Pancreatic malignancy is a lethal neoplasm, as well as one of the leading causes of cancer-associated mortality, having a 5-year overall survival rate of less than 10%. The average life expectancy of patients with advanced pancreatic cancer does not exceed six months. Although surgical excision is a favorable modality for long-term survival of pancreatic neoplasm, metastasis is initially identified in nearly 80% of the patients by the time of diagnosis, making the development of therapeutic policy for pancreatic cancer extremely daunting. Emerging evidence shows that pancreatic neoplastic cells interact intimately with a complicated microenvironment that can foster drug resistance, metastasis, or relapse in pancreatic cancer. As a result, the necessity of gaining further insight should be focused on the pancreatic microenvironment contributing to cancer progression. Numerous evidence reveals that perioperative factors, including surgical manipulation and anesthetics (e.g., propofol, volatile anesthetics, local anesthetics, epidural anesthesia/analgesia, midazolam), analgesics (e.g., opioids, non-steroidal anti-inflammatory drugs, tramadol), and anesthetic adjuvants (such as ketamine and dexmedetomidine), might alter the tumor microenvironment and cancer progression by affecting perioperative inflammatory or immune responses during cancer surgery.
- pancreatic cancer
- tumor microenvironment
- anesthesia
1. Introduction
2. Patient Factors: Hyperglycemia and Obesity
2.1. Hyperglycemia
Diabetes mellitus (DM) and PC are intimately related, as high blood glucose levels promote PC proliferation, invasion, EMT, and metastasis [13,14][13][14]. In addition, insulin resistance, hyperinsulinemia, hyperglycemia, and chronic inflammation are the mechanisms of type-2-DM-associated PC [15].2.1.1. Laboratory Studies
Recently, Otto et al. attributed a role to the type-2-DM-related hyperglycemic inflammatory micromilieu in the acquisition of malignancy-associated alterations in premalignant pancreatic ductal epithelial cells, thus providing new insights into how hyperglycemia might promote PC initiation [16]. It is well-known that EMT of pancreatic ductal epithelial cells develops in correlation with hyperglycemia or macrophages [17,18][17][18]. Moreover, hyperglycemia aggravates microenvironment hypoxia, accelerates EMT, and then promotes the metastatic ability of PC. PC is generally hypoxic due to its avascular morphology, and PC cells express high levels of HIF-1α and MMP-9 for promoting tumor growth, invasion and metastasis in a hypoxic environment [19]. In addition, the accumulation of HIF-1α induced by hyperglycemia might promote pancreatic glycolysis to facilitate cancer progression [20]. Zhou et al. reported that the high-glucose microenvironment accelerated PC growth [21]. With regard to the VAs, Guo et al. reported that isoflurane promoted glucose metabolism through upregulation of miR-21 and suppressed mitochondrial oxidative phosphorylation in ovarian cancer cells [22]. Dong et al. reported that dezocine, an opioid analgesic, promoted glucose metabolism and impaired the proliferation of lung cancer cells [23]. However, Codd et al. reported that opioid agonists did not elevate blood glucose and lacked an insulin-reducing effect [24]. Han et al. reported that indometacin, an inhibitor of cyclooxygenase (COX)-2, ameliorated high-glucose-induced proliferation and invasion via upregulation of E-cadherin in PC cells [25]. Current laboratory data on the effect of anesthesia on glucose metabolism in PC are limited, and further investigation is required.2.1.2. Clinical Studies
Insulin resistance, hyperinsulinemia, hyperglycemia, and chronic inflammation are the mechanisms of type-2-DM-associated PC [15]. Recently, type 2 DM was shown to reduce the likelihood of cancer survival, and was significantly correlated with comorbidity and poor prognosis in patients undergoing PC surgery [15]. In addition, metformin may lower the probability of PC. By contrast, insulin therapy may amplify the probability of PC [15]. In another study, approximately 85% of PC patients exhibited impaired glucose tolerance associated with DM and had a reduced overall survival rate [26]. Elderly patients with new-onset DM are at higher risk of developing PC than the general population [26]. Therefore, new-onset DM and hyperglycemia serve as important screening tools to diagnose asymptomatic PC and improve PC survival [26]. Sandini et al. reported that preoperative blood glucose ≥ 140 mg/dL was associated with poor long-term outcomes in patients undergoing resection for PC [27]. Conti et al. reported that anti-diabetic drugs represented a significant protective factor against mortality among older adults with metastatic PC [28]. However, in a recent meta-analysis study, blood glucose, fasting blood glucose, and glycated hemoglobin (HbA1c) levels were not associated with the survival of patients with PC [29]. Liu et al. reported that the blood glucose levels of the DM patients in the propofol group were significantly lower than those in the sevoflurane group during gastric cancer surgery. This result indicated that the effect of propofol on glucose metabolism under surgical stimulation was less than that of sevoflurane [30]. Epidural blockade with bupivacaine attenuated the hyperglycemic response to surgery by modifying glucose production in colorectal surgery [31]. Current clinical data on the effect of anesthesia on glucose metabolism in PC are limited. Further investigation is required to determine the effects of anesthetics and analgesics on glucose metabolism in PC (Figure 1).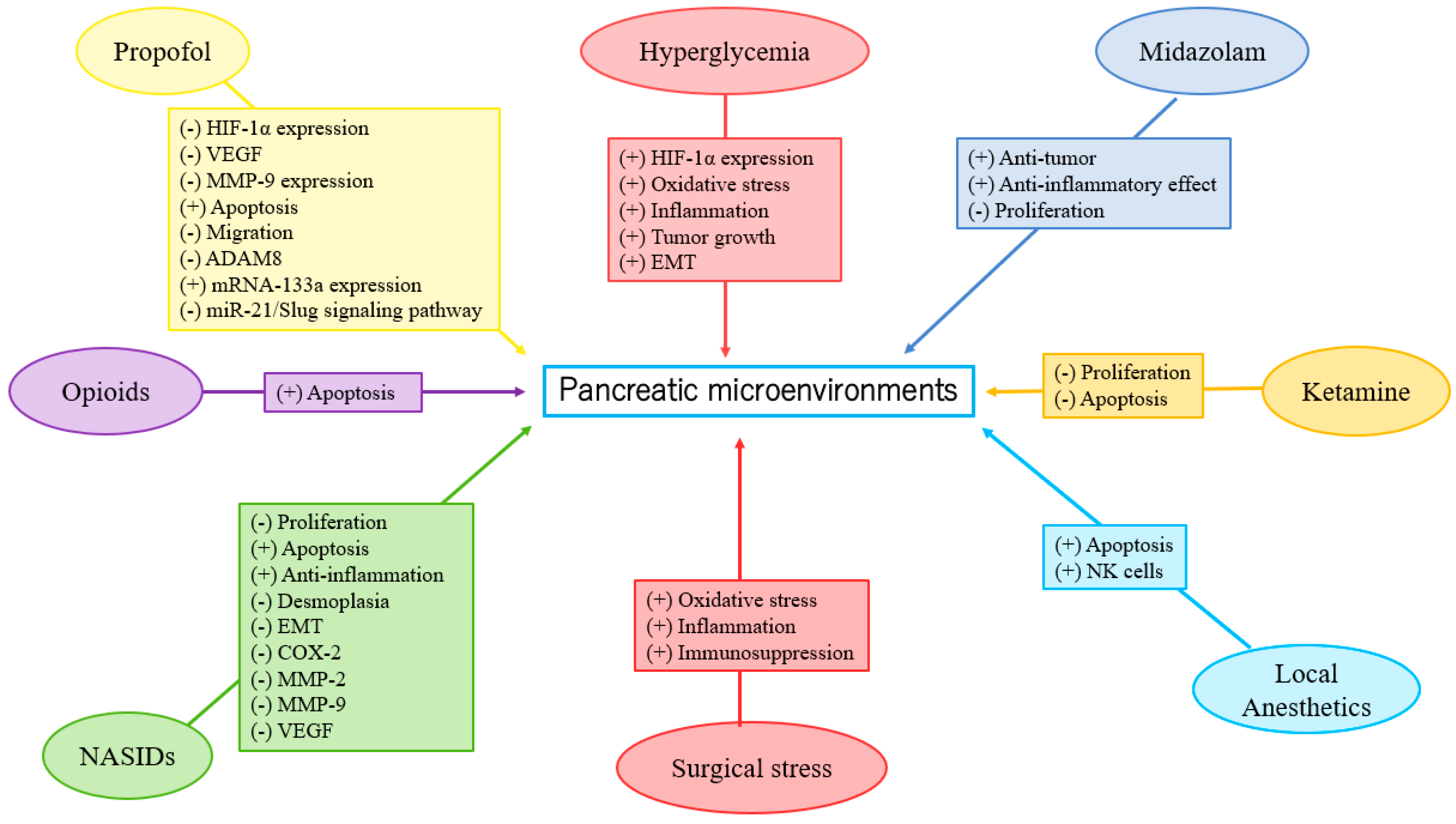
2.2. Obesity
Obesity-associated adipose tissue inflammation may play a central role in the development of PC and the promotion of PC growth [32]. Chronic inflammation, hormonal effects, circulating adipokines, and adipocyte-mediated inflammatory and immunosuppressive microenvironments are involved in the association of obesity with PC [33]. The tumor-promoting effects of obesity occur at the local level via adipose inflammation and associated alterations in the microenvironment, as well as systemically via circulating metabolic and inflammatory mediators associated with adipose inflammation [34]. In a review article, Heil et al. reported that anesthetics with the effect of inhibiting obesity-induced inflammation may improve postoperative outcomes [35]. Eley et al. concluded that VAs, ketamine, opioids, propofol, and regional anesthesia have been shown to modulate parts of the immune system in patients with obesity [36].2.2.1. Laboratory Study
Incio et al. reported that obesity-induced inflammation and desmoplasia promoted PC progression and resistance to chemotherapy [37]. Until now, there have been no laboratory studies on the effects of anesthetics in obesity-induced inflammation and PC progression, and further investigation is necessary.2.2.2. Clinical Studies
Recently, Zorbas et al. showed that obesity was significantly associated with higher risk of postoperative complications and mortality in patients with body mass index ≥ 40 after pancreatoduodenectomy [38]. Li et al. reported that the lean body-weight-based dosing of propofol had more potent antioxidant and anti-inflammatory effects on morbidly obese patients than the total body-weight-based dosing during anesthesia induction [39]. Until now, there have been no clinical studies on the effects of anesthetics in obesity-induced inflammation and PC progression, and further investigation is necessary.3. Tumor Factors: EMT, Hypoxia-Inducible Factor-1α (HIF-1α), Matrix Metalloproteinases (MMP)-9 Expression, Inflammation, Apoptosis, Autophagy, and Oxidative Stress
3.1. EMT
