Post-COVID syndrome or long COVID is defined as the persistence of symptoms after confirmed SARS-CoV-2 infection, the pathogen responsible for coronavirus disease. The content herein presented reviews the reported long-term consequences and aftereffects of COVID-19 infection and the potential strategies to adopt for their management. Recent studies have shown that severe forms of COVID-19 can progress into acute respiratory distress syndrome (ARDS), a predisposing factor of pulmonary fibrosis that can irreversibly compromise respiratory function. Considering that the most serious complications are observed in the airways, the inhalation delivery of drugs directly to the lungs should be preferred, since it allows to lower the dose and systemic side effects. Although further studies are needed to optimize these techniques, recent studies have also shown the importance of in vitro models to recreate the SARS-CoV-2 infection and study its sequelae. The information reported suggests the necessity to develop new inhalation therapies in order to improve the quality of life of patients who suffer from this condition.
- post-COVID syndrome
- long COVID
- inhalation therapy
- post-COVID sequelae
1. Introduction
2. Long-Term Consequences and After-effects of COVID Infection
Coronavirus disease 2019 (COVID-19) is caused by a novel coronavirus known as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and it was declared a pandemic by the World Health Organization on 11 March 2020 [10]. Coronaviruses are single-stranded, positive-sense RNA viruses that can infect animals and humans. COVID-19 is transmitted between people through small airborne droplets emanated by an infected individual, personal contact (shaking hands), and by touching infected surfaces [11]. This disease often causes no symptoms or mild symptoms in the patients affected by the virus; consequently, they usually have a good prognosis. However, many of these cases develop symptoms in a more severe form that can lead to complications that persist long after the infection [12]. In particular, although COVID-19-associated symptomatology