You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Sibel Süzen.
Nuclear factor-E2-related factor 2 (Nrf2) is a short-lived protein that works as a transcription factor and is related to the expression of many cytoprotective genes involved in xenobiotic metabolism and antioxidant responses. Nrf2 is a key regulator of OS defense and research supports a protective and defending role of Nrf2 against neurodegenerative conditions.
- ALS
- Nrf2
- oxidative stress
- antioxidant
- neurodegenerative
1. Introduction
Oxidative stress (OS) is a physiopathological state characterized by an imbalance between reactive oxygen (ROS) and nitrogen species (RNS) generation and cellular antioxidant capacity. Excess ROS formation causes critically important changes in cellular biomolecules, such as proteins, DNA, and lipids. There are numerous studies that confirm a major relationship between OS and neurodegenerative disorders [1,2][1][2] like Alzheimer’s disease (AD) [3], Huntington’s disease (HD) [4], Parkinson’s disease (PD) [5], Multiple sclerosis (MS) [6] and Amyotrophic Lateral Sclerosis (ALS) [7].
ROS/RNS are generated from many different sources in multiple compartments within the cell, either physiologically or because of exposure to toxic or pathologic conditions [8]. One of the most active types of ROS, superoxide (O2−), is produced by the one-electron reduction of O2 in mitochondria. Superoxide can also be produced by a family of NADPH oxidases (NOXs), using oxygen and NADPH as substrates in which superoxide is promptly disposed of [9]. Another important side-product of mitochondrial oxidative phosphorylation is hydroxyl radical (.OH). This is very unsteady, extremely reactive, and produces several reactive aldehydes from membrane lipid peroxidation (LP) that eventually cause cell death. Moreover, the ROS hydrogen peroxide (H2O2), is rapidly formed in the cytoplasm by superoxide dismutase 1 (SOD1), while the H2O2 outside the cell is generated by extracellular superoxide dismutase 3 (SOD3). To end up, H2O2 can be produced as a by-product during β-oxidation of fatty acids by cytochrome P450s. Although H2O2 is relatively more stable and less reactive, in the presence of Fe2+ or Cu+ (Fenton reaction), it can be transformed into hydroxyl radical [10].
The main RNS is ONOO− that rapidly decays into HO•, nitrogen dioxide radical (NO2•), and nitryl cation (NO2+) [11]. All of these are neurotoxic.
There are multiple steps in the production of the OS and in the imbalance of the endogenous cellular defense in neuronal cells that could be targeted therapeutically in the processing of neurodegenerative diseases.
Nuclear factor (erythroid-derived 2)-like2 (Nrf2), is a transcriptional factor associated with the essential defense mechanism of the cells against OS inducing expression of cytoprotective genes [12,13][12][13]. Moreover, the Nrf2 is crucial for blood cell differentiation and for the induction of a set of drug-metabolizing enzymes [14]. Proteins upregulated by Nrf2 signaling include heme oxygenase-1 (HO-1), SOD1, catalase, and enzymes involved in glutathione (GSH) metabolism, such as glutathione S-transferase (GST), glutathione cysteine ligase modifier subunit, and glutathione cysteine ligase catalytic subunit (GCLC) [15,16][15][16].
The capacity of Nrf2 to control intermediary metabolism and mitochondrial action leads that Nrf2 activation is a smart and comprehensive approach to the management of neurodegenerative disorders [17].
