Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Atrouli Chatterjee and Version 2 by Conner Chen.
Most of us get inspired by and interact with the world around us based on visual cues such as the colors and patterns that we see. In nature, coloration takes three primary forms: pigmentary coloration, structural coloration, and bioluminescence. Typically, pigmentary and structural coloration are used by animals and plants for their survival. These forms of coloration are enabled by a variety of intracellular biophotonic structures.
- structural color
- protein
- pigmentary color
- biophotonic
1. Introduction
From the words on this page to the scenery outside, we as a species are extremely dependent on, and in many ways, inspired by the colors and patterns that we see around us. Coloration in nature can be categorized into three forms: pigmentary coloration, structural coloration, and bioluminescence (Figure 1) [1][2][3][4][5][6][7][8]. Pigmentary coloration is typically angle-independent coloration, where natural pigments directly absorb or reflect specific wavelengths of light (Figure 1A) [1][2][3][4][5][6][7][8]. Structural coloration is often angle-dependent and involves the interference (i.e., reflection, transmittance, or scattering) of light by biological micro- or nano-structures with contrasting refractive indices (Figure 1B) [1][2][3][4][5][6][7][8]. In comparison, bioluminescence typically involves the exergonic reactions of oxygen with different substrates and enzymes that lead to the production of visible photons of light (Figure 1C) [1][2][3][4][5][6][7][8]. The variety of colors that result from these three photonic mechanisms in nature serves numerous functions, including camouflage, mimicry, aposematism, signaling, photosynthesis, and self-protection [1][2][3][4][5][6][7][8]. These natural utility of such structures have also inspired and led to the design and engineering of materials with dynamic color changing capabilities (e.g., for infrared camouflage), anti-counterfeiting technologies, and a variety of displays and sensors [9][10][11][12]. NHevertheless, the fundamental understanding of the molecular mechanisms that enable the formation (i.e., self-assembly) and dynamic tunability of these natural structures has been limited, which in turn has hindered the applications and engineering of biomolecule-based optical technologies. In the following contents,rein, we highlight a few examples of pigmentary and structural coloration in nature will be showcased, with emphasis on cephalopods as model organisms for dynamic structural colorationfound in nature.
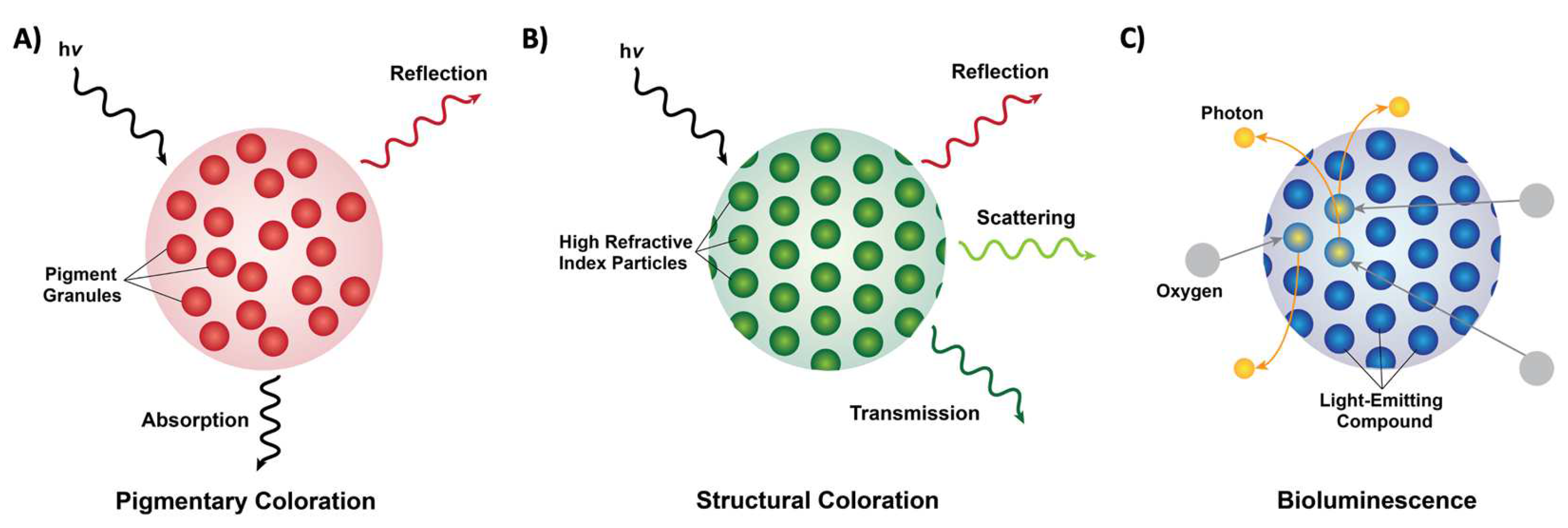
Figure 1. Forms of coloration in nature. (A) A schematic of pigmentary coloration in nature, where the incident light (hv) is absorbed or reflected by pigment granules (red circles), with no apparent long-range order. (B) A schematic of structural coloration in nature, where the incident light (hv) is reflected, scattered, or transmitted by high refractive index particles (green circles) showing long-range order. (C) A schematic of bioluminescence in nature, where oxygen (grey circles) reacts with a light-emitting compound such as luciferin (blue circles) and releases photons (yellow circles).