was more evident in individuals with severe disease, individuals with mild and moderate disease also reported a wide range of manifestations after the resolution of the clinical disease [13]. Since the new SARS-CoV-2 is genetically comparable to previously discovered coronavirus strains, such as SARS-CoV and MERS-CoV, it is highly expected that the consequences in patients recovered from COVID-19 are analogous to those of SARS and MERS [14]. Thus, a careful evaluation of the data available in follow-up studies of these infections could provide a useful scenario for identifying effective therapeutic protocols in the treatment of long-COVID syndromes. SARS-CoV-2 infection mostly affects the respiratory system [8] with complications ranging from mild fatigue to severe forms requiring long-term oxygen therapy or even lung transplantation [15]. The primary pulmonary manifestations of SARS-CoV-2 include hypoxemia, dyspnea, and cough while severe ones include hypoxemic respiratory failure and ARDS. ARDS may progress into pulmonary fibrosis, which in turn leads to irreversible impairment of respiratory function. Respiratory manifestations typical of post-COVID syndrome include chronic cough and persistent dyspnea [16]. Some patients develop important neuropsychiatric and musculoskeletal symptoms of COVID-19 including cerebrovascular accidents, olfactory and gustatory impairments, delirium, and myalgia. Some of the neuropsychiatric and musculoskeletal symptoms of post-COVID syndrome include sleep abnormalities, encephalopathy, chronic headache, delirium, brain fog, and small joint arthritis [17]. Regarding cardiovascular effects, during the acute phase of the infection, patients may report symptoms of shortness of breath, chest pain, and palpitations. These symptoms may persist up to 6 months after infection. Coagulopathies, thrombotic events that may become recurrent or persistent, hyperglycemia, acute kidney injury, and hepatocellular damage have also been observed (Figure 1) [16][18].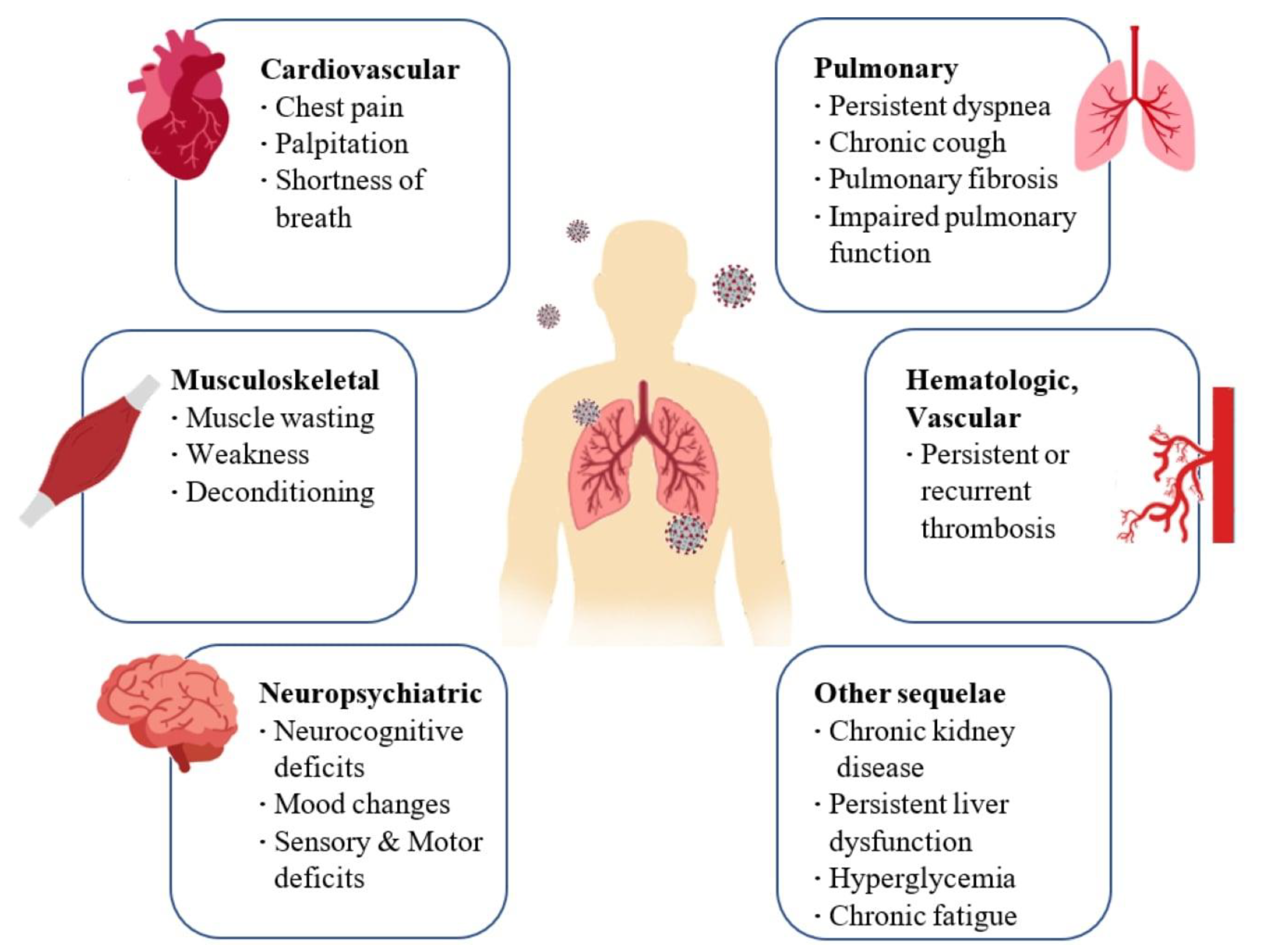 Figure 1. Long-term consequences and aftereffects of COVID-19 infections.
