Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 3 by Conner Chen.
Azadirachta indica (Neem) is a large tree that is native to India and is traditionally used due to its several properties, mainly to treat skin diseases, as well as its “herbicidal” activity. Its bark, leaves, seeds, fruits and flowers are widely used in medicinal treatment due to the presence of active secondary metabolites with biological effects, mainly limonoids and tetranortriterpenoids, such as azadirachtin. Thus, A. indica was studied in a variety of conditions, such as anticancer, antiseptic, anti-inflammatory and chemopreventive agents, as well as a biopesticide.
- natural cosmetics
- Neem biocompounds
1. Introduction
Azadirachta indica A. Juss, traditionally named Neem (Meliaceae), has been widely known for centuries as a source of active ingredients to develop products for health providers in remote areas. Thus, primary healthcare in developing countries has included treatments with this tree or its parts [1]. For instance, Indian traditional medicine reported cases of success that were not always scientifically tested [2]. A. indica is considered a multipurpose medicinal tree. Outstanding for its wide distribution in nature, as well as its low toxicity, Neem can be considered a natural source of cosmetic raw material for large-scale production. This tree is biologically close to Mahogany and all its parts (root, gum, leaves, flowers and fruits) can be used in agriculture, medicine and cosmetology since the seeds and leaves have a higher concentration of secondary metabolites, which are more accessible through different extraction processes. Therefore, its beneficial effects can be attributed to one or more phytochemicals, including flavonoids, for instance. In general, it has a better effect through the synergism of its constituents [3].
The concentration of phytocomponents may vary according to the mode of harvest, storage, moisture content, light, temperature and variations in pH. In recent literature, antiseptic, anti-inflammatory and chemopreventive activities were described [4]. Moreover, other pharmacological features, such as antidiabetic, hypolipidemic, hepatoprotective, antipyretic, antifertility, hypoglycemic, cardioprotective, antiulcer, neuroprotective, antioxidant, microbicidal, nematicidal and antileishmaniasis properties were described [1]. In addition, more recent scientific reports remarked on the possibility of using it as a biopesticide [1][5][6]. However, there are few studies describing its use in topical products for human health. For instance, it was evaluated in terms of the in vitro SPF (sun protection factor) values of creams containing A. indica oil in a recent study. The SPF values were higher than the commercial cream, thus, revealing this plant as an alternative for the production of multifunctional sunscreens. Moreover, such cream was inoffensive to representative skin flora [7]. Likewise, until the present date, there have been only a few efforts of in vivo testing with cosmetic samples containing A. indica ethanolic extract with satisfactory safety results [8].
2. Phytochemical Composition
The major representative phytochemical compounds are oxidized tetranortriterpenoids, such as azadirachtin A (azadirachtin), azadirachtin B (3-tigloylazadirachtol), azadirachtin D (1-tigloyl-3-acetyl-11-hydroxy-meliacarpin), azadirachtin H (11-demethoxycarbonyl azadirachtin), azadirachtin I (1-tigloyl-3acetyl-11-hydroxy-11-demethoxycarbonyl meliacarpin), azadirachtanin, azadiriadione, azadirachtolide, deacetylnimbin, epoxyazadiradione, isoazadirolide, margosinolide, nimbin, nimbolin A, nimbandiol, nimocinol, nimbinene, nimbocinone, nimbocinolide, nimocin, nimbolide, salannin and related derivatives [3][9][10][11]. Many of these have specific physiological functions, mainly in the defense against harmful environmental factors, such as light, predators, microorganisms and insects. Moreover, the different parts of Neem trees were used to isolate more than 135 phytocompounds. At least nine limonoids are triterpenes. Limonoids named azadirachtin A–G (produced in leaves) are the most studied and they have activities that allow them to be used as potent insecticides. Moreover, the bark is rich in lignans [3]. Other biological activities found in Neem oil studies are attributed to the following compounds: salannin, nimbin, meliantriol, meliacin, tignic acid, gedunin (also present in leaves), nimbidin, nimbidic acid, nimbidinin, nimbolide (found in leaves), valassin, meliacin, deacetylnimbin, linoleic acid, stearic acid, palmitic acid, oleic acid, azadiradione, hexadecanoic acid, caryophyllene oxide, linalool oxide, mahmoodin, margolone, azadirone, nimbolin, nimbinene and nimbosterol. Neem kernels have 30–50% oil, which is mainly used in soaps, biopesticides and pharmaceuticals [3][9][10][11]. Furthermore, it can be mentioned that the following nonisoprenoid compounds are present: proteins (amino acids), sulfurous compounds, carbohydrates (polysaccharides), polyphenolics such as flavonoids and their glycosides, rutin, dihydrochalcone, quercetin, carotenoids, catechin, ferulic acid, β-sitosterol, steroids (produced in leaves and/or bark), coumarin and tannins (produced in the bark), aliphatic compounds, ellagic acid, lupeol, saponins (leave), alkaloids (leave), resins, gums, margisine, cyclic trisulphide, steroids and ketones [3][4][11][12]. Hence, Table 1 shows the main secondary metabolites that are used in cosmetics and topical products with the corresponding reported biological activities.Table 1.