2. Pigmentary and Structural Coloration in Nature
Pigmentary coloration allows animals and plants to maintain bright coloration, which is often used to distinguish between species. Such forms of coloration are widely found in many mammals, birds, butterflies, fish and other marine organisms, and plants [13][14][15][16][17][18][19][20][21][22][23][24][25][26][27]. For example, mammalian skin and hair/fur typically contain melanin variants which give the animals their specific colorations [13][14]. Interestingly, pigments are also found in the eye, within retinal pigment epithelial (RPE) cells in humans, where melanin and lipofuscin serve to absorb light and thereby reduce light damage in the eye [15][16]. Similar to humans and other mammals, melanin is also a common pigment found in bird feathers and exposed structures (e.g., skin and legs) [17][18]. The colors of birds are particularly diverse because of their reliance on visual cues for communication [17][18]. Typically, birds produce both eumelanins (black, brown, and grey coloration) and pheomelanins (yellow and red coloration) via cells known as melanosomes, where coated vesicles are known to actively transport chemical precursors to induce the formation of different melanins [17][18]. In addition to melanins, birds are also known to utilize carotenoids (largely acquired via their diet), along with other pigments, including flavins and porphyrins [17][18]. On the other hand, butterflies, which also boast a wide variety of colors and patterns, have papiliochrome pigments (yellow coloration), the carotenoid lutein (blue–green coloration), and variants of these pigments depending on the species [19][20][21][22]. Notably, butterflies also use photoreceptors in their eyes to distinguish colors, likely in order to identify food sources, for mating, and to identify the locations of their host plants [23]. In contrast, fish have a variety of chromatophores (cells with pigment-based structures), including melanophores (which contain melanin), xanthophores (which contain yellow carotinoid-based pigments), and erythrophores (which contain red carotenoid-based pigments) [24][25]. More broadly, marine organisms also utilize carotenoids for their coloration, along with tetrapyrroles, quinones, azulenes, and melanins [26]. Some of the pigments found in these organisms are derived from their diets (e.g., carotenoids and tetrapyrroles), are found in the ink released by the organisms (e.g., tetrapyrroles in the purple ink of sea hares and melanin in the ink of cephalopods), or are produced by the organisms themselves (e.g., carotenoids in the lower trophic levels and anthraquinones in marine fungi) [26]. In comparison, the roles and diversity of pigments in plants likely surpass those known for animals, and specifically, plant pigmentation has a long history that is closely tied to the extraction and utilization of plant pigments as dyes and paints [27][28][29]. As one example, plants’ ability to perform photosynthesis is also critically dependent on pigment molecules such as chlorophylls (green pigments) and carotenoids (yellow, orange, and red pigments) [27][28][29]. Pigments such as flavonoids, including anthocyanins (pale yellow, pink/red, blue, and black pigments), and betalains (yellow or red pigments) can be found in the epidermal cells of flower petals, and are vital as visual signals for pollination and seed scattering, and thus in the interactions between plants and animals [27][28][29]. Altogether, pigments and pigment-based coloration represent a vibrant chemical modality for generating coloration; however, they generally produce angle-independent coloration that cannot be completely turned on or off, unlike structural coloration.