Figure 1. Long-term consequences and aftereffects of COVID-19 infections.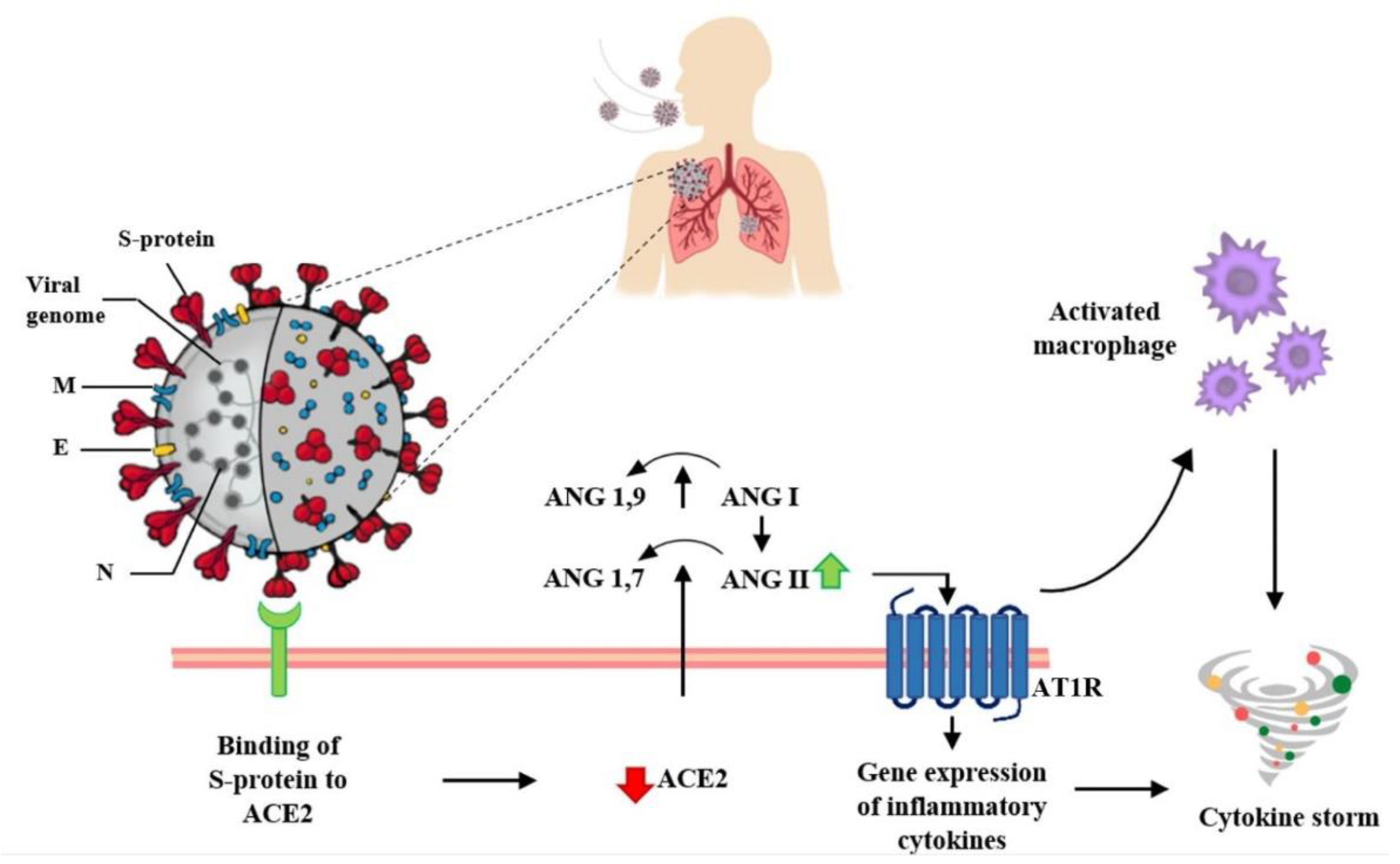 Figure 2. Interaction between SARS-CoV-2 and the Renin–Angiotensin System. SARS-CoV-2 enters host cells through the interaction of its spike protein with the ACE2 receptor. The downregulation of ACE2 receptors results in a decrease in the cleavage of angiotensin I and angiotensin II at Ang 1–9 and Ang 1–7, respectively. Ang II, through interaction with the AT1R receptor, stimulates the gene expression of various inflammatory cytokines and also influences the activation of macrophages that contribute to the “cytokine storm”.