The development of EMT originates in the conversion of epithelial cells to motile mesenchymal stem cells [40], which is based on many essential processes involving embryonic progression, tissue formation/fibrosis, and wound repairing [40]. Moreover, the initiation of EMT contributes to tumor growth, therapy resistance, and tumor spreading [40]. In the case of high EMT expression in tumors, deterioration of overall outcomes and metastases is inevitable. [40,41,42,43][40][41][42][43]. However, research on the direct effects of specific anesthetics on EMT of PC is currently lacking, and further investigation is required.3.1.1. Laboratory Studies
Anesthesia and analgesia may affect EMT [25,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61][25][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61]. Studies have reported that propofol suppressed EMT in esophageal cancer, choriocarcinoma, breast cancer, thyroid cancer, lung cancer, gastric cancer, hepatocellular carcinoma (HCC), renal cell carcinoma (RCC), prostate cancer, and oral squamous cell carcinoma cells [45,46,47,48,49,50,51,52,53,54][45][46][47][48][49][50][51][52][53][54]. By contrast, Ren et al. reported that desflurane induced EMT and metastasis in colorectal cancer through deregulation of the miR-34a/LOXL3 axis [44]. Opioids promoted EMT in breast and lung cancers via mu- or delta-opioid receptors [55,56][55][56]. Zhang et al. showed morphine-induced EMT in esophageal carcinoma cells [57]. However, sufentanil inhibited EMT by acting on NF-κB and Snail signaling pathways to inhibit proliferation and metastasis of esophageal cancer [58]. Lidocaine suppressed EMT in ovarian cancer cells [59]. However, high concentrations of levobupivacaine significantly increased EMT in the A549 lung cancer cell line, and enhanced metastasis in mice [60]. COX-2 inhibitors may suppress EMT in oral squamous cell carcinoma [61]. Han et al. reported that indometacin reduced the expression levels of MMP-2, MMP-9, and vascular endothelial growth factor (VEGF) by upregulation of E-cadherin, inhibiting proliferation and invasion of PC [25]. Zheng et al. observed the benefit of EMT inhibition due to the use of chemotherapy in PC treatment [62]. To the best of ouresearchers' knowledge, NSAIDs may inhibit EMT expression in PC. Propofol may inhibit EMT, but VAs may promote EMT in different cancers. On the other hand, opioids and LAs may induce uncertain effects (both positive and negative) on EMT. Laboratory research on the direct effects of specific anesthetics on EMT in PC is currently lacking. Further investigation is needed (see existing studies in Table 1 and Figure 1).| Type of Anesthetics/Analgesics | Effects |
|---|---|
| Clinical studies Propofol/VAs |
Propofol was associated with no or low-grade complication compared with desflurane in PC surgery [10]; propofol anesthesia was associated with better survival than desflurane anesthesia in PC surgery [11]. |
| NSAIDs | In a systematic review of observational studies, there was no signification association between aspirin use and mortality risk in PC [63]; aspirin use reduced risk of PC [64]; aspirin was associated with improved overall survival and improved disease-free survival in PC surgery [65]. |
| Opioids | High opioid consumption was related to decreased survival rates in newly diagnosed stage IV PC patients [66]; opioid prescription was associated with poor overall survival among PC patients [67]; there was an insignificant relationship between intraoperative opioid use and decreased survival in PC surgery [68]; administration of opioids was associated with prolonged survival in older adult patients with PC [69]. |
| LAs | Intraoperative administration of intravenous lidocaine was associated with improvement of overall survival in PC patients [12]; intraoperatively epidural ropivacaine infusion was associated with survival improvement in PC patients [70]; perioperative lidocaine administration might be beneficial to the function of NK cells in PC surgery [71]; peridural anesthesia with ropivacaine might improve the oncological outcome of PC patients [72]. |
| Experimental studies Propofol |
Propofol attenuated malignant potential by inhibiting HIF-1α and VEGF expression [73]; PC cell growth was inhibited by propofol via suppression of MMP-9 expression [74]; propofol inhibited migration and induced apoptosis [75]; propofol induced apoptosis in PC cells in vitro [76]; propofol inhibited PC progression by downregulating ADAM8 [77,78,79][77][78][79]; propofol suppressed proliferation and invasion of PC cells by upregulating microRNA-133a expression [80]; propofol inhibited growth and invasion of PC cells through regulation of the miR-21/Slug signaling pathway [81]. |
| NSAIDs | Indometacin ameliorated high glucose-induced proliferation and invasion by upregulating E-cadherin (EMT) in PC cells [25]; aspirin counteracted PC stem cell features and desmoplasia and gemcitabine resistance [82]; COX-2 inhibition promoted an immune-stimulatory microenvironment in preclinical models of PC [83]; sodium salicylate inhibited proliferation and induced G1 cell cycle arrest in human PC cell lines [84]; indometacin inhibited proliferation and activation of pancreatic stellate cells through the downregulation of COX-2 [85]. |
| Opioids | Fentanyl decreased gene expression of PC stem cell markers and increased expression of apoptosis-related genes [86]. |
| LAs | High concentrations of ropivacaine or bupivacaine revealed antiproliferative potency in PC cells [87]. |
| Midazolam | Midazolam exhibited antitumor (anti-proliferation) and anti-inflammatory effects in a mouse model of PC [88]. |
| Ketamine | Ketamine significantly inhibited proliferation in PC cells [89]; ketamine significantly inhibited proliferation and apoptosis in PC cells [90]. |
3.1.2. Clinical Studies
Clinical research on the direct effects of specific anesthetics on the EMT of PC is currently lacking. Further investigation is urgently required (Table 1 and Figure 1).3.2. HIF-1α
A review article showed that HIF-1α expression enhanced PC cell proliferation through multiple mechanisms by inducing neoplastic features and mediating tumorigenic crosstalk between tumor and stromal cells [91].3.2.1. Laboratory Studies
Propofol could attenuate PC cells’ malignant potential by inhibiting HIF-1α and VEGF expression [73]. VAs enhance angiogenesis through HIF-1α activity in prostate and lung cancers [92]. Isoflurane upregulated the levels of HIF-1α and exerted a protumorigenic effect on a human RCC cell line [93]. However, in the neuroglioma cell line, sevoflurane decreased HIF-1α expression via miR-210, while desflurane downregulated HIF1-α and MMP-9 expressions via miR-138 and miR-335, respectively [94]. Opioids were shown to promote tumor angiogenesis in a breast cancer cell by stimulation of δ-opioid receptors in breast cancer cells, leading to activation of HIF-1α and expression of COX-2 via PI3K/Akt stimulation [95]. However, Koodie et al. reported that morphine suppressed tumor angiogenesis by inhibiting HIF-1α expression in mouse Lewis lung carcinoma cells [96]. Okamoto et al. showed that HIF-1α activation conferred resistance to lidocaine-induced cell death in the RCC cell line [97]. Zhou et al. revealed that inhibition of HIF-1α by meloxicam (a selective COX-2 inhibitor) could suppress angiogenesis and enhance apoptosis of HCC cells [98]. To the best of ouresearchers' knowledge, propofol may reduce HIF-1α expression in PC. Based on the limited data, further investigation is required and encouraged to determine the effects of VAs, LAs, and NSAIDs on HIF-1α expression in PC (Table 1 and Figure 1). Recently, Yue et al. demonstrated that HIF-1α positively regulated miR-212 expression and resulted in pancreatic ductal adenocarcinoma progression [99]. Propofol inhibited ovarian cancer cells growth and glycolysis by elevating miR-212-5p expression [100]. Higher miR-212-5p expression showed a neuroprotective effect in rats with isoflurane-induced cognitive dysfunction by inhibiting neuroinflammation [101]. He et al. reported that δ-opioid receptor activation modified miR-212 expression in the rat kidney under prolonged hypoxia [102]. Until now, there have been no laboratory studies on the effects of anesthetics on miR-212 expression and PC progression; further investigation is necessary.3.2.2. Clinical Studies