Structural coloration is also found in a variety of organisms, but in comparison to pigmentary coloration, allows the organism to generate colors at very specific wavelengths depending on the geometry (sizes and order) of the structures formed. For example, guanine crystals are found in the iridocytes of giant clams (Tridacninae) and form hexagonal structures below the cuticles of Sapphirinid copepods (Figure 2), where the arrangement and geometries of the structures formed by the guanine crystals lead to very different spectra observed for the animals [30][31]. Other forms of structural colors in organisms include 1D photonic crystals, as observed in Japanese jewel beetles (Chrysochroa fulgidissima) and other buprestids, Cicindela scutellaris beetles, Papilio ulysses butterflies, and the Coeligena prunellei hummingbird (Figure 3A–C) [32][33][34]. These insects and birds have alternating layers of chitin/melanin, chitin/air, or air/melanin in a keratin matrix with different periodicities that result in either the metallic hues of the beetles or the blue–green colors observed in the butterflies and hummingbirds [32][33][34]. Other structures, such as the lamellar membrane morphology observed in the iridosomes of zebrafish and the Delabrea michieana fruit enable narrowband light reflectance or iridescence (Figure 3D,E) [35][36]. In general, the iridosomes reflect specific wavelengths of light with particulate structures formed from high-refractive-index materials arranged within ribbon-like structures that form a one dimensional photonic crystal-like architecture composed of alternating high and low refractive index areas in the cells [35][36]. Similar multilayer reflectors have also been observed in various other species of butterflies and fish [17][18][19][20][21][22]. Althoughgether these examples demonstrate the broad rangediversity of structures and thus examples of that enable structural coloration, the colors themselves are typically static (i.e., unchanging or changing over relatively long time-scales) or are influenced by the viewing angle of the observer rather than controlled by the organism itself in nature.
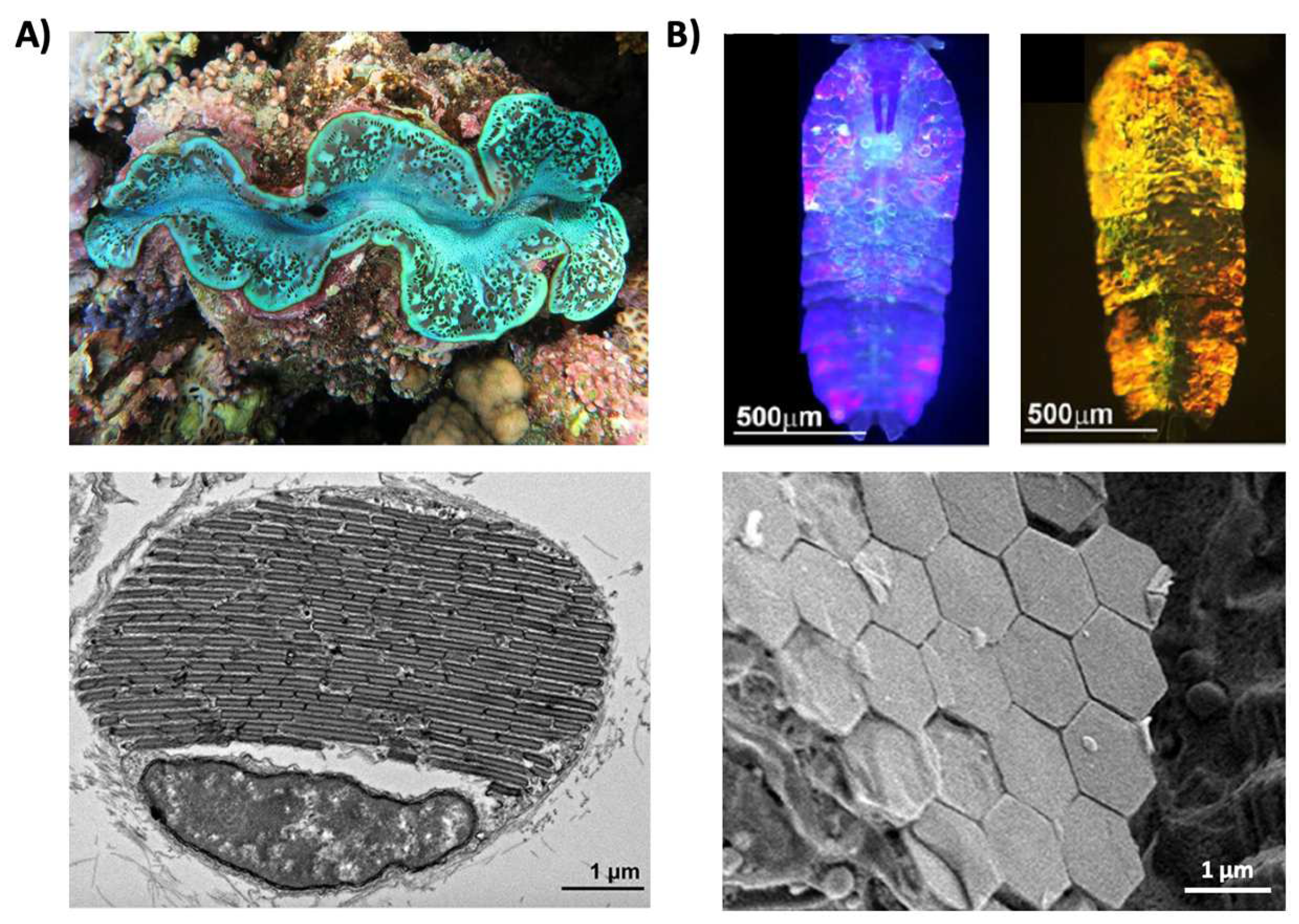
Figure 2. Structural color derived from guanine crystals. (A) (Top) An image of a giant clam (Tridacninae). (Bottom) An electron microscopy image of a giant clam iridocyte cell stacked with alternating layers of guanine crystal plates and cytoplasm sheets. (B) (Top) Images of male Sapphirinid copepods. (Bottom) A cryo-scanning electron microscopy image of an S. metallina copepod, showing hexagonal guanine crystals within iridophores below a chitin procuticle.