Figure 2. Interaction between SARS-CoV-2 and the Renin–Angiotensin System. SARS-CoV-2 enters host cells through the interaction of its spike protein with the ACE2 receptor. The downregulation of ACE2 receptors results in a decrease in the cleavage of angiotensin I and angiotensin II at Ang 1–9 and Ang 1–7, respectively. Ang II, through interaction with the AT1R receptor, stimulates the gene expression of various inflammatory cytokines and also influences the activation of macrophages that contribute to the “cytokine storm”.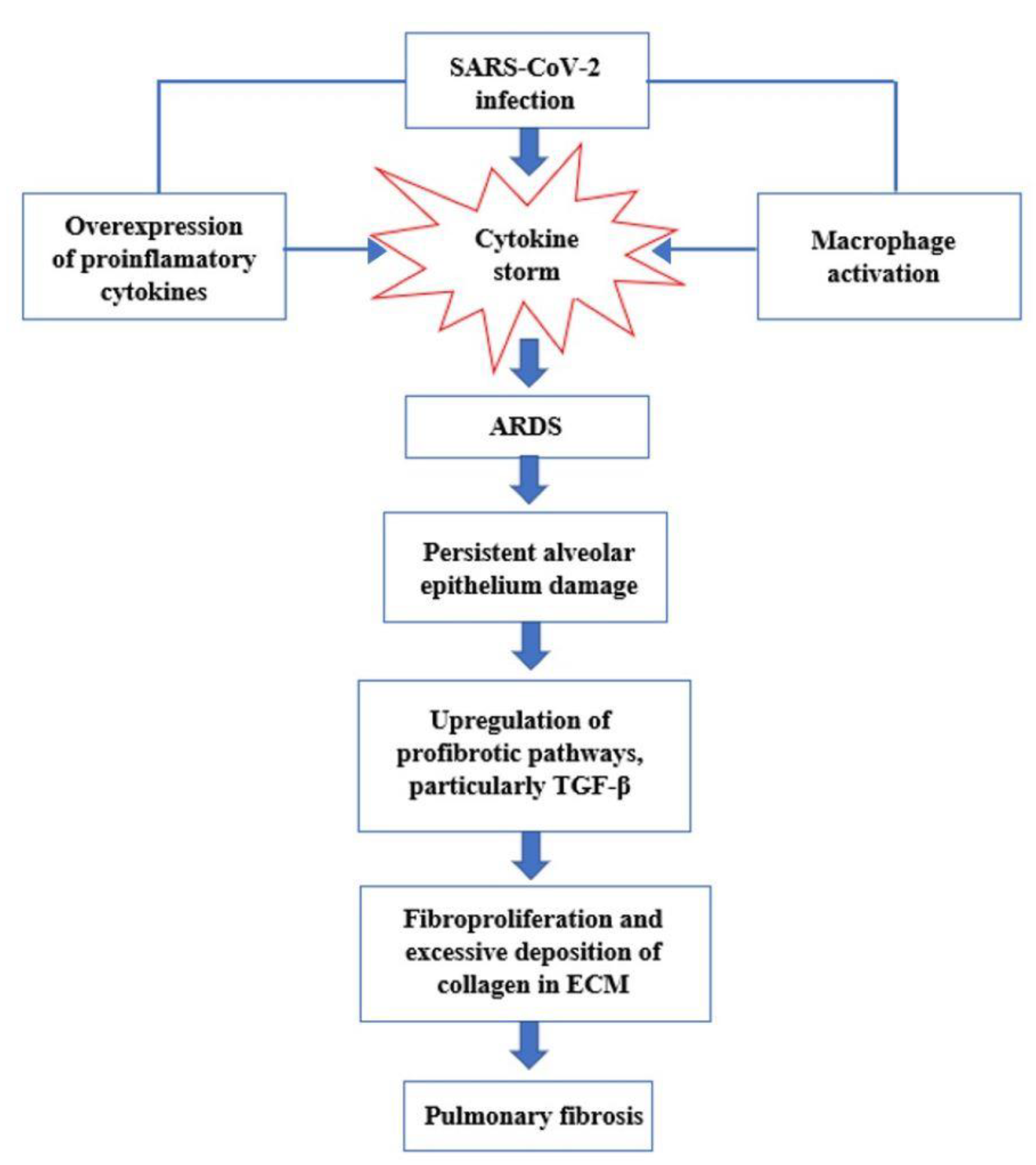 Figure 3. Key events in the progression of cytokine storm to acute respiratory distress syndrome (ARDS) and pulmonary fibrosis.