2. The Nrf2-ARE Pathway as a Therapeutic Target
Nrf2 organizes cellular protection mechanisms against oxidants via modifying the expression of more than 500 genes that are related to antioxidants, detoxification pathways, or metabolic enzymes. Kelch-like ECH-associated protein (KEAP1) is one of the main regulators of Nrf2 protein stability. Under normal homeostatic conditions, Nrf2 is located in the cytosol and binds KEAP1 [2]. Nrf2 is one of the members of the cap “n” collar (CNC) subfamily of basic-region leucine zipper (bZIP) transcription factors along with Nrf1, Nrf3, NF-E2 p45 subunits and the less related factors BTB domain and CNC homolog 1 and 2 (Bach1 and Bach2) [47,48][18][19]. Molecular structure characterization of Nrf2 revealed seven functional domains, called Nrf2ECH homology (Neh) domains 1–7 with distinct functions [49][20]. Neh1 domain is responsible for the binding to DNA [50][21] and contains a nuclear localization signal (NLS) for Nrf2 translocation from the cytoplasm to the nucleus [51][22]. The Neh2 domain is involved in the interaction with KEAP1, the main Nrf2 repressor with an essential part in regulating the Nrf2 signaling pathway [52][23]. Neh3 is responsible for the activation of the antioxidant response element (ARE), a cis-regulatory element that primarily responds to oxidative stress inducers. Neh4 and Neh5 are involved in the binding with different “cAMP (cyclic Adenosine MonoPhosphate) response element-binding” (CREB) proteins and activate transcription [53,54][24][25]. Neh6 domain is a negative regulatory domain that promotes Nrf2 ubiquitination [55][26]. The Neh7 domain inhibits the Nrf2-ARE signaling pathway by promoting the binding of Nrf2 to the Retinoic X Receptor (RXR) and disrupting binding between CBP (CREB-binding protein) and the Neh4 and Neh5 domains [56][27]. In physiological conditions, Nrf2 is retained in the cytoplasm by the KEAP1/Cullin-3/E3 Ubiquitin-Protein Ligase RBX complex, and undergoes proteasomal degradation, thus maintaining the expression of ARE-responsive genes at basal levels [57][28]. When cells are exposed to pro-oxidant conditions, they activate the Nrf2/KEAP1/ARE pathway [58,59][29][30]. Three cysteine residues (Cys151, Cys273, and Cys288) of KEAP1 are important for Nrf2 degradation. When these residues are oxidized, Nrf2 releases [60][31]. During OS, ROS causes structural changes on KEAP1, inhibiting its binding to Nrf2. Furthermore, the p62 protein, whose expression is prompted by ROS, also helps the stimulation of Nrf2 by docking straight onto KEAP1 through a KEAP1 binding area. This action results in blocking the binding between KEAP1 and Nrf2 [61,62][32][33]. Oxidative or electrophilic challenges distract the complex between Nrf2 and Keap1, leading to the translocation of Nrf2 to the nucleus. This allows Nrf2 to heterodimerise with musculoaponeurotic fibrosarcoma proteins (MAFs) and to attach to ARE in the promoter area of target genes [63,64][34][35]. Nrf2 is constantly produced and degraded, showing a half-life of just 20–30 min. The heterodimer identifies ARE that is existing in the regulatory areas of around 250 ARE-genes [65][36]. The sequence ARE was first identified on the promoter of the rat gene encoding the GST A2 subunit (GST A2) [66][37]. Nrf2 binds to the cis-acting enhancer ARE sequence (core sequence: 5′-TGACNNNGC-3′) existing in promoters of genes [67][38]. Of note, the nrf2 gene includes two ARE-like sequences in its promoter so that Nrf2 is able to autoregulate itself and make ARE-mediated gene expression longer [68][39]. The ARE is situated in the promoter area of a number of genes encoding phase II detoxifying enzymes, antioxidant enzymes, and proteins such as NAD(P)H:quinone oxidoreductase 1 (NQO1), GST, glutamate-cysteine ligase (GCL), HO-1, thioredoxin reductase-1, and thioredoxin [69][40]. Since the ARE core sequence has similarities to the sequence regulated by activator protein 1 (AP-1), it is possible that members of the Jun and Fos families of transcriptional factors could have a role in the transcriptional activation of the rat GST A2 subunit (GST A2) and quinone reductase (QR) genes. This suggestion was supported by the studies that Jun and Fos family members can be activated by OS [70,71][41][42]. Hence, Nrf2 is a transcription factor that responds to OS by binding to ARE in the promoter of genes coding for antioxidant enzymes and proteins for GSH synthesis [72,73][43][44]. NADPH is an essential cofactor for numerous drug-metabolizing enzymes and antioxidant systems, such as cytochromes p450 (CYP) enzymes and the Nrf2 target NQO1 [74][45]. Nrf2 supports NADPH production through the positive regulation of the principal NADPH-generating enzymes. Many different compounds like anethole derivatives such as anethole trithione, dithiolethiones, curcumin, isothiocyanates, caffeic acid, phenethyl esters, flavon derivatives, and triterpenoids (Figure 1) have been found to stimulate ARE and the phase II detoxifying enzymes [75,76][46][47].