In a systemic review and meta-analysis, Raji et al. found that miR-212 could be a novel potential biomarker in cancer diagnosis and prognosis [103]. High levels of miR-212 indicated poor prognosis in PC, and low levels of miR-212 indicated poor prognosis in other cancers [103]. Until now, there have been no clinical studies on the effects of anesthetics on HIF-1α or miR-212 expression and PC progression; further investigation is necessary.3.3. MMP-9
MMPs are part of the zinc-dependent proteolytic metalloenzyme family that may play a role in the early diagnosis and prognosis of PC. The higher expression of particular MMPs may also correlate with metastatic disease and/or poorer prognosis [104,105,106,107][104][105][106][107]. MMP-9, well-known as one of the most investigated MMPs, corrupts the extracellular matrix components, resulting in pathophysiologic alterations [108]. Impairment of MMP-9 expression and regulation affects various dysfunctions, including tumorigenesis, and MMP-9 suppression can be targeted in anticancer therapeutics [108]. Anesthesia may affect MMP-9 expression [74,94,109,110,111,112,113,114,115][74][94][109][110][111][112][113][114][115].3.3.1. Laboratory Studies
Yu et al. reported that propofol inhibits PC growth by suppressing MMP-9 expression [74]. Sevoflurane and desflurane inhibited glioma cell proliferation and migration via downregulation of MMP-9 [94]. Sevoflurane and desflurane reduced the invasion of colorectal and neuroglioma cancer cells through downregulation of MMP-9 [94,110][94][110]. Moreover, sevoflurane inhibited the proliferation and invasion of HCC cells through downregulation of MMP-9 [111]. Zhang et al. showed that fentanyl inhibited tumor growth and cell invasion in colorectal cancer by downregulation of miR-182 and MMP-9 expression [112]. In addition, the antitumor effects of morphine are associated with a reduction in the level of MMP-9 [113]. Both lidocaine and ropivacaine inhibited TNFα-induced invasion of lung adenocarcinoma cells in vitro by blocking MMP-9 expression [114]. Based on the limited data, further investigation is needed to clarify the effects of anesthetics and analgesics on MMP-9 expression in PC (Table 1 and Figure 1).3.3.2. Clinical Studies
Wang et al. reported that MMP-9 in the propofol group was significantly lower than in the sevoflurane group in lung cancer patients who received surgery [109]. In breast cancer patients, Kashefi et al. reported that novel NSAIDs may reduce MMP-2 and MMP-9 expression, which promotes angiogenesis and metastasis [115]. In summary, propofol may reduce MMP-9 expression in PC. Based on the limited data, further investigation is required to determine the effects of anesthetics and analgesics on MMP-9 expression in PC (Table 1 and Figure 1).3.4. Inflammation, and the Immune System
Inflammation, apoptosis, and autophagy can provide cellular defense, and impairments of these processes (rendering them deficient or overactivated) lead to pathological effects. Inflammation induces secretion of various cytokines and chemokines, and recruits various immune cells in reaction to oxidative stress or infection sites. Reflexively, enhancement of reactive oxygen species (ROS)-generation via inflammatory immune cells provokes oxidative stress and tissue injury. In addition, chronic inflammation not only produces high numbers of inflammatory mediators but also gives rise to oxidative stress [116]. Inflammatory processes have emerged as key elements in PC development and progression [117,118][117][118]. The relationship of chronic inflammation and cancer, as revealed in the pioneering work of Rudolf Virchow, has been observed for more than 150 years, especially in PC progression [118]. However, even in malignancy without preceding inflammation, cancer-induced inflammation, secretions of inflammatory factors, and immune cell infiltration are main characters in tumor initiation and advanced metastasis [118]. Anesthesia and analgesia may impact cellular immunity and inflammation during surgery, and thus affect cancer outcomes [119,120,121,122,123,124,125][119][120][121][122][123][124][125].3.4.1. Laboratory Studies
A recent laboratory study has shown that propofol enhances anti-inflammatory reactions and stimulatory effects on immune responses, which may be a potential benefit in the prevention of tumor recurrence. However, clinical evidence of the tumor suppression effects is inconclusive. [126]. Opioids influence the nervous system indirectly, as well as release biological amines that potentially impair innate immunity by suppressing natural killer (NK) cell cytotoxicity. [127]. However, a mu-opioid receptor (MOR) partial agonist, buprenorphine, intercepted the inhibition of NK cell cytotoxicity and progression caused by surgery in a rat mammary adenocarcinoma cell line [128]. Additionally, neoplasm is related to inflammation, and anti-inflammatory properties are identified in LAs. LAs may be able to reduce metastasis risk, but the molecular mechanism is not fully understood. [129]. With regard to NSAIDs, aspirin was associated with a decreased expression of markers for progression, inflammation, and desmoplasia in PC cell lines [82]. NSAIDs also reduced inflammation and induced apoptosis in rat osteosarcoma cells in vitro [130]. Thus, NSAIDs may attenuate inflammation in PC. Further laboratory research is necessary (see extant research in Table 1 and Figure 1).3.4.2. Clinical Studies
The surgical treatment of PC is complicated by the prolonged nature of the surgery, the magnitude of the surgical stress, inflammatory response, immunosuppression, anesthesia-/epidural-induced hypotension, and blood loss, all of which cause oxidative stress [92,131][92][131]. In a retrospective study based on clinical pathological analysis, Huang et al. showed that the survival probability was reduced in patients with TNM stage III to IV, lymph node metastasis, higher CD4+IL-17+ level, and lower CD8+ expression, which implied that the tumor immune microenvironment may affect the outcome of PC [132]. Recently, Li et al. reported that high systemic immune-inflammation index levels were regarded as negative with regard to PC overall survival and cancer-specific survival [133]. Yamaguchi et al. demonstrated that propofol reduced the number of CD8+ T cells, whereas sevoflurane augmented the percentage of regulatory T cells in lung-cancer surgery patients [121]. Sevoflurane was revealed to devastate multiple pulmonary functions by releasing a series of inflammatory secretions in lung cancer patients undergoing perioperative one-lung ventilation [134]. However, another clinical study reported that sevoflurane inhibited pulmonary inflammatory cytokines [135]. Propofol combined with epidural anesthesia and epidural analgesia demonstrated less interference with the immune system (compared to propofol with intravenous analgesia) and led to fast recovery in patients undergoing radical resection of pulmonary carcinoma [125]. However, in a clinical study, Fant et al. reported that thoracic epidural analgesia with bupivacaine inhibits the neurohormonal but not the acute inflammatory stress response after radical retropubic prostatectomy [136]. Based on the published data, further investigation is required to determine the effects of anesthetics and analgesics on inflammation and cellular immunity in PC progression (Table 1 and Figure 1).References
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer. J. Clin. 2018, 68, 7–30.
