Chitosan is obtained from chitin that in turn is recovered from marine crustacean wastes. The recovery methods and their varying types and the advantages of the recovery methods are briefly discussed. CThitin is the major component of cuticles of insects (cockroach, beetle, true fly, and worm), fungal cell walls (Aspergillus niger, Mucor rouxii, Penicillum notatum, ye bioactive properties of chitosan, which emphasize the unequivocal deliverables contained by this biopolymer, have been concisely presented. The variations of chitosan and its derivast) and green algaetives and their unique properties are discussed. The recovery methodsantioxidant properties of chitosan have been presented and their varying types ande need for more work targeted towards harnessing the advantages of the recovery methods are briefly discussntioxidant property of chitosan has been emphasized.
- chitosan
- chitin
- crustacean shells
- waste
- antioxidant
- derivatives
- applications
- nanotechnology
1. Introduction
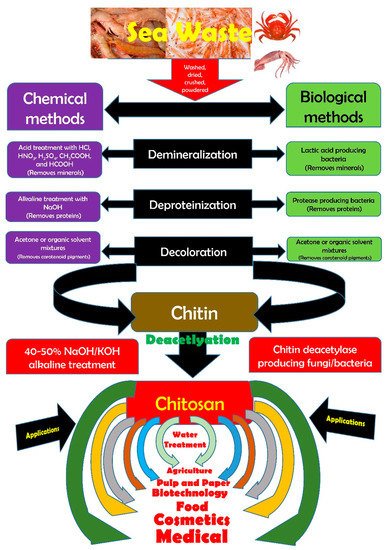
2. Chitin–Chitosan Metamorphosis
The seafood processing industry produces large quantities of byproducts and discards such as heads, tails, skins, scales, viscera, backbones, and shells of marine organisms. Although these are waste residues, they still are an excellent source of lipids, proteins, pigments, and small molecules, and moreso a source of chitinous materials. One of the limitations in the use of chitin on a large-scale is its water insolubility, this is why water-soluble derivatives have been sought after. Chitosan is the most important of these. Chitosan is obtained from chitin by a process called deacetylation, whereby acetyl groups (CH3-CO) are removed resulting in a molecule that is soluble in most diluted acids [27]. The deacetylation process releases amine groups (-NH2) rendering chitosan a cationic nature. Chitosan, a linear polysaccharide is made up of β-(1–4)-linked d-glucosamine and N-acetyl-d-glucosamine moieties [28][29][30][31][32][33][34][28,29,30,31,32,33,34]. Chitosan is derived from chitin by chemical or enzymatic deacetylations. Although chemical deacetylation is preferentially cheaper and warrants mass production, the major disadvantage is the energy consumption and high environmental pollution risks. Alternatives in the form of enzymatic methods that utilize chitin deacetylases have been explored via enzymatic deacetylation of chitin. Research has identified that selected fungal, viral, and bacterial chitin deacetylases could produce partially acetylated chitosan tetramers with a defined degree of acetylation and a pattern of acetylation [35]. With the recent progress in extraction methodologies and instrumentation sophistication, chitosan extraction from marine crustaceans has also been achieved outside of chemical extraction through autoclave-based methods [36][37][38][39][40][41][42][36,37,38,39,40,41,42], microwave-based methods [39][43][44][45][46][39,43,44,45,46], ultrasonication-based methods [47][48][49][47,48,49], and Graviola extract combined with magnetic stirring on hot plate [50]. Chitin and chitosan exhibit several biological properties such as anti-cancer [51][109], antioxidant [52][110], antimicrobial [53][111], and anti-coagulant [54][112] properties. In addition, they are used as biomaterials in a wide range of applications: for biomedical purposes such as for artificial skin, bones, and cartilage regeneration [55][56][113,114], for food preservation such as for edible films [57][115], and for pharmaceutical purposes such as for drug delivery [58][116]. Chitin is a versatile, environmentally friendly, modern material [59][117]. Chitin and chitin derivatives have been used in virtually every significant segment of the economy (e.g., water treatment, pulp and paper industry, biomedical devices and therapies, cosmetics, biotechnology, agriculture, food science, and membrane technology) [60][118]. Chitin and chitosan are important bioactive materials, with many highly potent activities such as immune function, hemostasis and wound healing, antioxidant action, antimicrobial activity, and heavy metal and other pollutant removal [61][119]. Therefore, as renewable resources, chitin and its derivatives have a wide range of applications in food and nutrition [62][120], pharmaceutical [63][121], biotechnological [64][122], cosmetic [65][123], packaging [66][124], textile, wastewater treatment [67][125], and agricultural [68][126] industries.3. Snap Shot of the Bioactive Properties of Chitosan