Phytochemical structures of
Azadirachta indica
(Neem) parts with their cosmetic or topical applications.
| Phytochemical | Neem Part | Structural Formula | Biological Activity | References |
|---|---|---|---|---|
| Nimbolide | Oil from leaves and seeds, fruit | 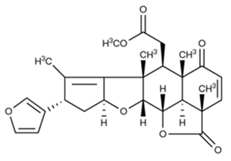 |
Psoriasis, antibacterial | [13][14] |
| Gedunin | Oil from leaves and seeds | 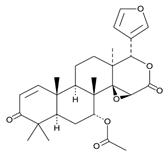 |
Antifungal | [13][14] |
| Mahmoodin | Oil from seeds | 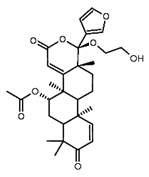 |
Antibacterial | [13] |
| Margolone | Oil from seeds and stem bark | 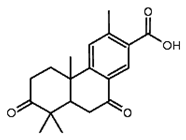 |
Antibacterial | [13][14] |
| Cyclic trisulphide | Oil from seeds and leaves | 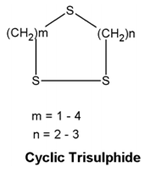 |
Antifungal | [14] |
| 4 azadiradione-type limonoids | Leaf extract | 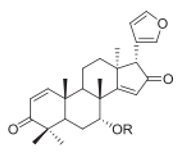 |
Melanogenesis inhibition | [15] |
| 2 salannin-type limonoids | Leaf extract | 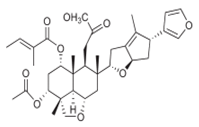 |
Melanogenesis inhibition | [15] |
| 5 nimbin-type limonoids |
Root extract | 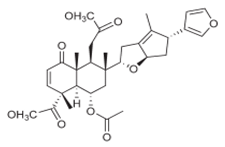 |
Melanogenesis inhibition | [15] |
| Limonoid | Leaves extract | 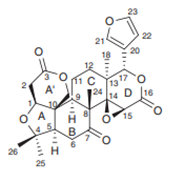 |
Anti-inflammatory | [16] |
3. Recent Developments in Cell Culture to Produce Molecules for Cosmetic Use
The biotechnological aspects of differentiated plant cell tissue in A. indica cultivation, as well as the biosynthesis of interesting secondary metabolites under different nutritional and environmental conditions, are well described in the literature. Recent efforts have focused on modifying the nutritional necessities of cells, optimizing the culture medium composition, and evaluating the amount of both the elicitor and precursor, which could improve not only cell growth but also secondary metabolites formation. Such studies intended to produce enhanced azadirachtin and related limonoids (AZRL) [5]. The rheological properties with their main parameters, such as the Reynolds number, apparent viscosity, volumetric power and phase state of A. indica cell cultures, were evaluated in 250 mL shake flasks that were 5 cm in diameter. The morphological aspects were evaluated and it was demonstrated that the isodiametric round shape of their huge individual cells, as well as their tendency to produce cell clusters, determined the capacity of such cell cultures to store secondary metabolites with high commercial interest in terms of developing both pharmaceuticals and cosmetics [17]. Moreover, the culturing of plant cells of A. indica was developed to continuously produce its principal secondary metabolites in recent years [18][19]. For instance, azadirachtin, mevalonic acid and squalene were successfully extracted from an A. indica cell suspension culture by using a green analytical method for ultrasound-assisted extraction with ethanol as solvent [18]. Another recent study investigated the effect of different culture types, as well as some concentrations of both cytokinins and auxins on cell biomass production, cell growth and azadirachtin production with further accumulation in cell suspension, as well as callus cultures of A. indica [19]. Furthermore, an A. indica culture as a cell suspension was carried out in shake flasks and bioreactors using a modified culture medium, as well as precursors and elicitors. They achieved an increase from 0.77 to 4.52 mg limonoids/g dry cell weight (DCW), with the consequent limonoids volumetric production increasing more than threefold using a two-stage culture (the one-stage process produced 13.82 mg limonoids/L, while the two-stage one produced 41.44 mg/L) in the bioreactors [20]. Thus, hydrodynamic stress under different agitation speeds was evaluated in A. indica cultivations using shake flasks. Some interesting parameters, such as limonoid concentration, cell viability and growth, reactive oxygen species (ROS) production and glutathione peroxidase (GPx) enzymatic activity, confirmed the effect of mechanical stimulus on the production of specific secondary metabolites. In addition to this, the most suitable agitation was 400 rpm with no cell integrity damage and a maximum biomass concentration of 10.53 ± 1.03 g DW containing 82.66 ± 32.96 mg limonoid/g DW [21].4. In Vitro Tests with Dermocosmetic Applications