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Prime. 2016, 2, 16022.
- Deplanque, G.; Demartines, N. Pancreatic cancer: Are more chemotherapy and surgery needed? Lancet 2017, 389, 985–986.
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 1039–1049.
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620.
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The tumour microenvironment in pancreatic cancer—Clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540.
- Ramirez, M.F.; Gorur, A.; Cata, J.P. Opioids and cancer prognosis: A summary of the clinical evidence. Neurosci. Lett. 2021, 746, 135661.
- Tat, T.; Jurj, A.; Selicean, C.; Pasca, S.; Ionescu, D. Antiproliferative effects of propofol and lidocaine on the colon adenocarcinoma microenvironment. J. Buon 2019, 24, 106–115.
- Soliz, J.M.; Ifeanyi, I.C.; Katz, M.H.; Wilks, J.; Cata, J.P.; McHugh, T.; Fleming, J.B.; Feng, L.; Rahlfs, T.; Bruno, M.; et al. Comparing postoperative complications and inflammatory markers using total intravenous anesthesia versus volatile gas anesthesia for pancreatic cancer surgery. Anesthesiol. Pain Med. 2017, 7, e13879.
- Lai, H.C.; Lee, M.S.; Liu, Y.T.; Lin, K.T.; Hung, K.C.; Chen, J.Y.; Wu, Z.F. Propofol-based intravenous anesthesia is associated with better survival than desflurane anesthesia in pancreatic cancer surgery. PLoS ONE 2020, 15, e0233598.
- Zhang, H.; Yang, L.; Zhu, X.; Zhu, M.; Sun, Z.; Cata, J.P.; Chen, W.; Miao, C. Association between intraoperative intravenous lidocaine infusion and survival in patients undergoing pancreatectomy for pancreatic cancer: A retrospective study. Br. J. Anaesth. 2020, 125, 141–148.
- Li, W.; Zhang, L.; Chen, X.; Jiang, Z.; Zong, L.; Ma, Q. Hyperglycemia promotes the epithelial-mesenchymal transition of pancreatic cancer via hydrogen peroxide. Oxid. Med. Cell Longev. 2016, 2016, 5190314.
- Li, J.; Ma, J.; Han, L.; Xu, Q.; Lei, J.; Duan, W.; Li, W.; Wang, F.; Wu, E.; Ma, Q.; et al. Hyperglycemic tumor microenvironment induces perineural invasion in pancreatic cancer. Cancer Biol. Ther. 2015, 16, 912–921.
- Li, Y.; Bian, X.; Wei, S.; He, M.; Yang, Y. The relationship between pancreatic cancer and type 2 diabetes: Cause and consequence. Cancer Manag. Res. 2019, 11, 8257–8268.
- Otto, L.; Rahn, S.; Daunke, T.; Walter, F.; Winter, E.; Möller, J.L.; Rose-John, S.; Wesch, D.; Schäfer, H.; Sebens, S. Initiation of pancreatic cancer: The interplay of hyperglycemia and macrophages promotes the acquisition of malignancy-associated properties in pancreatic ductal epithelial cells. Int. J. Mol. Sci. 2021, 22, 5086.
- Helm, O.; Held-Feindt, J.; Grage-Griebenow, E.; Reiling, N.; Ungefroren, H.; Vogel, I.; Krüger, U.; Becker, T.; Ebsen, M.; Röcken, C.; et al. Tumor-associated macrophages exhibit pro- and anti-inflammatory properties by which they impact on pancreatic tumorigenesis. Int. J. Cancer. 2014, 135, 843–861.
- Helm, O.; Mennrich, R.; Petrick, D.; Goebel, L.; Freitag-Wolf, S.; Röder, C.; Kalthoff, H.; Röcken, C.; Sipos, B.; Kabelitz, D.; et al. Comparative characterization of stroma cells and ductal epithelium in chronic pancreatitis and pancreatic ductal adenocarcinoma. PLoS ONE 2014, 9, e94357.
- Li, W.; Liu, H.; Qian, W.; Cheng, L.; Yan, B.; Han, L.; Xu, Q.; Ma, Q.; Ma, J. Hyperglycemia aggravates microenvironment hypoxia and promotes the metastatic ability of pancreatic cancer. Comput. Struct. Biotechnol. J. 2018, 16, 479–487.
- Cheng, L.; Qin, T.; Ma, J.; Duan, W.; Xu, Q.; Li, X.; Han, L.; Li, W.; Wang, Z.; Zhang, D.; et al. Hypoxia-inducible factor-1α mediates hyperglycemia-induced pancreatic cancer glycolysis. Anticancer Agents Med. Chem. 2019, 19, 1503–1512.
- Zhou, C.; Qian, W.; Li, J.; Ma, J.; Chen, X.; Jiang, Z.; Cheng, L.; Duan, W.; Wang, Z.; Wu, Z.; et al. High glucose microenvironment accelerates tumor growth via SREBP1-autophagy axis in pancreatic cancer. J. Exp. Clin. Cancer Res. 2019, 38, 302.
- Guo, N.L.; Zhang, J.X.; Wu, J.P.; Xu, Y.H. Isoflurane promotes glucose metabolism through up-regulation of miR-21 and suppresses mitochondrial oxidative phosphorylation in ovarian cancer cells. Biosci. Rep. 2017, 37, BSR20170818.
- Dong, W.; Zhang, D.; Zhu, A.; Hu, Y.; Li, W. High concentration of Dezocine induces immune escape of lung cancer and promotes glucose metabolism through up-regulating PD-L1 and activating NF-κB pathway. Curr. Mol. Med. 2021; Epub ahead of print.
- Codd, E.E.; Baker, J.; Brandt, M.R.; Bryant, S.; Cai, C.; Carson, J.R.; Chevalier, K.M.; Colburn, R.W.; Coogan, T.P.; Dax, S.L.; et al. Diabetogenic effect of a series of tricyclic delta opioid agonists structurally related to cyproheptadine. Toxicol. Sci. 2010, 117, 493–504.