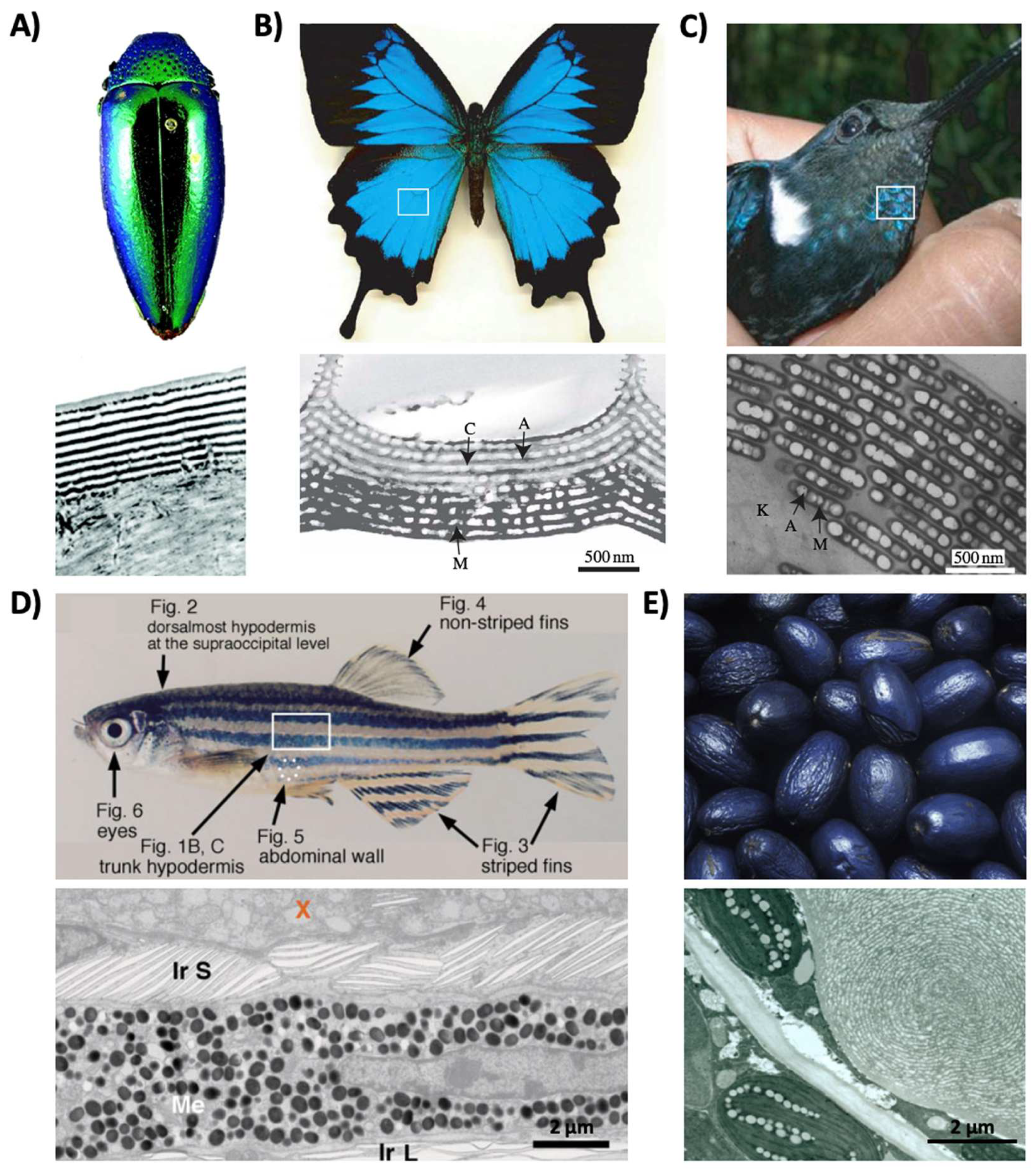
Figure 3. Structural color derived from 1D photonic crystal-like structures and iridophore cells. (A) (Top) An image of multilayer color in a buprestid or jewel beetle. (Bottom) An electron microscopy image of the cuticle of a Cicindela scutellaris beetle. (B) (Top) An image of a P. ulysses butterfly. (Bottom) A transmission electron microscopy image of the blue scale of the butterfly showing chitin (C) and air (A) arrays; the lower portion contains diffuse melanin (M). (C) (Top) An image of the C. prunellei hummingbird. (Bottom) A transmission microscopy image of a green barbule from a C. iris hummingbird, showing ordered layers of air (A) and melanin (M) in a keratin (K) matrix. (D) (Top) An image of a zebrafish. (Bottom) A transmission electron microscopy image of a portion of the zebrafish hypodermis showing xanthophores (X), type-S iridophores (Ir S), melanophores (Me), and type-L iridophores (Ir L). (E) (Top) An image of a D. michieana fruit. (Bottom) A transmission electron microscopy image of a transverse cross-section of a D. michieana fruit showing iridosomes.
References
- Barrows, F.P.; Bartl, M.H. Photonic structures in biology: A possible blueprint for nanotechnology. Nanomater. Nanotechnol. 2014, 4, 1.
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, 37.
- Vukusic, P.; Sambles, J.R. Photonic structures in biology. Nature 2003, 424, 852–855.
- Sun, J.; Bhushan, B.; Tong, J. Structural coloration in nature. RSC Adv. 2013, 3, 14862–14889.