Figure 3. Key events in the progression of cytokine storm to acute respiratory distress syndrome (ARDS) and pulmonary fibrosis.3. Management of Patients with Post-COVID Syndrome and after Effects of SARS-CoV-2 Infection
Considering the events occurring after the infection, several classes of active ingredients may be useful in relieving the effects of infection in the airways (Table 1).
| Drug | Category | Mode of Action | References |
|---|---|---|---|
| Flavonoids (luteolin, apigenin, kaempferol, fisetin, quercetin, genistein, and epigallocatechin gallate) | Mast cell level Stabilizers |
Anti-inflammatory and mast cell-stabilizing effects | [38][39][40] |
| Antihistamine drugs (olopatadine, rupatadine, and ketotifen) | Mast cell level Stabilizers |
Anti-inflammatory and mast cell-stabilizing effects | [38][39][41] |
| Clarithromycin | Mast cell level Stabilizers |
Anti-inflammatory and mast cell-stabilizing effects | [42][43] |
| Dexamethasone | Corticosteroids | Decreases the inflammation linked with cytokine release syndrome | [44] |
| Ciclesonide | Corticosteroids | Anti-inflammatory action | [45] |
| Azithromycin | Antibiotics | Inhibit the proliferation of fibroblasts, reduce the production of collagen and the levels of TGF-β | [46] |
| Pirfenidone | Antifibrotic | Inhibit the synthesis of collagen induced by TGF-β; suppresses the production of TNF-α, IFN-γ, IL-1β- and IL-6; suppresses the differentiation of fibroblasts associated with TGF-β | [47] |
| Curcumin | Antifibrotic | Decreasing the expression of the TGF-β II receptor (TGF-ß RII), as well as in directly reducing the expression of the TGF-β protein and its mRNA |
[21] |
| N-Acetylcysteine (NAC) | Antioxidants | Inhibits virus replication and expression of pro-inflammatory molecules. Boosting a type of cell in the immune system that attacks infections |
[48] |
| GSH | Antioxidants | Blocks viral replication through redox state modulation |
[49] |
| Molnupiravir | Antivirals | Inhibits the replication of SARS-CoV-2, acting on the enzyme that the virus uses to generate copies of itself by introducing errors into its genetic code |
[50] |
| Zofin | Derived from human amniotic fluid | Suppressor of cytokine activation |
[51] |
| Ampion | Biological Drug | Modulate inflammatory cytokine levels |
[52] |
Recently, Ampio Pharmaceuticals launched a phase I randomized study to evaluate the safety, tolerability, and efficacy of nebulized Ampion in improving the clinical outcomes of 40 patients hospitalized with COVID-19 infections, with persistent respiratory symptoms. Details on the study will be published as soon as they are ready [69] Ampion is the low molecular weight filtrate of human serum albumin and as an immunomodulatory agent with anti-inflammatory effects; it has the potential to modulate inflammatory cytokine levels related to COVID-19 disease and respiratory complications, such as respiratory distress syndrome. acute (ARDS). Administration of Ampion to patients by inhalation allows the drug to reach the target site directly and attenuate lung inflammation [11].
46. Conclusions
Post-COVID syndrome is a new condition that can adversely affect quality of life, regardless of age and the presence of pre-existing diseases. Unfortunately, it is not yet possible to know which patients are most at risk of developing long-term consequences and whether these problems will solve, improve, or become permanent. This research examined the reports of the scientific community on the long-term consequences of COVID-19 and its after-effects, particularly in the lung, as the main site of infection, and possible treatment options useful for alleviating its symptoms. Active ingredients demonstrating a biological logic in the treatment of post-COVID sequelae have been reported, concluding that the most appropriate type of formulation for their administration is inhalation, allowing forthe release of the drug directly on the site of action with a reduction in dose and systemic side effects. Considering also that pulmonary fibrosis has been reported as one of the most serious consequences, the development of new in vitro experimental models, able to faithfully recreate the infection, will help scientists and pharmaceutical companies around the world to develop therapeutic strategies for similar conditions; although, further studies are needed to overcome the limitations of these techniques.
References
- Yan, Z.; Yang, M.; Lai, C.-L. Long COVID-19 syndrome: A comprehensive review of its effect on various organ systems and recommendation on rehabilitation plans. Biomedicines 2021, 9, 966.
- Jiang, D.H.; McCoy, R.G. Planning for the post-COVID syndrome: How payers can mitigate long-term complications of the pandemic. J. Gen. Intern. Med. 2020, 35, 3036–3039.