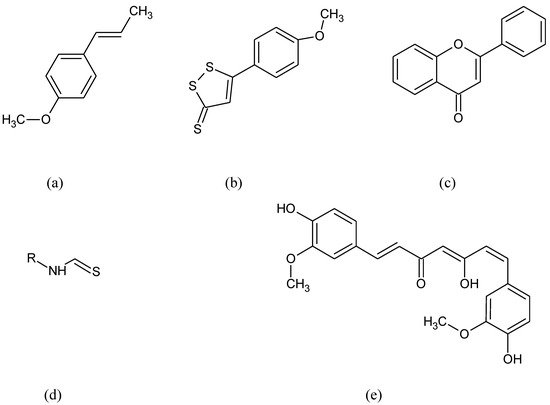
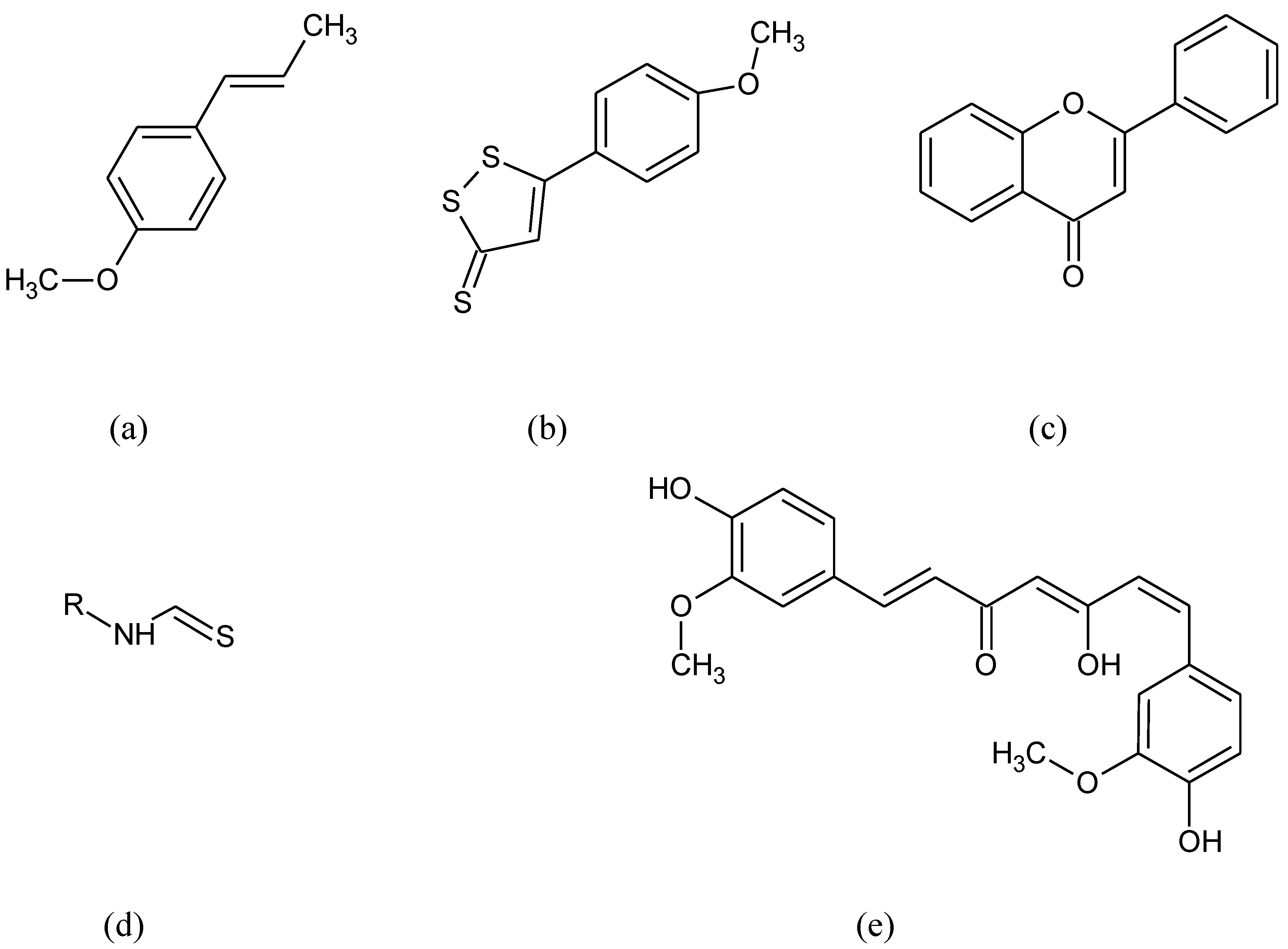
Figure 1.
Chemical formula of (
a
) anetol, (
b
) anethole trithione, (
c
) flavon, (
d
) isothiocyanates, (
e) curcumin.
) curcumin.
References
- Reynolds, A.; Laurie, C.; Mosley, R.L.; Gendelman, H.E. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int. Rev. Neurobiol. 2007, 82, 297–325.
- Seminotti, B.; Grings, M.; Tucci, P.; Leipnitz, G.; Saso, L. Nuclear Factor Erythroid-2-Related Factor 2 Signaling in the Neuropathophysiology of Inherited Metabolic Disorders. Front. Cell Neurosci. 2021, 15, 785057.
- Tejo, F.V.; Quintanilla, R.A. Contribution of the Nrf2 Pathway on Oxidative Damage and Mitochondrial Failure in Parkinson and Alzheimer’s Disease. Antioxidants 2021, 10, 1069.
- Moretti, D.; Tambone, S.; Cerretani, M.; Fezzardi, P.; Missineo, A.; Sherman, L.T.; Munoz-Sajuan, I.; Harper, S.; Dominquez, C.; Pacifici, R.; et al. NRF2 activation by reversible KEAP1 binding induces the antioxidant response in primary neurons and astrocytes of a Huntington’s disease mouse model. Free Radic. Biol. Med. 2021, 162, 243–254.
- Petrillo, S.; Schirinzi, T.; Di Lazzaro, G.; D’Amico, J.; Colona, V.L.; Bertini, E.; Pierantozzi, M.; Mari, L.; Mercuri, N.B.; Piemonte, F.; et al. Systemic activation of Nrf2 pathway in Parkinson’s disease. Mov. Disord. 2020, 35, 180–184.
- Fischer, M.T.; Sharma, R.; Lim, J.L.; Haider, L.; Frischer, J.M.; Drexhage, J.; Mahad, D.; Bradl, M.; van Horssen, J.; Lassmann, H. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain 2012, 135, 886–899.
- Xiao, Y.; Karam, C.; Yi, J.; Zhang, L.; Li, X.; Yoon, D.; Wang, H.; Dhakal, K.; Ramlow, P.; Yu, T.; et al. ROS-related mitochondrial dysfunction in skeletal muscle of an ALS mouse model during the disease progression. Pharmacol. Res. 2018, 138, 25–36.
- Finkel, T. Signal Transduction by Mitochondrial Oxidants. J. Biol. Chem. 2012, 287, 4434–4440.
- Nauseef, W.M. Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 757–767.