4.1. Antimicrobial Activity
Plants have substances called secondary metabolites that can develop antibacterial, antifungal and/or antioxidant activities (such as glycosides, alkaloids, flavonoids, saponins, among others), and can be also part of a defense mechanism against pathogens [12][22]. Such phytochemicals are extracted according to the molecule polarity and the characteristics of the vegetable part used. Thus, studies with methanolic extracts from A. indica leaves inhibited the action of Bacillus, while oils from seeds, bark and leaves could inhibit the growth and/or viability of Gram-negative and Gram-positive bacteria. Moreover, β-sitosterol is recognized for its wide spectrum in treating skin diseases [4]. In addition to the compounds mentioned above, nimbolide and nimbidin were found in A. indica, which showed antibacterial activity against the following species: Staphylococcus coagulase, S. aureus, Staphylococcus sp. and Serratia. Moreover, various concentrations of an aqueous extract of Neem leaves inhibited the growth of Bacteroides intermedius, B. gingivalis, Streptococcus viridans and S. salivarius in vitro [23]. Furthermore, nimbidin isolated from bark showed antifungal activity [4][12]. A. indica was classified as the most effective medicinal plant for dermatophytosis in traditional treatment due to its components. A study revealed the minimum fungicidal concentration (MFC) and the minimum inhibitory concentration (MIC) for the leaf and seed extracts of A. indica against various dermatophytes, such as Trichophyton mentagrophytes, T. rubrum and Microsporum nanum. The growth curve of such dermatophytes was affected in the presence of A. indica extracts, whose effect was unexpected when compared with the dermatophytes without extracts [24]. Moreover, gedunin has antifungal activity and deoxygedunin has moderate antibacterial action, both of which were isolated from Neem seed oil [4]. A. indica leaf and seed grain extracts were effective against the human fungi Candida, Geotrichum, Epidermophyton, Trichophyton, Microsporum and Trichosporon [12]. In addition to this, the antifungal properties of A. indica leaf extracts were attributed to the inhibition of dermatophytes. The effect was attributed due to the presence of quercetin [12]. Thus, various concentrations of petroleum ether extracts of the Neem leaves inhibited the growth of Microsporum canis, M. gypseum, Epidermophyton floccosum, Trichophyton concentricum, T. rubrum and T. violaceum [24]. Furthermore, the leaf extracts and seed oil inhibited the growth of E. floccosum T. mentagrophytes, M. canis and T. rubrum, where the extract from Neem leaves had the highest antifungal activity of both [25]. Sulfur-containing compounds isolated from the steam distillation of fresh and ripe Neem leaves revealed antifungal activity against Trichophyton mentagrophytes [4]. In addition, some A. indica extracts that contained flavonoids with known antioxidant activity were evaluated. For instance, rutin was one of the flavonoids found in A. indica leaves, where the antibacterial action of quercetin combined with rutin was superior in relation to the same isolates [12]. Studies demonstrated that tetranortriterpenoid and some other phytocompounds, such as azadiradione, 6-deacetylnimbin, salannin, nimbin and epoxyazadiradione, were the main antifungal compounds from A. indica. However, the triterpenoids from Neem oil in its pure form (separately) showed basically no antifungal activity, while the combined effect was demonstrated against the three fungi tested, which may be indicative of such compounds having additive or synergistic effects [26]. Furthermore, nimbolide showed the greatest zone of inhibition against some bacteria species, such as S. epidermis, S. aureus, P. aeruginosa, K. pneumoniae and S. aureus (MR—methicillin resistant), in agar diffusion assay. The highest inhibition level was found against K. pneumonia, as well as the synergistic action of nimbolide, when combined with the pre-existing antibiotics cephalexin (97% pure) and cefazolin (98% pure). Thus, a greater antimicrobial effect in the synergy with these antibiotics compared to the other tested compounds (such as deacetylnimbin) was shown [27].4.2. Antioxidant Activity