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615.
- Hackett, M.L.; Glozier, N.S.; Martiniuk, A.L.; Jan, S.; Anderson, C.S. Sydney Epilepsy Incidence Study to Measure Illness Consequences: The SESIMIC Observational Epilepsy Study Protocol. BMC Neurol. 2011, 11, 3.
- Ahmed, H.; Patel, K.; Greenwood, D.; Halpin, S.; Lewthwaite, P.; Salawu, A.; Eyre, L.; Breen, A.; O’Connor, R.; Jones, A.; et al. Long-Term Clinical Outcomes in Survivors of Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome Coronavirus Outbreaks after Hospitalisation or ICU Admission: A Systematic Review and Meta-Analysis. J. Rehabil. Med. 2020, 52, jrm00063.
- Hui, D.S. Impact of Severe Acute Respiratory Syndrome (SARS) on Pulmonary Function, Functional Capacity and Quality of Life in a Cohort of Survivors. Thorax 2005, 60, 401–409.
- Daugherty, S.E.; Guo, Y.; Heath, K.; Dasmariñas, M.C.; Jubilo, K.G.; Samranvedhya, J.; Lipsitch, M.; Cohen, K. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: Retrospective cohort study. BMJ 2021, 373, n1098.
- Garg, M.; Maralakunte, M.; Garg, S.; Dhooria, S.; Sehgal, I.; Bhalla, A.S.; Vijayvergiya, R.; Grover, S.; Bhatia, V.; Jagia, P.; et al. The conundrum of ‘Long-COVID-19’: A narrative review. IJGM 2021, 14, 2491–2506.
- Michalski, J.E.; Kurche, J.S.; Schwartz, D.A. From ARDS to pulmonary fibrosis: The next phase of the COVID-19 pandemic? Transl. Res. 2022, 241, 13–24.
- Boechat, J.L.; Chora, I.; Morais, A.; Delgado, L. The immune response to SARS-CoV-2 and COVID-19 immunopathology—Current perspectives. Pulmonology 2021, 27, 423–437.
- Eedara, B.B.; Alabsi, W.; Encinas-Basurto, D.; Polt, R.; Ledford, J.G.; Mansour, H.M. Inhalation delivery for the treatment and prevention of COVID-19 infection. Pharmaceutics 2021, 13, 1077.
- Fratta Pasini, A.M.; Stranieri, C.; Cominacini, L.; Mozzini, C. Potential role of antioxidant and anti-inflammatory therapies to prevent severe SARS-CoV-2 complications. Antioxidants 2021, 10, 272.
- Salamanna, F.; Veronesi, F.; Martini, L.; Landini, M.P.; Fini, M. Post-COVID-19 Syndrome: The Persistent Symptoms at the Post-Viral Stage of the Disease. A Systematic Review of the Current Data. Front. Med. 2021, 8, 653516.
- Sivashanmugam, K.; Kandasamy, M.; Subbiah, R.; Ravikumar, V. Repurposing of histone deacetylase inhibitors: A promising strategy to combat pulmonary fibrosis promoted by TGF-β signalling in COVID-19 survivors. Life Sci. 2021, 266, 118883.
- Ali, R.M.M.; Ghonimy, M.B.I. Post-COVID-19 pneumonia lung fibrosis: A worrisome sequelae in surviving patients. Egypt. J. Radiol. Nucl. Med. 2021, 52, 101.
- Lutchmansingh, D.D.; Knauert, M.P.; Antin-Ozerkis, D.E.; Chupp, G.; Cohn, L.; Dela Cruz, C.S.; Ferrante, L.E.; Herzog, E.L.; Koff, J.; Rochester, C.L.; et al. A clinic blueprint for post-coronavirus disease 2019 RECOVERY. Chest 2021, 159, 949–958.
- Mehandru, S.; Merad, M. Pathological Sequelae of Long-Haul COVID. Nat. Immunol. 2022, 23, 194–202.
- Rezkalla, S.H.; Kloner, R.A. Post-acute sequelae of SARS-COVID-2 syndrome: Just the beginning. Cardiol Res 2021, 12, 279–285.