- Parker, A.R. 515 million years of structural colour. J. Opt. A Pure Appl. Opt. 2000, 2, 15.
- Vukusic, P.; Stavenga, D.G. Physical methods for investigating structural colors in biological systems. J. R. Soc. Interface 2009, 6, S133–S148.
- Haddock, S.H.D.; Moline, M.A.; Case, J.F. Bioluminescence in the Sea. Annu. Rev. Mar. Sci. 2010, 2, 443–493.
- Wilson, T.; Hastings, J.W. Bioluminescence. Annu. Rev. Cell Dev. Biol. 1998, 14, 197–230.
- Shang, L.; Zhang, W.; Xu, K.; Zhao, Y. Bio-inspired intelligent structural color materials. Mater. Horiz. 2019, 6, 945–958.
- Kreit, E.; Mathger, L.M.; Hanlon, R.T.; Dennis, P.B.; Naik, R.R.; Forsythe, E.; Heikenfeld, J. Biological versus electronic adaptive coloration : How can one inform the other? J. R. Soc. Interface 2013, 10, 20120601.
- Phan, L.; Kautz, R.; Leung, E.M.; Naughton, K.L.; Dyke, Y.V.; Gorodetsky, A.A. Dynamic materials inspired by cephalopods. Chem. Mater. 2016, 19, 6804–6816.
- Chatterjee, A.; Norton-Baker, B.; Bagge, L.E.; Patel, P.; Gorodetsky, A.A. An introduction to color-changing systems from the cephalopod protein reflectin. Bioinspir. Biomim. 2018, 13, 045001.
- Pawelek, J.; Wong, G.; Sansone, M.; Morowitz, J. Molecular controls in mammalian pigmentation. Yale J. Biol. Med. 1973, 46, 430–443.
- Aspengren, S.; Hedberg, D.; Skold, H.N.; Wallin, M. New insights into melanosome transport in vertebrate pigment cells. Int. Rev. Cell Mol. Biol. 2009, 272, 245–302.
- Yang, S.; Zhou, J.; Li, D. Functions and diseases of the retinal pigment epithelium. Front. Parmacol. 2021, 12, 1976.
