesis, mode-of-action, new natural products, and synthesis. Nat. Prod. Rep. 2012, 29, 264–325.
- Messaoudi, O.; Sudarman, E.; Bendahou, M.; Jansen, R.; Stadler, M.; Wink, J. Kenalactams A–E, polyene macrolactams isolated from Nocardiopsis CG3. J. Nat. Prod. 2019, 82, 1081–1088.
- Zhao, T.; Chang, Y.; Zhu, T.; Li, J.; Gu, Q.; Li, D.; Che, Q.; Zhang, G. α-Pyrone derivatives with cyto-protective activity from two Takla Makan desert soil derived actinomycete Nocardiopsis strains recovered in seawater based medium. Nat. Prod. Res. 2019, 33, 2498–2506.
- Bolling, B.W.; Parkin, K.L. Limited contribution of isoflavones to hepatocellular phase II enzyme-inducing activity of soybean (Glycine max) extracts. Food Chem. 2009, 113, 1069–1075.
- Park, J.S.; Jung, J.S.; Jeong, Y.H.; Hyun, J.W.; Le, T.K.V.; Kim, D.H.; Choi, E.C.; Kim, H.S. Antioxidant mechanism of isoflavone metabolites in hydrogen peroxide-stimulated rat primary astrocytes: Critical role of hemeoxygenase-1 and NQO1 expression. J. Neurochem. 2011, 119, 909–919.
- Wang, J.-X.; Sun, C.-X.; Shah, M.; Zhang, G.-J.; Gu, Q.-Q.; Zhu, T.-J.; Che, Q.; Li, D.-H. New metabolites from a Mariana Trench-derived actinomycete Nocardiopsis sp. HDN 17-237. J. Asian Nat. Prod. Res. 2020, 22, 1031–1036.
- Zhou, L.; Chen, X.; Sun, C.; Chang, Y.; Huang, X.; Zhu, T.; Zhang, G.; Che, Q.; Li, D. Saliniquinone derivatives, saliniquinones G−I and heraclemycin E, from the marine animal-derived Nocardiopsis aegyptia HDN19-252. Mar. Drugs 2021, 19, 575.
- Shin, J.; Yang, S.-H.; Du, Y.E.; Park, K.; Kim, D.; Shin, D.; Kim, J.; Kim, S.-H.; Kim, Y.K.; Shin, J. Borrelidin from saltern-derived halophilic Nocardiopsis sp. dissociates amyloid-β and tau fibrils. J. Alzheimer’s Dis. Rep. 2021, 5, 7–13.
- Castro-Falcon, G.; Millan-Aguinaga, N.; Roullier, C.; Jensen, P.R.; Hughes, C.C. Nitrosopyridine probe to detect polyketide natural products with conjugated alkenes: Discovery of novodaryamide and nocarditriene. ACS Chem. Biol. 2018, 13, 3097–3106.
- Kim, T.; Lee, S.-A.; Noh, T.; Choi, P.; Choi, S.-J.; Song, B.G.; Kim, Y.; Park, Y.-T.; Huh, G.; Kim, Y.-J.; et al. Synthesis, structure revision, and cytotoxicity of nocarbenzoxazole G. J. Nat. Prod. 2019, 82, 1325–1330.
- Sun, M.; Zhang, X.; Hao, H.; Li, W.; Lu, C. Nocarbenzoxazoles A–G, benzoxazoles produced by halophilic Nocardiopsis lucentensis DSM 44048. J. Nat. Prod. 2015, 78, 2123–2127.
- Yang, C.L.; Zhang, B.; Xue, W.W.; Li, W.; Xu, Z.F.; Shi, J.; Shen, Y.; Jiao, R.H.; Tan, R.X.; Ge, H.M. Discovery, biosynthesis, and heterologous production of loonamycin, a potent anticancer indolocarbazole alkaloid. Org. Lett. 2020, 22, 4665–4669.
- Zhang, X.; Chen, S.; Zhang, L.; Zhang, Q.; Zhang, W.; Chen, Y.; Zhang, W.; Zhang, H.; Zhang, C. Dassonmycins A and B, polycyclic thioalkaloids from a marine sponge-derived Nocardiopsis dassonvillei SCSIO 40065. Org. Lett. 2021, 23, 2858–2862.
- Miao, L.; Qian, S.; Qi, S.; Jiang, W.; Dong, K. Culture medium optimization and active compounds investigation of an anti-quorum sensing marine actinobacterium Nocardiopsis dassonvillei JS106. Microbiology 2021, 90, 112–123.
More