- Proal, A.D.; VanElzakker, M.B. Long COVID or post-acute sequelae of COVID-19 (PASC): An overview of biological factors that may contribute to persistent symptoms. Front. Microbiol. 2021, 12, 698169.
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20.
- Yo, E.C.; Kadharusman, M.M.; Karman, A.P.; Louisa, M.; Arozal, W. Potential pharmacological options and new avenues using inhaled curcumin nanoformulations for treatment of post-COVID-19 fibrosis. Syst. Rev. Pharm. 2021, 12, 1119–1128.
- Banu, N.; Panikar, S.S.; Leal, L.R.; Leal, A.R. Protective role of ACE2 and its downregulation in SARS-CoV-2 infection leading to macrophage activation syndrome: Therapeutic implications. Life Sci. 2020, 256, 117905.
- Ramasamy, S.; Subbian, S. Critical determinants of cytokine storm and type I interferon response in COVID-19 pathogenesis. Clin. Microbiol. Rev. 2021, 34, e00299-20.
- Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017, 39, 529–539.
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 562–572.
- Suess, C.; Hausmann, R. Gross and histopathological pulmonary findings in a COVID-19 associated death during self-isolation. Int. J. Leg. Med. 2020, 134, 1285–1290.
- Oronsky, B.; Larson, C.; Hammond, T.C.; Oronsky, A.; Kesari, S.; Lybeck, M.; Reid, T.R. A review of persistent post-COVID syndrome (PPCS). Clin. Rev. Allergy Immunol. 2021, 1–9.
- Plataki, M.; Hubmayr, R.D. The physical basis of ventilator-induced lung injury. Expert Rev. Respir. Med. 2010, 4, 373–385.
- Cabrera-Benitez, N.E.; Laffey, J.G.; Parotto, M.; Spieth, P.M.; Villar, J.; Zhang, H.; Slutsky, A.S. Mechanical ventilation–associated lung fibrosis in acute respiratory distress syndrome. Anesthesiology 2014, 121, 189–198.
- Abodonya, A.M.; Abdelbasset, W.K.; Awad, E.A.; Elalfy, I.E.; Salem, H.A.; Elsayed, S.H. Inspiratory muscle training for recovered COVID-19 patients after weaning from mechanical ventilation: A pilot control clinical study. Medicine 2021, 100, e25339.
- Umemura, Y.; Mitsuyama, Y.; Minami, K.; Nishida, T.; Watanabe, A.; Okada, N.; Yamakawa, K.; Nochioka, K.; Fujimi, S. Efficacy and safety of Nintedanib for pulmonary fibrosis in severe pneumonia induced by COVID-19: An interventional study. Int. J. Infect. Dis. 2021, 108, 454–460.
- Wang, F.; Kream, R.M.; Stefano, G.B. Long-term respiratory and neurological sequelae of COVID-19. Med. Sci. Monit. 2020, 26, e928996-1–e928996-10.
- Udwadia, Z.; Koul, P.; Richeldi, L. Post-COVID lung fibrosis: The tsunami that will follow the earthquake. Lung India 2021, 38, 41.
- Esendağli, D.; Yilmaz, A.; Akçay, Ş.; Özlü, T. Post-COVID syndrome: Pulmonary complications. Turk. J. Med. Sci. 2021, 51, 3359–3371.
- Ong, K.-C.; Ng, A.W.-K.; Lee, L.S.-U.; Kaw, G.; Kwek, S.-K.; Leow, M.K.-S.; Earnest, A. 1-Year Pulmonary Function and Health Status in Survivors of Severe Acute Respiratory Syndrome. Chest 2005, 128, 1393–1400.
- Torres-Castro, R.; Vasconcello-Castillo, L.; Alsina-Restoy, X.; Solis-Navarro, L.; Burgos, F.; Puppo, H.; Vilaró, J. Respiratory Function in Patients Post-Infection by COVID-19: A Systematic Review and Meta-Analysis. Pulmonology 2021, 27, 328–337.
- Gerardo, A.M.; Almeida, T.; Maduro, S.; Carvalho, M.; Boléo-Tomé, J.P.; Liberato, H. Pulmonary function, functional capacity and health status in COVID-19 survivors. Rev. Med. Clínica 2021, 5, e11052105023.