- Han, L.; Peng, B.; Ma, Q.; Ma, J.; Li, J.; Li, W.; Duan, W.; Chen, C.; Liu, J.; Xu, Q.; et al. Indometacin ameliorates high glucose-induced proliferation and invasion via modulation of e-cadherin in pancreatic cancer cells. Curr. Med. Chem. 2013, 20, 4142–4152.
- Khadka, R.; Tian, W.; Hao, X.; Koirala, R. Risk factor, early diagnosis and overall survival on outcome of association between pancreatic cancer and diabetes mellitus: Changes and advances, a review. Int. J. Surg. 2018, 52, 342–346.
- Sandini, M.; Strobel, O.; Hank, T.; Lewosinska, M.; Nießen, A.; Hackert, T.; Büchler, M.W.; Schimmack, S. Pre-operative dysglycemia is associated with decreased survival in patients with pancreatic neuroendocrine neoplasms. Surgery 2020, 167, 575–580.
- Conti, C.; Pamoukdjian, F.; Aparicio, T.; Mebarki, S.; Poisson, J.; Manceau, G.; Taieb, J.; Rance, B.; Katsahian, S.; Charles-Nelson, A.; et al. Overall survival and prognostic factors among older patients with metastatic pancreatic cancer: A retrospective analysis using a hospital database. Cancers 2022, 14, 1105.
- Wang, X.; Xu, W.; Hu, X.; Yang, X.; Zhang, M. The prognostic role of glycemia in patients with pancreatic carcinoma: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 780909.
- Liu, J.; Yang, L. Effects of propofol and sevoflurane on blood glucose, hemodynamics, and inflammatory factors of patients with type 2 diabetes mellitus and gastric cancer. Oncol. Lett. 2020, 19, 1187–1194.
- Lattermann, R.; Carli, F.; Wykes, L.; Schricker, T. Epidural blockade modifies perioperative glucose production without affecting protein catabolism. Anesthesiology 2002, 97, 374–381.
- Eibl, G.; Rozengurt, E. Obesity and pancreatic cancer: Insight into mechanisms. Cancers 2021, 13, 5067.
- Zhou, B.; Wu, D.; Liu, H.; Du, L.T.; Wang, Y.S.; Xu, J.W.; Qiu, F.B.; Hu, S.Y.; Zhan, H.X. Obesity and pancreatic cancer: An update of epidemiological evidence and molecular mechanisms. Pancreatology 2019, 19, 941–950.
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and cancer mechanisms: Tumor microenvironment and inflammation. J. Clin. Oncol. 2016, 34, 4270–4276.
- Heil, L.B.B.; Silva, P.L.; Pelosi, P.; Rocco, P.R.M. Immunomodulatory effects of anesthetics in obese patients. World J. Crit. Care Med. 2017, 6, 140–152.
- Eley, V.A.; Thuzar, M.; Navarro, S.; Dodd, B.R.; van Zundert, A.A. Obesity, metabolic syndrome, and inflammation: An update for anaesthetists caring for patients with obesity. Anaesth. Crit. Care Pain Med. 2021, 40, 100947.
- Incio, J.; Liu, H.; Suboj, P.; Chin, S.M.; Chen, I.X.; Pinter, M.; Ng, M.R.; Nia, H.T.; Grahovac, J.; Kao, S.; et al. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 2016, 6, 852–869.
- Zorbas, K.; Wu, J.; Reddy, S.; Esnaola, N.; Karachristos, A. Obesity affects outcomes of pancreatoduodenectomy. Pancreatology 2021, 21, 824–832.
- Li, J.; Li, J.Y.; Wu, Z.; Peng, X. Reduced antioxidant and anti-inflammatory effects of propofol at high-dose on morbidly obese patients. Pak. J. Pharm. Sci. 2021, 34, 561–565.
- Babaei, G.; Aziz, S.G.; Jaghi, N.Z.Z. EMT, cancer stem cells and autophagy; The three main axes of metastasis. Biomed. Pharmacother. 2021, 133, 110909.
- Brown, M.S.; Muller, K.E.; Pattabiraman, D.R. Quantifying the epithelial-to-mesenchymal transition (EMT) from bench to bedside. Cancers 2022, 14, 1138.
- Armstrong, A.J.; Marengo, M.S.; Oltean, S.; Kemeny, G.; Bitting, R.L.; Turnbull, J.D.; Herold, C.I.; Marcom, P.K.; George, D.J.; Garcia-Blanco, M.A. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol. Cancer Res. 2011, 9, 997–1007.
- Markiewicz, A.; Topa, J.; Nagel, A.; Skokowski, J.; Seroczynska, B.; Stokowy, T.; Welnicka-Jaskiewicz, M.; Zaczek, A.J. Spectrum of Epithelial-Mesenchymal Transition Phenotypes in Circulating Tumour Cells from Early Breast Cancer Patients. Cancers 2019, 11, 59.
- Ren, J.; Wang, X.; Wei, G.; Meng, Y. Exposure to desflurane anesthesia confers colorectal cancer cells metastatic capacity through deregulation of miR-34a/LOXL3. Eur. J. Cancer Prev. 2021, 30, 143–153.
- Guo, R.; Lu, X.; Xu, G.; Luo, S. Propofol suppresses hypoxia-induced esophageal cancer cell migration, invasion, and EMT through regulating lncRNA TMPO-AS1/miR-498 axis. Thorac. Cancer 2020, 11, 2398–2405.
- Sun, H.; Wang, Y.; Zhang, W. Propofol inhibits proliferation and metastasis by up-regulation of miR-495 in JEG-3 choriocarcinoma cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1738–1745.
- Du, Q.; Zhang, X.; Zhang, X.; Wei, M.; Xu, H.; Wang, S. Propofol inhibits proliferation and epithelial-mesenchymal transition of MCF-7 cells by suppressing miR-21 expression. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1265–1271.
- Li, Y.; Zeng, Q.G.; Qiu, J.L.; Pang, T.; Wang, H.; Zhang, X.X. Propofol suppresses migration, invasion, and epithelial-mesenchymal transition in papillary thyroid carcinoma cells by regulating miR-122 expression. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5101–5110.
- Liu, W.Z.; Liu, N. Propofol inhibits lung cancer A549 Cell growth and epithelial-mesenchymal transition process by upregulation of microRNA-1284. Oncol. Res. 2018, 27, 1–8.
- Liu, F.; Qiu, F.; Fu, M.; Chen, H.; Wang, H. Propofol reduces epithelial to mesenchymal transition, invasion and migration of gastric cancer cells through the microRNA-195-5p/Snail axis. Med. Sci. Monit. 2020, 26, e920981.
- Liu, Y.; Wang, X.; Li, H.; Guan, E.; Luo, K. Propofol ameliorates the proliferation and epithelial-mesenchymal transition of hepatoma carcinoma cells via non-coding RNA activated by DNA damage (NORAD)/microRNA (miR)-556-3p/migration and invasion enhancer 1 (MIEN1) axis. J. Environ. Pathol. Toxicol. Oncol. 2021, 40, 87–97.
- Shi, H.; Yan, C.; Chen, Y.; Wang, Z.; Guo, J.; Pei, H. Propofol inhibits the proliferation, migration, invasion and epithelial to mesenchymal transition of renal cell carcinoma cells by regulating microRNA-363/Snail1. Am. J. Transl. Res. 2021, 13, 2256–2269.
- Qian, J.; Shen, S.; Chen, W.; Chen, N. Propofol reversed hypoxia-induced docetaxel resistance in prostate cancer cells by preventing epithelial-mesenchymal transition by inhibiting hypoxia-inducible factor 1α. Biomed. Res. Int. 2018, 2018, 4174232.
- Cao, C.; Zhang, X.; Xu, Y. Propofol prevents the aggressive progression of oral squamous cell carcinoma via regulating circ_0005623/miR-195-5p/HOXB7 axis. Biotechnol. Appl. Biochem. 2021; Epub ahead of print.