Chitosan has three reactive groups, the primary amine group and the primary and secondary hydroxyl groups at C-2, C-3, and C-6 positions, respectively [69][127]. Among the three groups, the primary amine at the C-2 position is reported to be responsible for the bioactivity of chitosan [70][128]. The chemical modification of chitosan adds unique functional properties for use towards biological and biomedical applications [71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161]. The biodegradability, biocompatibility, mucoadhesion, hemostatic, analgesic, adsorption enhancer, antimicrobial, anticholesterolemic, and antioxidant attributes of chitosan are those which make it suitable for biomedical applications. Chitosan has been well established as an alternative to antibiotics, undertaking antimicrobial and antifungal roles. Because of its cationic properties, chitosan is able to lead to membrane-disrupting effects [104][105][106][107][162,163,164,165], which are higher against Gram-positives than Gram-negatives [107][165]. The antibacterial activity of chitosan is influenced by the molecular weight of chitosan and allied physicochemical properties. A number of chitosan derivatives with different modifications have shown improved antibacterial activity; in this way, chitosan micro/nanoparticles display unique physical and chemical features [108][166]. The chitosan nanoparticles penetrate inside the cell, interacting with phosphorus- and sulfur-containing compounds such as DNA and protein, eventually causing damage to the cells [105][106][163,164]. Successful experiments were performed using chitosan and reticulated chitosan microparticles against aquaculture related trouble-makers: Lactococcus garvieae (Gram +), Vibrio parahaemolyticus, and Vibrio alginolyticus (Gram −). These microorganisms are the most predominant bacterial pathogens of mariculture industry and are responsible for crucial economic losses in cultured fish and seafood worldwide [109][167]. The antimicrobial activity of chitin, chitosan, and their derivatives against different groups of microorganisms, such as bacteria, yeast, and fungi, has received considerable attention in recent years [62][110][111][120,168,169]. Traditional chemotherapeutic agents kill actively dividing cells, characteristic of most cancer cells. Cytotoxic drugs continue to play a crucial role in cancer therapy, although side effects such as the destruction of lymphoid and bone marrow cells is inevitable. In this direction, constant efforts to improve cancer therapy-based side effects are sought after. This is why biocompatible anticancer drugs are needed for cancer therapy. The introduction of several groups into chitosan modifies its structure significantly, thereby increasing the biological activity of chitosan. The introduction of sulfates and phenyl groups in carboxymethyl benzylamide dextrans into chitosan, lead to enhanced anticancer activity in breast cancer cells. Sulfated chitosan (SCS) and sulfated benzaldehyde chitosan (SBCS) significantly inhibited cell proliferation, induced apoptosis, and blocked the fibroblast growth factors (FGF)-2-induced phosphorylation of extracellular signal-regulated kinase (ERK) in Middle cranial fossa (MCF)-7 cells [112][170]. Dialkylaminoalkylation and reductive amination followed by quaternization of chitosan could elicit inhibitory effects on the proliferation of tumor cell lines [113][171].4. Antioxidant Activity of Chitosan
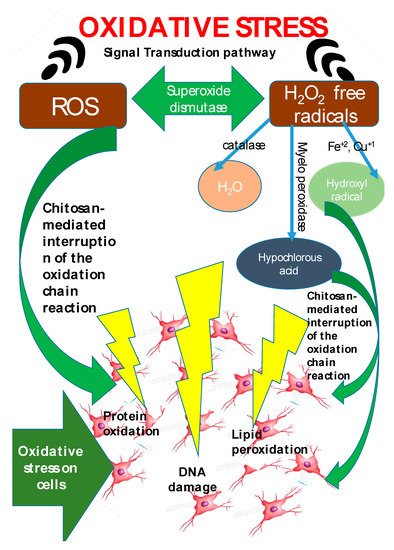
Being extracted from crustacean’s exoskeleton and fungi cell walls, chitosan products are biocompatible and biodegradable, and their range of applications include food, wastewater treatment, cell culture, cosmetics, textiles, agrochemicals, and medical devices [30]. Additionally, chitosan also exhibits antioxidant activity [114][115][184,185] and can be used as a replacement for synthetic antioxidants such as butylated hydroxytoluene (BHT), butylated hydroxy-anisole (BHA), propyl gallate, and tert-butylhydroquinone (TBHQ) [116][186]. Reactive oxygen species (ROS) such as H2O2, hydroxyl radicals, and superoxides lead to oxidative stress which is the key behind a wide range of pathologies: cancer [117][187], cardiovascular disease [118][119][188,189], premature aging [120][190], rheumatoid arthritis, and inflammation [121][191]. Chitin, similar to vitamin C, exhibits antioxidant effects [122][192] and can be used as an ingredient for the production of functional foods in order to circumvent age-related and diet-related diseases [123][193]. Due to oxidation of lipids in food, off-flavors and rancidity manifests, this is why BHT and BHA (synthetic antioxidants) are used. BHT and BHA are well known for their potential health hazards [124][194], and hence safe and natural alternatives are being sought [125][195]. The addition of 1% chitosan resulted in 70% decrease in the 2-thiobarbituric acid reactive substance (TBARS) values of frozen meat. Chitosan addition is reported to have lead to chelation of the free iron in heme proteins of meat that are released during processing [126][196]. In seafoods, oxidation of high concentrations of prooxidants such as hemoglobin and metal ions in fish muscles is also reported [127][197]. Antioxidant effect of chitosan is reported [128][198] to be directly proportional to its molecular weight, concentration, and viscosity. Chitosans from crab shell wastes were tested on herring flesh and chitosan with different viscosity were also tested on fish samples. The highest activity was observed with low viscosity chitosan (14 cP) and its action was