- Hafezi, B.; Chan, L.; Knapp, J.P.; Karimi, N.; Alizadeh, K.; Mehrani, Y.; Bridle, B.W.; Karimi, K. Cytokine storm syndrome in SARS-CoV-2 infections: A functional role of mast cells. Cells 2021, 10, 1761.
- Kilinç, E.; Baranoğlu, Y. Mast Cell Stabilizers as a Supportive Therapy Can Contribute to Alleviate Fatal Inflammatory Responses and Severity of Pulmonary Complications in COVID-19 Infection. Anadolu Klin. Tıp Bilimleri Derg. 2020, 25, 111–118.
- Kakavas, S.; Karayiannis, D.; Mastora, Z. The Complex Interplay between Immunonutrition, Mast Cells, and Histamine Signaling in COVID-19. Nutrients 2021, 13, 3458.
- Baba, A.; Tachi, M.; Maruyama, Y.; Kazama, I. Olopatadine Inhibits Exocytosis in Rat Peritoneal Mast Cells by Counteracting Membrane Surface Deformation. Cell. Physiol. Biochem. 2015, 35, 386–396.
- Kazama, I. Stabilizing mast cells by commonly used drugs: A novel therapeutic target to relieve post-COVID syndrome? Drug Discov. Ther. 2020, 14, 259–261.
- Kazama, I.; Saito, K.; Baba, A.; Mori, T.; Abe, N.; Endo, Y.; Toyama, H.; Ejima, Y.; Matsubara, M.; Yamauchi, M. Clarithromycin Dose-Dependently Stabilizes Rat Peritoneal Mast Cells. Chemotherapy 2016, 61, 295–303.
- The RECOVERY Collaborative Group Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704.
- Clemency, B.M.; Varughese, R.; Gonzalez-Rojas, Y.; Morse, C.G.; Phipatanakul, W.; Koster, D.J.; Blaiss, M.S. Efficacy of Inhaled Ciclesonide for Outpatient Treatment of Adolescents and Adults with Symptomatic COVID-19: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 42.
- Echeverría-Esnal, D.; Martin-Ontiyuelo, C.; Navarrete-Rouco, M.E.; De-Antonio Cuscó, M.; Ferrández, O.; Horcajada, J.P.; Grau, S. Azithromycin in the Treatment of COVID-19: A Review. Expert Rev. Anti Infect. Ther. 2021, 19, 147–163.
- Zhang, F.; Wei, Y.; He, L.; Zhang, H.; Hu, Q.; Yue, H.; He, J.; Dai, H. A Trial of Pirfenidone in Hospitalized Adult Patients with Severe Coronavirus Disease 2019. Chin. Med. J. 2022, 135, 368–370.
- Memorial Sloan Kettering Cancer Center Phase II Study of N-Acetylcysteine in Severe or Critically Ill Patients with Refractory COVID-19 Infection. 2021. Available online: clinicaltrials.gov (accessed on 18 May 2022).
- Horowitz, R.I.; Freeman, P.R.; Bruzzese, J. Efficacy of Glutathione Therapy in Relieving Dyspnea Associated with COVID-19 Pneumonia: A Report of 2 Cases. Respir. Med. Case Rep. 2020, 30, 101063.
- Molnupiravir and Remdesivir Available for the Treatment of Non-Hospitalized COVID-19 Patients at High Risk of Progressing to Severe Disease. Available online: https://www.aifa.gov.it/en/-/disponibilit%C3%A0-molnupiravir-e-remdesivir-trattamento-pazienti-non-ospedalizzati-covid-19-1 (accessed on 19 May 2022).
- Mitrani, M.I.; Bellio, M.A.; Meglin, A.; Khan, A.; Xu, X.; Haskell, G.; Arango, A.; Shapiro, G.C. Treatment of a COVID-19 long hauler with an amniotic fluid-derived extracellular vesicle biologic. Respir. Med. Case Rep. 2021, 34, 101502.
- Ampio Pharmaceuticals Receives Investigational Review Board Approval for Its Phase I Long COVID-19 Trial (AP-018). Available online: https://www.biospace.com/article/ampio-pharmaceuticals-receives-investigational-review-board-approval-for-its-phase-i-long-covid-19-trial-ap-018-/ (accessed on 19 May 2022).