- Tripolt, S.; Neubauer, H.A.; Knab, V.M.; Elmer, D.P.; Aberger, F.; Moriggl, R.; Fux, D.A. Opioids drive breast cancer metastasis through the δ-opioid receptor and oncogenic STAT3. Neoplasia 2021, 23, 270–279.
- Lennon, F.E.; Mirzapoiazova, T.; Mambetsariev, B.; Poroyko, V.A.; Salgia, R.; Moss, J.; Singleton, P.A. The Mu opioid receptor promotes opioid and growth factor-induced proliferation, migration and epithelial mesenchymal transition (EMT) in human lung cancer. PLoS ONE 2014, 9, e91577.
- Zhang, J.; Yao, N.; Tian, S. Morphine stimulates migration and growth and alleviates the effects of chemo drugs via AMPK-dependent induction of epithelial-mesenchymal transition in esophageal carcinoma cells. Biol. Pharm. Bull. 2020, 43, 774–781.
- Tang, H.; Li, C.; Wang, Y.; Deng, L. Sufentanil inhibits the proliferation and metastasis of esophageal cancer by inhibiting the NF-κB and snail signaling Pathways. J. Oncol. 2021, 2021, 7586100.
- Liu, C.; Yu, M.; Li, Y.; Wang, H.; Xu, C.; Zhang, X.; Li, M.; Guo, H.; Ma, D.; Guo, X. Lidocaine inhibits the metastatic potential of ovarian cancer by blocking NaV 1.5-mediated EMT and FAK/Paxillin signaling pathway. Cancer Med. 2021, 10, 337–349.
- Chan, S.M.; Lin, B.F.; Wong, C.S.; Chuang, W.T.; Chou, Y.T.; Wu, Z.F. Levobuipivacaine-induced dissemination of A549 lung cancer cells. Sci. Rep. 2017, 7, 8646.
- Santoro, A.; Bufo, P.; Russo, G.; Cagiano, S.; Papagerakis, S.; Bucci, P.; Aquino, G.; Longo, F.; Feola, A.; Giordano, A.; et al. Expression and clinical implication of cyclooxygenase-2 and E-cadherin in oral squamous cell carcinomas. Cancer Biol Ther. 2020, 21, 667–674.
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530.
- Sun, J.; Li, Y.; Liu, L.; Jiang, Z.; Liu, G. Aspirin use and pancreatic cancer risk: A systematic review of observational studies. Medicine 2019, 98, e18033.
- Risch, H.A.; Lu, L.; Streicher, S.A.; Wang, J.; Zhang, W.; Ni, Q.; Kidd, M.S.; Yu, H.; Gao, Y.T. Aspirin use and reduced risk of pancreatic cancer. Cancer Epidemiol. Biomark. Prev. 2017, 26, 68–74.
- Pretzsch, E.; D’Haese, J.G.; Renz, B.; Ilmer, M.; Schiergens, T.; Miksch, R.C.; Albertsmeier, M.; Guba, M.; Angele, M.K.; Werner, J.; et al. Effect of platelet inhibition with perioperative aspirin on survival in patients undergoing curative resection for pancreatic cancer: A propensity score matched analysis. BMC Surg. 2021, 21, 98.
- Steele, G.L.; Dudek, A.Z.; Gilmore, G.E.; Richter, S.A.; Olson, D.A.; Eklund, J.P.; Zylla, D.M. Impact of pain, opioids, and the mu-opioid receptor on progression and survival in patients with newly diagnosed stage IV pancreatic cancer. Am. J. Clin. Oncol. 2020, 43, 591–597.
- Abdel-Rahman, O.; Karachiwala, H.; Easaw, J.C. Outcomes of patients with advanced gastrointestinal cancer in relationship to opioid use: Findings from eight clinical trials. J. Natl. Compr. Cancer Netw. 2020, 18, 575–581.
- Call, T.R.; Pace, N.L.; Thorup, D.B.; Maxfield, D.; Chortkoff, B.; Christensen, J.; Mulvihill, S.J. Factors associated with improved survival after resection of pancreatic adenocarcinoma: A multivariable model. Anesthesiology 2015, 122, 317–324.
- Zylberberg, H.M.; Woodrell, C.; Rustgi, S.D.; Aronson, A.; Kessel, E.; Amin, S.; Lucas, A.L. Opioid prescription is associated with increased survival in older adult patients with pancreatic cancer in the United States: A propensity score analysis. JCO Oncol. Pract. 2022, 18, e659–e668.
- Chen, W.; Xu, Y.; Zhang, Y.; Lou, W.; Han, X. Positive impact of intraoperative epidural ropivacaine infusion on oncologic outcomes in pancreatic cancer patients undergoing pancreatectomy: A retrospective cohort study. J. Cancer 2021, 12, 4513–4521.
- Cata, J.P.; Ramirez, M.F.; Velasquez, J.F.; Di, A.I.; Popat, K.U.; Gottumukkala, V.; Black, D.M.; Lewis, V.O.; Vauthey, J.N. Lidocaine stimulates the function of natural killer cells in different experimental settings. Anticancer Res. 2017, 37, 4727–4732.
- Alexander, A.; Lehwald-Tywuschik, N.; Rehders, A.; Rabenalt, S.; Verde, P.E.; Eisenberger, C.F.; Picker, N.; Knoefel, W.T.; Kienbaum, P. Peridural anesthesia and cancer-related survival after surgery for pancreatic cancer-A retrospective cohort study. Clin. Pract. 2021, 11, 532–542.
- Chen, X.; Wu, Q.; You, L.; Chen, S.; Zhu, M.; Miao, C. Propofol attenuates pancreatic cancer malignant potential via inhibition of NMDA receptor. Eur. J. Pharmacol. 2017, 795, 150–159.
- Yu, X.; Shi, J.; Wang, X.; Zhang, F. Propofol affects the growth and metastasis of pancreatic cancer via ADAM8. Pharmacol. Rep. 2020, 72, 418–426.
- Wang, H.; Jiao, H.; Jiang, Z.; Chen, R. Propofol inhibits migration and induces apoptosis of pancreatic cancer PANC-1 cells through miR-34a-mediated E-cadherin and LOC285194 signals. Bioengineered 2020, 11, 510–521.
- Du, Q.H.; Xu, Y.B.; Zhang, M.Y.; Yun, P.; He, C.Y. Propofol induces apoptosis and increases gemcitabine sensitivity in pancreatic cancer cells in vitro by inhibition of nuclear factor-κB activity. World J. Gastroenterol. 2013, 19, 5485–5492.
- Yu, X.; Gao, Y.; Zhang, F. Propofol inhibits pancreatic cancer proliferation and metastasis by up-regulating miR-328 and down-regulating ADAM8. Basic Clin. Pharmacol. Toxicol. 2019, 125, 271–278.
- Gao, Y.; Yu, X.; Zhang, F.; Dai, J. Propofol inhibits pancreatic cancer progress under hypoxia via ADAM8. J. Hepatobiliary Pancreat Sci. 2019, 26, 219–226.
- Gao, Y.; Zhou, Y.; Wang, C.; Sample, K.M.; Yu, X.; Ben-David, Y. Propofol mediates pancreatic cancer cell activity through the repression of ADAM8 via SP1. Oncol. Rep. 2021, 46, 249.
- Wang, Z.T.; Gong, H.Y.; Zheng, F.; Liu, D.J.; Dong, T.L. Propofol suppresses proliferation and invasion of pancreatic cancer cells by upregulating microRNA-133a expression. Genet. Mol. Res. 2015, 14, 7529–7537.
- Liu, Z.; Zhang, J.; Hong, G.; Quan, J.; Zhang, L.; Yu, M. Propofol inhibits growth and invasion of pancreatic cancer cells through regulation of the miR-21/Slug signaling pathway. Am. J. Transl. Res. 2016, 8, 4120–4133.