It is known that ROS and free radicals are involved in cancer, DNA damage and even aging [1], and the addition of antioxidants in dermocosmetics can minimize these effects. Thus, some extracts that contained flavonoids with known antioxidant effects and other extracts derived from A. indica flowers and young leaves showed strong antioxidant potential [4]. Moreover, the aqueous fraction of the Neem bark had greater antioxidant activity than the leaf extract because of the higher concentration of phenolic compounds [28]. The topical application of A. indica leaf ethanolic extract was tested on hairless mice exposed to UVB irradiation to prevent the formation of wrinkles. To carry out this study, dry Neem leaves (10 g) were used, which were pulverized and extracted three times with 1 L of 50% ethanol over 24 h at room temperature. Then, the extract was analyzed via liquid chromatography, using methanol as the mobile phase. Moreover, a significant amount of rutin was found in the dried leaf extract, showing a high capacity to eliminate free radicals, which indicated the antioxidant activity. Finally, it was found that mice exposed to UVB irradiation and received the treatment with A. indica leaf extract had less wrinkle formation than the other groups, indicating a possible antiaging effect of the A. indica extracts [29].4.3. Skin-Soothing and Melanogenesis Inhibition Activities
A detailed investigation of two species from Meliaceae plants, namely, Azadirachta indica (Neem, AI) and Azadirachta indica var. siamensis (Siamese Neem, AIS), isolated and categorized 81 limonoids, 11 flavonoids and 1 diterpenoid, and revealed inhibitory activity against melanogenesis in B16 and tumor promoter assay (TPA)-induced inflammation in mice [15]. As it is well known, skin color depends mainly on the pigment melanin. The biosynthetic pathway named melanogenesis is responsible for melanin production in human skin. Furthermore, a form of hyperpigmentation condition named melasma was often reported as brown or gray patches on facial skin. Thus, there is an increasing demand for dermocosmetics to diminish skin hyperpigmentation, e.g., those containing melanogenesis inhibitors. Many of the AI and AIS compounds have revealed inhibitory activity against melanogenesis in B16 4A5 (mouse melanoma cells) stimulated with [30] or without α-melanocyte-stimulating hormone (α-MSH) [31]. Hair and skin pigmentation are a response to the stimulation of α-MSH. Among the AI and AIS compounds, some of them (4 azadiradione-type limonoids, 5 nimbin-type limonoids and 2 salannin-type limonoids) showed higher melanogenesis inhibition (79.1–108.1% cell viabilities) than the reference arbutin (4-hydroxyphenyl β-D-glucopyranoside; 100.1% cell viabilities), which was used as a depigmentation alternative in skin whitening for the cosmetic sector. Likewise, tyrosinase, which catalyzes the oxidation of L-DOPA to L-DOPA quinone, as well as the hydroxylation of L-tyrosine to L-(3,4-dihydroxyphenyl) alanine (L-DOPA), tyrosinase-related protein-1 (TRP-1), TRP-2 and tyrosinase, are involved in melanin biosynthesis. Furthermore, a nimbin-type limonoid from an AIS root extract, which was reported as the most abundant limonoid, was the most effective melanogenesis inhibitor [15]. Furthermore, the TPA-induced inflammatory ear edema in mice was studied to investigate the limonoids from AI and AIS extracts. A single application of TPA induced skin inflammation that was mainly defined by edema, polymorphonuclear leukocyte infiltration and erythema. The tested limonoids showed higher anti-inflammatory activity (50% inhibitory dose, ID50 = 0.19–0.75 μmol/ear) than indomethacin (ID50 = 0.91 μmol/ear) [15].5. In Vivo Tests (Subjects) for Cosmetic Applications