- George, S.M.; Lu, F.; Rao, M.; Leach, L.L.; Gross, J.M. The retinal pigment epithelium: Development, injury responses, and regenerative potential in mammalian and non-mammalian systems. Prog. Retin. Eye Res. 2021, 85, 100969.
- Ralph, C.L. The control of color in birds. Am. Zool. 1969, 9, 521–530.
- Galvan, I.; Solano, F. Bird integumentary melanins: Biosynthesis, forms, function and evolution. Int. J. Mol. Sci. 2016, 17, 520.
- Rembold, H.; Rascher, J.; Eder, J.; Umebachi, Y. Partial structure of the papiliochrome, the yellow wing pigment of the papilionid butterflies. Z. Nat. C 1978, 33, 498–503.
- Koch, P.B.; Behnecke, B.; Weigmann-Lenz, M.; Ffrench-Constant, R.H. Insect pigmentation: Activities of beta-alanyldopamine synthase in wing color patterns of wild-type and melanic mutant swallowtail butterfly Papilio glaucus. Pigment Cell Res. 2000, 13, 54–58.
- Stavenga, D.G.; Giraldo, M.A.; Leertouwer, H.L. Butterfly wing colors: Glass scales of Graphium sarpedon cause polarized iridescence and enhance blue/green pigment coloration of the wing membrane. J. Exp. Biol. 2010, 213, 1731–1739.
- Wilts, B.D.; Matsushita, A.; Arikawa, K.; Stavenga, D.G. Spectrally tuned structural and pigmentary coloration of birdwing butterfly wing scales. J. R. Soc. Interface 2015, 12, 20150717.
- Blackiston, D.; Briscoe, A.D.; Weiss, M.R. Color vision and learning in the monarch butterfly, Danaus plexippus (Nymphalidae). J. Exp. Biol. 2011, 214, 509–520.
- Sugimoto, M. Morphological color changes in fish: Regulation of pigment cell density and morphology. Micros. Res. Tech. 2002, 58, 496–503.
- Hirata, M.; Nakamura, K.; Kondo, S. Pigment cell distributions in different tissues of the zebrafish, with special reference to the striped pigment pattern. Dev. Dyn. 2005, 234, 293–300.
- Pereira, D.M.; Valentao, P.; Andrade, P.B. Marine natural pigments: Chemistry, distribution and analysis. Dyes Pigm. 2014, 111, 124–134.
- Mlodzinska, E. Survey of plant pigments: Molecular and environmental determinants of plant colors. Acta Biol. Crac. Ser. Bot. 2009, 51, 7–16.
- Sandmann, G. Carotenoid biosynthesis and biotechnological application. Arch. Biochem. Biophys. 2001, 385, 4–12.
- Pourcel, L.; Routaboul, J.-M.; Cheynier, V.; Lepiniec, L.; Debeaujon, I. Flavonoid oxidation in plants: From biochemical properties to physiological functions. Trends Plant Sci. 2007, 12, 29–36.
- Rossbach, S.; Subedi, R.C.; Ng, T.K.; Ooi, B.S.; Duarte, C.M. Iridocytes mediate photonic cooperation between giant clams (Tridacninae) and their photosynthetic symbionts. Front. Mar. Sci. 2020, 7, 465.
- Gur, D.; Leshem, B.; Pierantoni, M.; Farstey, V.; Oron, D.; Weiner, S.; Addadi, L. Structural basis for the brilliant colors of the Sapphirinid Copepods. J. Am. Chem. Soc. 2015, 137, 8408–8411.
- Kinoshita, S.; Yoshioka, S.; Miyazaki, J. Physics of structural colors. Rep. Prog. Phys. 2008, 71, 76401–76500.
- Seago, A.E.; Brady, P.; Vigneron, J.P.; Schultz, T.D. Gold bugs and beyond: A review of iridescence and structural colour mechanisms in beetles (Coleoptera). J. R. Soc. Interface 2008, 6, S165–S184.
- Shawkey, M.D.; Morehouse, N.I.; Vukusic, P. A protean palette: Colour materials and mixing in birds and butterflies. J. R. Soc. Interface 2009, 6, S221–S231.
- Frohnhofer, H.G.; Krauss, J.; Maischein, H.-M.; Nusslein-Volhard, C. Iridophores and their interactions with other chromatophores are required for stripe formation in zebrafish. Development 2013, 140, 2997–3007.
- Vignolini, S.; Moyroud, E.; Glover, B.J.; Steiner, U. Analysing photonic structures in plants. J. R. Soc. Interface 2013, 10, 20130394.
More