- Zhang, Y.; Liu, L.; Fan, P.; Bauer, N.; Gladkich, J.; Ryschich, E.; Bazhin, A.V.; Giese, N.A.; Strobel, O.; Hackert, T.; et al. Aspirin counteracts cancer stem cell features, desmoplasia and gemcitabine resistance in pancreatic cancer. Oncotarget 2015, 6, 9999–10015.
- Zhang, Y.; Kirane, A.; Huang, H.; Sorrelle, N.B.; Burrows, F.J.; Dellinger, M.T.; Brekken, R.A. Cyclooxygenase-2 inhibition potentiates the efficacy of vascular endothelial growth factor blockade and promotes an immune stimulatory microenvironment in preclinical models of pancreatic cancer. Mol. Cancer Res. 2019, 17, 348–355.
- Perugini, R.A.; McDade, T.P.; Vittimberga, F.J., Jr.; Duffy, A.J.; Callery, M.P. Sodium salicylate inhibits proliferation and induces G1 cell cycle arrest in human pancreatic cancer cell lines. J. Gastrointest. Surg. 2000, 4, 24–32.
- Sun, L.; Chen, K.; Jiang, Z.; Chen, X.; Ma, J.; Ma, Q.; Duan, W. Indometacin inhibits the proliferation and activation of human pancreatic stellate cells through the downregulation of COX-2. Oncol. Rep. 2018, 39, 2243–2251.
- Celik, F.; Duran, T. Effects of fentanyl on pancreatic cancer cell proliferation and cancer stem cell differentiation. Cell Mol. Biol. 2019, 65, 21–25.
- Bundscherer, A.; Malsy, M.; Gebhardt, K.; Metterlein, T.; Plank, C.; Wiese, C.H.; Gruber, M.; Graf, B.M. Effects of ropivacaine, bupivacaine and sufentanil in colon and pancreatic cancer cells in vitro. Pharmacol. Res. 2015, 95, 126–131.
- Oshima, Y.; Sano, M.; Kajiwara, I.; Ichimaru, Y.; Itaya, T.; Kuramochi, T.; Hayashi, E.; Kim, J.; Kitajima, O.; Masugi, Y.; et al. Midazolam exhibits antitumour and anti-inflammatory effects in a mouse model of pancreatic ductal adenocarcinoma. Br. J. Anaesth. 2022, 128, 679–690.
- Malsy, M.; Graf, B.; Bundscherer, A. The effects of analgesics and local anesthetics on gene transcription mediated by NFATc2 and Sp1 in pancreatic carcinoma. Anticancer Res. 2019, 39, 4721–4728.
- Malsy, M.; Gebhardt, K.; Gruber, M.; Wiese, C.; Graf, B.; Bundscherer, A. Effects of ketamine, s-ketamine, and MK 801 on proliferation, apoptosis, and necrosis in pancreatic cancer cells. BMC Anesthesiol. 2015, 15, 111.
- Jin, X.; Dai, L.; Ma, Y.; Wang, J.; Liu, Z. Implications of HIF-1α in the tumorigenesis and progression of pancreatic cancer. Cancer Cell Int. 2020, 20, 273.
- Kim, R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J. Transl. Med. 2018, 16, 8.
- Benzonana, L.L.; Perry, N.J.; Watts, H.R.; Yang, B.; Perry, I.A.; Coombes, C.; Takata, M.; Ma, D. Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology 2013, 119, 593–605.
- Ishikawa, M.; Iwasaki, M.; Zhao, H.; Saito, J.; Hu, C.; Sun, Q.; Sakamoto, A.; Ma, D. Inhalational anesthetics inhibit neuroglioma cell proliferation and migration via miR-138, -210 and -335. Int. J. Mol. Sci. 2021, 22, 4355.
- Schoos, A.; Gabriel, C.; Knab, V.M.; Fux, D.A. Activation of HIF-1α by δ-opioid receptors induces COX-2 expression in breast cancer cells and leads to paracrine activation of vascular endothelial cells. J. Pharmacol. Exp. Ther. 2019, 370, 480–489.
- Koodie, L.; Ramakrishnan, S.; Roy, S. Morphine suppresses tumor angiogenesis through a HIF-1alpha/p38MAPK pathway. Am. J. Pathol. 2010, 177, 984–997.
- Okamoto, A.; Sumi, C.; Tanaka, H.; Kusunoki, M.; Iwai, T.; Nishi, K.; Matsuo, Y.; Harada, H.; Takenaga, K.; Bono, H.; et al. HIF-1-mediated suppression of mitochondria electron transport chain function confers resistance to lidocaine-induced cell death. Sci. Rep. 2017, 7, 3816.
- Zhou, Y.; Dong, X.; Xiu, P.; Wang, X.; Yang, J.; Li, L.; Li, Z.; Sun, P.; Shi, X.; Zhong, J. Meloxicam, a selective COX-2 inhibitor, mediates hypoxia-inducible factor- (HIF-) 1α signaling in hepatocellular carcinoma. Oxid. Med. Cell Longev. 2020, 2020, 7079308.
- Yue, H.; Liu, L.; Song, Z. miR-212 regulated by HIF-1α promotes the progression of pancreatic cancer. Exp. Ther. Med. 2019, 17, 2359–2365.
- Qu, D.; Zou, X.; Liu, Z. Propofol modulates glycolysis reprogramming of ovarian tumor via restraining circular RNA-zinc finger RNA-binding protein/microRNA-212-5p/superoxide dismutase 2 axis. Bioengineered 2022, 13, 11881–11892.
- Si, J.; Jin, Y.; Cui, M.; Yao, Q.; Li, R.; Li, X. Neuroprotective effect of miR-212-5p on isoflurane-induced cognitive dysfunction by inhibiting neuroinflammation. Toxicol. Mech. Methods 2021, 31, 501–506.
- He, X.; Yang, Y.; Zhi, F.; Moore, M.L.; Kang, X.; Chao, D.; Wang, R.; Balboni, G.; Salvadori, S.; Kim, D.H.; et al. δ-Opioid receptor activation modified microRNA expression in the rat kidney under prolonged hypoxia. PLoS ONE 2013, 8, e61080.
- Raji, S.; Sahranavard, M.; Mottaghi, M.; Sahebkar, A. MiR-212 value in prognosis and diagnosis of cancer and its association with patient characteristics: A systematic review and meta-analysis. Cancer Cell Int. 2022, 22, 163.
- Xie, J.; Zhou, X.; Wang, R.; Zhao, J.; Tang, J.; Zhang, Q.; Du, Y.; Pang, Y. Identification of potential diagnostic biomarkers in MMPs for pancreatic carcinoma. Medicine 2021, 100, e26135.
- Bramhall, S.R.; Neoptolemos, J.P.; Stamp, G.W.; Lemoine, N.R. Imbalance of expression of matrix metalloproteinases (MMPs) and tissue inhibitors of the matrix metalloproteinases (TIMPs) in human pancreatic carcinoma. J. Pathol. 1997, 182, 347–355.
- Jones, L.E.; Humphreys, M.J.; Campbell, F.; Neoptolemos, J.P.; Boyd, M.T. Comprehensive analysis of matrix metalloproteinase and tissue inhibitor expression in pancreatic cancer: Increased expression of matrix metalloproteinase-7 predicts poor survival. Clin. Cancer Res. 2004, 10, 2832–2845.
- Matsuyama, Y.; Takao, S.; Aikou, T. Comparison of matrix metalloproteinase expression between primary tumors with or without liver metastasis in pancreatic and colorectal carcinomas. J. Surg. Oncol. 2002, 80, 105–110.
- Mondal, S.; Adhikari, N.; Banerjee, S.; Amin, S.A.; Jha, T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur. J. Med. Chem. 2020, 194, 112260.