Some preclinical trials that investigated the antidiabetic, anti-inflammatory, analgesic, antinociceptive and insecticidal/pesticidal activities of A. indica extracts using different parts are described in the literature [1]. Neem oil was used in an emollient cream to assess its photoprotective effect, rheological characterization, toxicity and effect on the natural cutaneous flora health. Volunteers were used as donors of their skin’s bacterial flora, rubbing it with sterile swabs and grown on agar with nutrients and incubated for 24 h at 37 °C. As a result, the rheological characterization was similar to the commercial creams. Such Neem formulation had no cytotoxicity in 3T3 cell lines, as well as no damage to the natural cutaneous flora. In addition to this, when titanium dioxide was added to this cream, it showed greater photoprotective activity than the commercial product [7]. A randomized double-blind study with a commercial nutritional supplementation using turmeric polyherbal formulation decreased facial redness compared with the turmeric or placebo in volunteers in a study within 4 weeks. The turmeric polyherbal tablets included 500 mg of a blend of the following certified organic herbs: A. indica (Neem) leaf, H. indicus (anantamul) root, C. longa (turmeric) root, R. cordifola (manjistha) root, C. asiatica (brahmi/gotu kola) leaf, Glycyrrhiza glabra (licorice) root, Tinospora cordifolia (guduchi) stem, P. amarus (bhumyamalaki) herb and P. emblica (amalaki) fruit. Thus, the beneficial effects of this formulation were attributed to a synergic effect of each plant, such as anti-inflammatory, antioxidant, photoprotective and anti-aging properties, which enhance both the prevention and treatment of different skin conditions with different origins [32]. A polyherbal cream was developed as a vaginal cream containing A. indica (Neem) dry seed extract, Sapindus pericarp extract and quinine hydrochloride, which gave the cream a spermicidal action, as well as antimicrobial properties. The mentioned ingredients were incorporated into a water-soluble cream base and stabilized with the addition of antioxidant and preservative components. Then, an in vivo test was performed with intravaginal application in both rabbits and monkeys. Finally, such cream was tested and considered devoid of both sensitization and irritation with the Draize test using a 21-day duration cumulative skin sensitivity test in volunteers [33]. In a more recent study, a polyherbal gel was developed with ethanolic extracts of the following species: A. indica, Piper betle, Adhatoda vasica, Pongamia pinnata and Ocimum tenuiflorum to evaluate its antimicrobial action. Moreover, its drug content, physical appearance, viscosity, pH, spreadability, washing ability and skin irritation were tested. To do so, twenty healthy volunteers were submitted to the patch test, which was applied in the forearm of each participant to detect the possible reactions to formulations A, B, C (with 0.1, 0.3 and 0.5% ethanolic extracts, respectively) and the control after 48 h. It was concluded that the combination of ethanolic extracts did not demonstrate any serious adverse reaction, and thus, they are considered safe in those conditions. In addition, the most effective concentration tested was 0.5% [8]. Furthermore, a 6-week study was performed to evaluate the effectiveness of Neem extract dental gel with a positive control containing chlorhexidine gluconate (0.2% w/v) mouthwash. It showed that the Neem extract dental gel exhibited a higher reduction in the bacterial presence, as well as the plaque index than the control group [34].References
- Saleem, S.; Muhammad, G.; Hussain, M.A.; Bukhari, S.N.A. A comprehensive review of phytochemical profile, bioactives for pharmaceuticals, and pharmacological attributes of Azadirachta indica. Phyther. Res. 2018, 32, 1241–1272.
- Sen, S.; Chakraborty, R. Revival, modernization and integration of Indian traditional herbal medicine in clinical practice: Importance, challenges and future. J. Tradit. Complement. Med. 2017, 7, 234–244.