- Rojkind, M.; Domínguez-Rosales, J.A.; Nieto, N.; Greenwel, P. Role of hydrogen peroxide and oxidative stress in healing responses. CMLS Cell Mol. Life Sci. 2002, 59, 1872–1891.
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Malgorzata, F. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 4094–4125.
- Wasik, U.; Milkiewicz, M.; Kempinska-Podhorodecka, A.; Milkiewicz, P. Protection against oxidative stress mediated by the Nrf2/Keap1 axis is impaired in Primary Biliary Cholangitis. Sci. Rep. 2017, 7, 44769.
- Telkoparan-Akillilar, P.; Panieri, E.; Cevik, D.; Suzen, S.; Saso, L. Therapeutic Targeting of the NRF2 Signaling Pathway in Cancer. Molecules 2021, 26, 1417.
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426.
- Stachurska, A.; Ciesla, M.; Kozakowska, M.; Wolffram, S.; Boesch-Saadatmandi, C.; Rimbach, G.; Jozkowicz, A.; Dulak, J.; Loboda, A. Cross-talk between microRNAs, nuclear factor E2-related factor 2, and heme oxygenase-1 in ochratoxin A-induced toxic effects in renal proximal tubular epithelial cells. Mol. Nutr. Food Res. 2013, 57, 504–515.
- Panieri, E.; Telkoparan-Akillilar, P.; Suzen, S.; Saso, L. The NRF2/KEAP1 Axis in the Regulation of Tumor Metabolism: Mechanisms and Therapeutic Perspectives. Biomolecules 2020, 10, 791.
- Esteras, N.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 activation in the treatment of neurodegenerative diseases: A focus on its role in mitochondrial bioenergetics and function. Biol. Chem. 2016, 397, 383–400.
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745.
- Pan, H.; Guan, D.; Liu, X.; Li, J.; Wang, L.; Wu, J.; Zhou, J.; Zhang, W.; Ren, R.; Zhang, W.; et al. SIRT6 safeguards human mesenchymal stem cells from oxidative stress by coactivating NRF2. Cell Res. 2016, 26, 190–205.
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Cerebrovascular and Neurological Disorders: Protective Role of NRF2. Int. J. Mol. Sci. 2019, 20, 3433.
- Sun, Z.; Chin, Y.E.; Zhang, D.D. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol. Cell Biol. 2009, 29, 2658–2672.
- Theodore, M.; Kawai, Y.; Yang, J.; Kleshchenko, Y.; Reddy, S.P.; Villalta, F.; Arinze, I.J. Multiple nuclear localization signals function in the nuclear import of the transcription factor Nrf2. J. Biol. Chem. 2008, 283, 8984–8994.
- Suzuki, T.; Motohashi, H.; Yamamoto, M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol. Sci. 2013, 34, 340–346.
- Nioi, P.; Nguyen, T.; Sherratt, P.J.; Pickett, C.B. The carboxy-terminal Neh3 domain of Nrf2 is required for transcriptional activation. Mol. Cell Biol. 2005, 25, 10895–10906.
- Katoh, Y.; Itoh, K.; Yoshida, E.; Miyagishi, M.; Fukamizu, A.; Yamamoto, M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells 2001, 6, 857–868.
- Chowdhry, S.; Zhang, Y.; McMahon, M.; Sutherland, C.; Cuadrado, A.; Hayes, J.D. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene 2013, 32, 3765–3781.
- Wang, H.; Liu, K.; Geng, M.; Gao, P.; Wu, X.; Hai, Y.; Li, Y.; Li, Y.; Luo, L.; Hayes, J.D.; et al. RXRα inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res. 2013, 73, 3097–3108.
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777.
- Panieri, E.; Saso, L. Potential Applications of NRF2 Inhibitors in Cancer Therapy. Oxid. Med. Cell Longev. 2019, 2019, 8592348.
- Sova, M.; Saso, L. Design and development of Nrf2 modulators for cancer chemoprevention and therapy: A review. Drug Des. Devel. Ther. 2018, 12, 3181–3197.