similar to that of BHA, BHT, and TBHQ. Chitosans are speculated to slowdown lipid oxidation by chelating ferrous ions in fish. This eliminates the prooxidant activity of ferrous ions by preventing their conversion to ferric ion [127][197]. Kim and Thomas [129][199] have also reported identical inferences in Atlantic salmon (Salmo salar). Free radical reaction is connected with several specific human diseases and has gained paramount attention. In the human body, reactive oxygen species (ROS) produced during metabolic process oxidize lipids, proteins, carbohydrates and nucleic material, resulting in oxidative stress [122][192]. The ROS generated, may activate enzymes that eventually manifest as life-threatening disorders such as cancer, aging, cardiovascular diseases wrinkle formation, rheumatoid arthritis, inflammation, hypertension, dyslipidemia, atherosclerosis, myocardial infraction, angina pectoris, heart failure, and neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and amyotrophic lateral sclerosis [130][131][132][133][134][200,201,202,203,204]. The term ROS refers to oxygen-derived free radicals like superoxide, hydroxyl radical, and nitric oxide, and is extended to nonradical oxygen derivatives of high reactivity like singlet oxygen, hydrogen peroxide, peroxynitrite, and hypochlorite [135][136][205,206]. Mitochondria in biological cells are responsible for ROS generation [137][207]. The cellular defense includes enzymes such as catalase, superoxide dismutase, and glutathione peroxidase [130][200]. When excessive ROS are generated, the defense mechanism is unable to respond appropriately and thus oxidative stress manifests. The antioxidant activity of chitosan has gained paramount importance, with chitosan exhibiting confirmed scavenging activity against various radical species. The degree of deactylation (DDA) and molecular weight (MW) of chitosan determine the scavenging capacity of chitosan [138][208] and the chitosan NH2 groups are responsible for free radical scavenging effect. Mahdy Samar et al. confirmed that a high rate of DDA and low MW of chitosan exhibits higher antioxidant activity [44]. Hajji et al. studied chitosan obtained from Tunisian marine shrimp (Penaeus kerathurus) waste (DDA: 88%), crab (Carcinus mediterraneus) shells (DDA: 83%), and cuttlefish (Sepia officinalis) bones (DDA: 95%) [139][74] and tested their antioxidant activities. Cuttlefish with 95% DDA exhibited the highest antioxidant activity. Sun et al. [140][209], reported that chitosan oligomers exhibited stronger scavenging activity with lower MW. Chang et al. [141][210] demonstrated that lower MW chitosan (~2.2 kDa) greatly impacts the scavenging ability. Although the antioxidant activity of chitosan has been proven, yet it is limited by the lack of an H-atom donor, to serve as a good chain-breaking antioxidant [142][211]. The scavenging capacity of free radicals is related to the bond dissociation energy of O–H or N–H and the stability of the formed radicals. Due to the presence of strong intramolecular and intermolecular hydrogen bonds in chitosan molecules, the OH and NH2 groups find it difficult to dissociate and react with hydroxyl radicals [143][212]. This is why various modifications of chitosan molecules by grafting functional groups into the molecular structure have evolved. Modification of chitosan by grafting polyphenols, has been observed to enhance the antioxidant activity. This has resulted owing to the synergetic effects obtained from both chitosan and polyphenols [144][213]. Chito-oligosaccharides (COS) are known to be highly promising compounds for use as natural antioxidants in biological systems [145][146][62,214]. Li et al. 2018 [147][215] have recently investigated the preparation and potential free radical scavenging activity of chitosan derivatives with 1,2,3-triazoles and 1,2,3-triazoliums. Their results indicated that all the chitosan derivatives exhibited higher radical scavenging activity than chitosan and the scavenging ability was further enhanced following the N-methylation of 1,2,3-triazole moieties. Other researchers [148][149][216,217] have also reported the antioxidant activity of quaternary ammonium groups in chitosan derivatives. Antioxidant agents like chitosan play a role in scavenging the free radicals and by inhibiting the oxidative damage caused by free radicals (Figure 2). Antioxidant mechanism of chitosan functions by protecting the host against oxidative stress induced damages via interfering with the oxidation chain reaction. The exact mechanism of free radical scavenging activity of chitosan has still not been established. However, Riaz et al., 2019 [150][218] put forth a plausible theory that the unsteady free radicals may react with the OH group and the amino group at C-2, C-3, and C-6 positions of the pyranose ring to produce a stable macromolecule. This rentryview calls to attention that this (elucidating the mechanism of antioxidant activity of chitosan) is one of the gray areas worth working on with respect to the antioxidant activity of chitosan.