- Mossini, S.A.G.; Kemmelmeier, C. A árvore Nim (Azadirachta indica A. Juss): Múltiplos Usos. Acta Farm. Bonaer. 2005, 24, 139–148.
- Biswas, K.; Chattopadhyay, I.; Banerjee, R.K.; Bandyopadhyay, U. Biological activities and medicinal properties of Neem (Azadirachta indica). Curr. Sci. 2002, 82, 1336–1345.
- Thakore, D.; Srivastava, A.K. Production of biopesticide azadirachtin using plant cell and hairy root cultures. Eng. Life Sci. 2017, 17, 997–1005.
- Gonzalez-Coloma, A.; Reina, M.; Diaz, C.E.; Fraga, B.M. Natural Product-Based Biopesticides for Insect Control. In Comprehensive Natural Products II; Mander, L., Liu, H.-W., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 237–268.
- Banerjee, K.; Thiagarajan, N.; Thiagarajan, P. Formulation optimization, rheological characterization and suitability studies of polyglucoside-based Azadirachta indica A. Juss emollient cream as a dermal base for sun protection application. Indian J. Pharm. Sci. 2017, 79, 914–922.
- Bhinge, S.D.; Bhutkar, M.A.; Randive, D.S.; Wadkar, G.H.; Kamble, S.Y.; Kalel, P.D.; Kadam, S.S. Formulation and evaluation of polyherbal gel containing extracts of Azadirachta indica, Adhatoda vasica, Piper betle, Ocimum tenuiflorum and Pongamia pinnata. J. Res. Pharm. 2019, 23, 44–54.
- Chaturvedi, R.; Razdan, M.K.; Bhojwani, S.S. An efficient protocol for the production of triploid plants from endosperm callus of Neem, Azadirachta indica A. Juss. J. Plant Physiol 2003, 160, 557–564.
- Mak-Mensah, E.; Firempong, C. Chemical characteristics of toilet soap prepared from Neem (Azadirachta indica A. Juss) seed oil. Asian J. Plant Sci. Res. 2011, 1, 1–7.
- Gupta, A.; Malviya, R.; Singh, T.P.; Sharma, P.K. Indian medicinal plants used in hair care cosmetics: A short review. Pharmacogn. J. 2010, 2, 361–364.
- Shivanand, P.; Nilam, M.; Viral, D. Herbs play an important role in the field of cosmetics. Int. J. PharmTech Res. CODEN 2010, 2, 632–639.
- Hatti, K.S.; Muralitharan, L.; Hegde, R.; Kush, A. NeeMDB: Convenient database for Neem secondary metabolites. Bioinformation 2014, 10, 314–315.
- Pimple, B.P.; Badole, S.L.; Menaa, F. Exploring Neem (Azadirachta indica) for antidermatophytic activity. In Bioactive Dietary Factors and Plant Extracts in Dermatology; Watson, R., Zibadi, S., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2013; pp. 459–470.
- Akihisa, T.; Zhang, J.; Manosroi, A.; Kikuchi, T.; Manosroi, J.; Abe, M. Limonoids and other secondary metabolites of Azadirachta indica (Neem) and Azadirachta indica var. siamensis (Siamese Neem), and their bioactivities. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2021; Volume 68, pp. 29–65.
- Gerolino, E.F.; Chierrito, T.P.C.; Santana Filho, A.; Souto, E.R.; Gonçalves, R.A.C.; Oliveira, A.J.B. Evaluation of limonoid production in suspension cell culture of Citrus sinensis. Rev. Bras. Farmacogn. 2015, 25, 455–461.
- Alvarez-Yela, A.C.; Chiquiza-Montaño, L.N.; Hoyos, R.; Orozco-Sánchez, F. Rheology and mixing analysis of plant cell cultures (Azadirachta indica, Borojoa patinoi and Thevetia peruviana) in shake flasks. Biochem. Eng. J. 2016, 114, 18–25.
- Farjaminezhad, R.; Garoosi, G. Establishment of green analytical method for ultrasound-assisted extraction of azadirachtin, mevalonic acid and squalene from cell suspension culture of Azadirachta indica using response surface methodology. Ind. Crops Prod. 2020, 144, 111946.
- Farjaminezhad, R.; Garoosi, G. New biological trends on cell and callus growth and azadirachtin production in Azadirachta indica. 3 Biotech 2019, 9, 309.