- Silva-Palacios, A.; Ostolga-Chavarría, M.; Zazueta, C.; Königsberg, M. Nrf2: Molecular and epigenetic regulation during aging. Ageing Res. Rev. 2018, 47, 31–40.
- Jain, A.; Lamark, T.; Sjøttem, E.; Larsen, K.B.; Awuh, J.A.; Øvervatn, A.; McMahon, M.; Hayes, J.D.; Johansen, T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J. Biol. Chem. 2010, 285, 22576–22591.
- Llanos-González, E.; Henares-Chavarino, Á.A.; Pedrero-Prieto, C.M.; García-Carpintero, S.; Frontiñán-Rubio, J.; Sancho-Bielsa, F.J.; Alcain, F.J.; Peinado, J.R.; Rabanal-Ruíz, Y.; Durán-Prado, M. Interplay between Mitochondrial Oxidative Disorders and Proteostasis in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 1444.
- Liddell, J.R. Are Astrocytes the Predominant Cell Type for Activation of Nrf2 in Aging and Neurodegeneration? Antioxidants 2017, 6, 65.
- Carvalho, A.N.; Firuzi, O.; Gama, M.J.; van Horssen, J.; Saso, L. Oxidative Stress and Antioxidants in Neurological Diseases: Is There Still Hope? Curr. Drug Targets 2017, 18, 705–718.
- Jiménez-Villegas, J.; Ferraiuolo, L.; Mead, R.J.; Shaw, P.J.; Cuadrado, A.; Rojo, A.I. NRF2 as a therapeutic opportunity to impact in the molecular roadmap of ALS. Free Radic. Biol. Med. 2021, 173, 125–141.
- Rushmore, T.H.; Pickett, C.B. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J. Biol. Chem. 1990, 265, 14648–14653.
- Nioi, P.; Mcmahon, M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: Reassessment of the ARE consensus sequence. Biochem. J. 2003, 374, 337–348.
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116.
- Jeong, W.S.; Jun, M.; Kong, A.N.T. Nrf2: A potential molecular target for cancer chemoprevention by natural compounds. Antioxid. Redox Signal. 2006, 8, 99–106.
- Abate, C.; Patel, L.; Rauscher, F.J.; Curran, T. Redox Regulation of Fos and Jun DNA-Binding Activity In Vitro. Science 1990, 249, 1157–1161.
- Nguyen, T.; Rushmore, T.H.; Pickett, C.B. Transcriptional regulation of a rat liver glutathione S-transferase Ya subunit gene. Analysis of the antioxidant response element and its activation by the phorbol ester 12-O-tetradecanoylphorbol-13-acetate. J. Biol. Chem. 1994, 269, 13656–13662.
- Vomhof-DeKrey, E.E.; Picklo, M.J. The Nrf2-antioxidant response element pathway: A target for regulating energy metabolism. J. Nutr. Biochem. 2012, 23, 1201–1206.
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295.
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218.
- Yates, M.S.; Tauchi, M.; Katsuoka, F.; Flanders, K.C.; Liby, K.T.; Honda, T.; Gribble, G.W.; Johnson, D.A.; Johnson, J.A.; Burton, N.C.; et al. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol. Cancer Ther. 2007, 6, 154–162.
- Shahcheraghi, S.H.; Salemi, F.; Peirovi, N.; Ayatollahi, J.; Alam, W.; Khan, H.; Saso, L. Nrf2 Regulation by Curcumin: Molecular Aspects for Therapeutic Prospects. Molecules 2021, 27, 167.
- Lane, D.J.R.; Metselaar, B.; Greenough, M.; Bush, A.I.; Ayton, S.J. Ferroptosis and NRF2: An emerging battlefield in the neurodegeneration of Alzheimer’s disease. Essays Biochem. 2021, 65, 925–940.
- Hensley, K.; Harris-White, M.E. Redox regulation of autophagy in healthy brain and neurodegeneration. Neurobiol. Dis. 2015, 84, 50–59.
More