- Wang, G.; Liu, J.; Gao, J.; Zheng, X. Comparison of the effects of sevoflurane and propofol anesthesia on pulmonary function, MMP-9 and postoperative cognition in patients receiving lung cancer resection. Oncol. Lett. 2019, 17, 3399–3405.
- Müller-Edenborn, B.; Roth-Zʼgraggen, B.; Bartnicka, K.; Borgeat, A.; Hoos, A.; Borsig, L.; Beck-Schimmer, B. Volatile anesthetics reduce invasion of colorectal cancer cells through down-regulation of matrix metalloproteinase-9. Anesthesiology 2012, 117, 293–301.
- Cao, Y.; Lv, W.; Ding, W.; Li, J. Sevoflurane inhibits the proliferation and invasion of hepatocellular carcinoma cells through regulating the PTEN/Akt/GSK-3β/β-catenin signaling pathway by downregulating miR-25-3p. Int. J. Mol. Med. 2020, 46, 97–106.
- Zhang, X.L.; Chen, M.L.; Zhou, S.L. Fentanyl inhibits proliferation and invasion of colorectal cancer via β-catenin. Int. J. Clin. Exp. Pathol. 2015, 8, 227–235.
- Afsharimani, B.; Baran, J.; Watanabe, S.; Lindner, D.; Cabot, P.J.; Parat, M.O. Morphine and breast tumor metastasis: The role of matrix-degrading enzymes. Clin. Exp. Metastasis 2014, 31, 149–158.
- Piegeler, T.; Schläpfer, M.; Dull, R.O.; Schwartz, D.E.; Borgeat, A.; Minshall, R.D.; Beck-Schimmer, B. Clinically relevant concentrations of lidocaine and ropivacaine inhibit TNFα-induced invasion of lung adenocarcinoma cells in vitro by blocking the activation of Akt and focal adhesion kinase. Br. J. Anaesth. 2015, 115, 784–791.
- Kashefi, S.; Ahmadi, H.; Omranipour, R.; Mahmoodzadeh, H.; Jafarnezhad-Ansariha, F.; Tofighi Zavareh, F.; Mirshafiey, A. The anti-tumoral effect of β-D-mannuronic acid (M2000) as a novel NSAID on Treg cells frequency and MMP-2, MMP-9, CCL22 and TGFβ1 gene expression in pre-surgical breast cancer patients. Iran. J. Allergy Asthma Immunol. 2019, 18, 80–90.
- Lee, Y.M.; Song, B.C.; Yeum, K.J. Impact of volatile anesthetics on oxidative stress and inflammation. Biomed. Res. Int. 2015, 2015, 242709.
- Gukovsky, I.; Li, N.; Todoric, J.; Gukovskaya, A.; Karin, M. Inflammation, autophagy, and obesity: Common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology 2013, 144, 1199–1209.
- Shadhu, K.; Xi, C. Inflammation and pancreatic cancer: An updated review. Saudi J. Gastroenterol. 2019, 25, 3–13.
- Wall, T.; Sherwin, A.; Ma, D.; Buggy, D.J. Influence of perioperative anaesthetic and analgesic interventions on oncological outcomes: A narrative review. Br. J. Anaesth. 2019, 123, 135–150.
- Ackerman, R.S.; Luddy, K.A.; Icard, B.E.; Piñeiro Fernández, J.; Gatenby, R.A.; Muncey, A.R. The effects of anesthetics and perioperative medications on immune function: A narrative review. Anesth. Analg. 2021, 133, 676–689.
- Yamaguchi, A.; Kawagoe, I.; Inoue, S.; Kochiyama, T.; Fukuda, M.; Saito, M.; Hayashida, M. Propofol decreases CD8+ T cells and sevoflurane increases regulatory T cells after lung cancer resection: A randomized controlled trial. J. Thorac. Dis. 2021, 13, 5430–5438.
- Zhu, R.; Xiang, J.; Tan, M. Effects of different anesthesia and analgesia on cellular immunity and cognitive function of patients after surgery for esophageal cancer. Minerva Chir. 2020, 75, 449–456.
- Zhou, M.; Liu, W.; Peng, J.; Wang, Y. Impact of propofol epidural anesthesia on immune function and inflammatory factors in patients undergoing gastric cancer surgery. Am. J. Transl. Res. 2021, 13, 3064–3073.
- Xu, Q.; Shi, N.J.; Zhang, H.; Zhu, Y.M. Effects of combined general-epidural anesthesia and total intravenous anesthesia on cellular immunity and prognosis in patients with non-small cell lung cancer: A comparative study. Mol. Med. Rep. 2017, 16, 4445–4454.
- Chen, J.; Luo, F.; Lei, M.; Chen, Z. A study on cellular immune function of patients treated with radical resection of pulmonary carcinoma with two different methods of anesthesia and analgesia. J. Buon 2017, 22, 1416–1421.
- Jiang, S.; Liu, Y.; Huang, L.; Zhang, F.; Kang, R. Effects of propofol on cancer development and chemotherapy: Potential mechanisms. Eur. J. Pharmacol. 2018, 831, 46–51.
- Boland, J.W.; Pockley, A.G. Influence of opioids on immune function in patients with cancer pain: From bench to bedside. Br. J. Pharmacol. 2018, 175, 2726–2736.
- Franchi, S.; Panerai, A.E.; Sacerdote, P. Buprenorphine ameliorates the effect of surgery on hypothalamus-pituitary-adrenal axis, natural killer cell activity and metastatic colonization in rats in comparison with morphine or fentanyl treatment. Brain Behav. Immun. 2007, 21, 767–774.
- Votta-Velis, E.G.; Piegeler, T.; Minshall, R.D.; Aguirre, J.; Beck-Schimmer, B.; Schwartz, D.E.; Borgeat, A. Regional anaesthesia and cancer metastases: The implication of local anaesthetics. Acta Anaesthesiol. Scand. 2013, 57, 1211–1229.
- Zuckerman, L.M.; Frames, W.L.; Elsissy, J.G.; Shields, T.G.; de Necochea-Campion, R.; Mirshahidi, H.R.; Williams, N.L.; Mirshahidi, S. The effect of non-steroidal anti-inflammatory drugs on osteosarcoma cells. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2681–2690.
- Kundović, S.A.; Rašić, D.; Popović, L.; Peraica, M.; Črnjar, K. Oxidative stress under general intravenous and inhalation anaesthesia. Arh. Hig. Rada Toksikol. 2020, 71, 169–177.
- Huang, H.; Sun, J.; Li, Z.; Zhang, X.; Li, Z.; Zhu, H.; Yu, X. Impact of the tumor immune microenvironment on the outcome of pancreatic cancer: A retrospective study based on clinical pathological analysis. Gland Surg. 2022, 11, 472–482.
- Li, X.; Lin, H.; Ouyang, R.; Yang, Y.; Peng, J. Prognostic significance of the systemic immune-inflammation index in pancreatic carcinoma patients: A meta-analysis. Biosci. Rep. 2021, 41, BSR20204401.
- Jin, Y.; Zhao, X.; Li, H.; Wang, Z.; Wang, D. Effects of sevoflurane and propofol on the inflammatory response and pulmonary function of perioperative patients with one-lung ventilation. Exp. Ther. Med. 2013, 6, 781–785.
- Schilling, T.; Kozian, A.; Senturk, M.; Huth, C.; Reinhold, A.; Hedenstierna, G.; Hachenberg, T. Effects of volatile and intravenous anesthesia on the alveolar and systemic inflammatory response in thoracic surgical patients. Anesthesiology 2011, 115, 65–74.
- Fant, F.; Tina, E.; Sandblom, D.; Andersson, S.O.; Magnuson, A.; Hultgren-Hörnkvist, E.; Axelsson, K.; Gupta, A. Thoracic epidural analgesia inhibits the neuro-hormonal but not the acute inflammatory stress response after radical retropubic prostatectomy. Br. J. Anaesth. 2013, 110, 747–757.