- Vásquez-Rivera, A.; Chicaiza-Finley, D.; Hoyos, R.A.; Orozco-Sánchez, F. Production of Limonoids with insect antifeedant activity in a two-stage bioreactor process with cell suspension culture of Azadirachta indica. Appl. Biochem. Biotechnol. 2015, 177, 334–345.
- Villegas-Velásquez, S.; Martínez-Mira, A.D.; Hoyos, R.; Rojano, B.; Orozco-Sánchez, F. Hydrodynamic stress and limonoid production in Azadirachta indica cell culture. Biochem. Eng. J. 2017, 122, 75–84.
- Amadioha, A.C. Controlling rice blast in vitro and in vivo with extracts of Azadirachta indica. Crop Prot. 2000, 19, 287–290.
- Wolinsky, L.E.; Mania, S.; Nachnani, S.; Ling, S. The inhibiting effect of aqueous Azadirachta indica (Neem) extract upon bacterial properties influencing in vitro plaque formation. J. Dent. Res. 1996, 75, 816–822.
- Natarajan, V.; Venugopal, P.V.; Menon, T. Effect of Azadirachta indica (neem) on the growth pattern of dermatophytes. Indian J. Med. Microbiol. 2003, 21, 98–101.
- Ospina Salazar, D.I.; Hoyos Sánchez, R.A.; Orozco Sánchez, F.; Arango Arteaga, M.; Gómez Londoño, L.F. Antifungal activity of Neem (Azadirachta indica: Meliaceae) extracts against dermatophytes. Acta Biológica Colomb. 2015, 20, 201–207.
- Govindachari, T.R.; Suresh, G.; Gopalakrishnan, G.; Banumathy, B.; Masilamani, S. Identification of antifungal compounds from the seed oil of Azadirachta indica. Phytoparasitica 1998, 26, 109–116.
- Dhanya, S.R.; Kumar, S.N.; Sankar, V.; Raghu, K.G.; Kumar, B.S.D.; Nair, M.S. Nimbolide from Azadirachta indica and its derivatives plus first-generation cephalosporin antibiotics: A novel drug combination for wound-infecting pathogens. RSC Adv. 2015, 5, 89503–89514.
- Ghimeray, A.K.; Jin, C.-W.; Ghimire, B.K.; Cho, D.H. Antioxidant activity and quantitative estimation of azadirachtin and nimbin in Azadirachta indica A. Juss grown in foothills of Nepal. African J. Biotechnol. 2009, 8, 3084–3091.
- Ngo, H.T.T.; Hwang, E.; Seo, S.A.; Park, B.; Sun, Z.W.; Zhang, M.; Shin, Y.K.; Yi, T.H. Topical application of Neem leaves prevents wrinkles formation in UVB-exposed hairless mice. J. Photochem. Photobiol. B Biol. 2017, 169, 161–170.
- Akihisa, T.; Horiuchi, M.; Matsumoto, M.; Ogihara, E.; Ishii, K.; Zhang, J. Melanogenesis-inhibitory activities of isomeric C-seco limonoids and deesterified limonoids. Chem. Biodivers. 2016, 13, 1410–1421.
- Akihisa, T.; Takahashi, A.; Kikuchi, T.; Takagi, M.; Watanabe, K.; Fukatsu, M.; Fujita, Y.; Banno, N.; Tokuda, H.; Yasukawa, K. The melanogenesis-inhibitory, anti-inflammatory, and chemopreventive effects of limonoids in n-hexane extract of Azadirachta indica A. Juss. (Neem) Seeds. J. Oleo Sci. 2011, 60, 53–59.
- Vaughn, A.R.; Pourang, A.; Clark, A.K.; Burney, W.; Sivamani, R.K. Dietary supplementation with turmeric polyherbal formulation decreases facial redness: A randomized double-blind controlled pilot study. J. Integr. Med. 2019, 17, 20–23.
- Garg, S.; Taluja, V.; Upadhyay, S.; Talwar, G. Studies on the contraceptive efficacy of Praneem polyherbal cream. Contraception 1993, 48, 591–596.
- Alzohairy, M.A. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. Evid. -Based Complement. Altern. Med. 2016, 2016, 7382506.
More
