Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Aránzazu Zarzuelo Castañeda and Version 2 by Conner Chen.
Microorganisms of fungal origin currently show resistance to the different antifungals of conventional use, which is undoubtedly altering the oral health of human beings, but there are new therapeutic possibilities such as the active principles of various natural species. In this situation, a therapeutic option of great validity could be the use of various active components that are found in different vegetable species, which is an alternative that might decrease both the side effects that are present when using conventional drugs and the resistance to different medicines
- antifungal agents
- phytotherapy
1. Mycotic and Antifungal Agents in the Oral Cavity
Fungi are part of the oral microbiota, wherein they constitute a minor component; however, if their presence surpasses the balance, they become pathological agents that provide a place to numerous diseases with a significative influence on the population.
It is necessary to point out that progress regarding the use of antifungal drugs has evolved over time (Figure 12) [1][7]. Table 1 lists the classification of antifungals and their mechanisms of action.
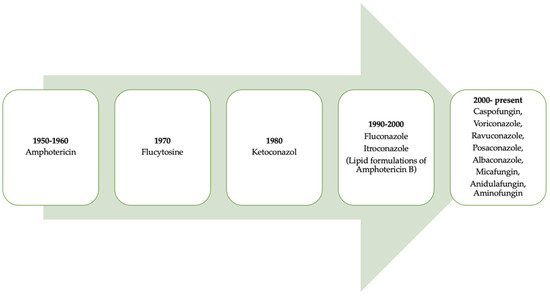
Figure 12. A retrospective look at the evolution of antifungals throughout history.
| Inside the Structure | Mechanism of Action | |
|---|---|---|
| Polyene | Antifungal classification: a look inside the structure Nystatin, natamycin, and amphotericin B |
Antifungals that act on the cytoplasmatic membrane |
| Azoles | Imidazole: miconazole, clotrimazole, and ketoconazole Triazoles: fluconazole and itraconazole (ketoconazole) |
Antifungals that act on the cytoplasmatic membrane |
| Allylamines | Terbinafine and naftifine | Antifungals that act on the cytoplasmatic membrane |
| Lipopeptides | Papulacandins and glycosylated triterpenes echinocandins: caspofungin, anidulafungin, and micafungin | Antifungals that act on the cell wall |
| Pyrimidines | Flucytosine | Antifungals that act on the cell nucleus |
| Other | Ciclopirox potassium iodide, tolnaftate, and griseofulvin | Antifungals that act on the cell nucleus |
In the oral cavity, when conditions are adversely presented, such as pH imbalance, lack of hygiene, or cases of immunosuppression, several pathologies occur, and even the replication of several opportunistic and pathogenic species such as fungi and bacteria, being the first ones the subject of interest in this review. Fungi in the oral cavity are presented in biofilms of multiple kinds, exhibiting features of an antifungal-resistant phenotype as a result of some factors that change their components and structure. The elimination of these biofilms and their treatments are based on the use of antifungal drugs that allow their treatment and prevention. In addition, antiseptics such as chlorhexidine and triclosan are often used to reduce the microbial load present in the oral cavity, reducing bacteria, viruses, and fungi. However, these therapeutic options in many cases fail to fully improve oral health due to inadequate use and microbial load, enhancing antimicrobial resistance.
2. Resistance to Antifungals and Their Effect on Human Health
Fungal infections are a public health problem. About 1.7 billion people worldwide suffer from a fungal infection; most of these infections occur at the mucosa or skin level. Candida species represent one of the predominant causes of fungal infections with high morbidity and mortality rates [2][3][8,9]. Candida species are considered one of the most frequently isolated microorganisms in microbiological cultures of fungal-infected patients. The most frequent Candida species is Candida albicans; however, the incidence of other species such as Candida tropicalis, Candida parapsilosis, and Candida glabrata has been increasing, as well as Aspergillus spp. species. This high incidence is caused by the inadequate use of antifungal drugs since the available therapeutic options limit specialized treatment, producing resistance to antifungal drugs. Antifungal resistance generally occurs to echinocadins and azoles, although cross-resistance to two or more antifungal classes, this situation is of concern and requires appropriate countermeasures. Therefore, new strategies are needed to combat this type of pathogens through more potent molecules of plant origin [4][10].
3. Drugs That Produce Antifungal Resistance
Treatment of mucosal or invasive candidiasis is based on a set of limited antifungal agents, which include polyenes, azoles, and echinocandins [5][11].
Amphotericin B, an antifungal drug widely used in the 1980s, is toxic due to its lack of selectivity; since fungal cells are eukaryotic, it is currently only used for the treatment of serious fungal infections that threaten life. This fact has led to the search for new active principles, highlighting the azoles, which lack toxicity but are fungistatic drugs, which degenerate into resistance.
In 2001, a new class of antifungals came onto the market, the echinocandins (caspofungin), considered a broad-spectrum antifungal and effective against infections caused by species of Candida spp. or Aspergillus section Fumigatti. Unfortunately, their clinical use is limited to the treatment of systemic candidiasis and causes the development of resistance, especially to C. glabrata, in some cases. According to investigations, resistance occurs in between 2.9% and 3.1% of cases [6][12], typically corresponding to acquired resistance after exposure to echinocandins [7][8][13,14].
The effect of nystatin, an antifungal from the polyene group that is able to act as a fungistatic or fungicide, depends on the concentration. An example of this effect is on the ergosterols of a fungus’ cell membrane, where it shows greater affinity, modifying it spatially, affectation of the cellular metabolism, and also has an affinity for cholesterol, making it a drug with toxic potential limiting its use [9][15].
Fluconazole, on the contrary, has limitations due to the natural resistance of some fungi toward the compound (Candida krusei), the power of some species of Candida to achieve resistance to established doses, along with the necessary adjustments in drugs, in renal patients, and the mutual action that can be exerted with another medication. Following this line, evidence indicates that certain species of Candida tend to develop resistance to fluconazole by the influence of intrinsic factors such as pathogenicity.
4. Resistance Mechanisms to Anti-Fungal
Resistance mechanisms can be primary or secondary, depending on the intrinsic characteristics of each fungus [10][16]. The sequential administration of specific drugs generates the appearance of isolates resistant to specific molecules, proving—by in vivo assays—the rapid appearance of mutants resistant to multiple drugs under the condition of combined therapy, a precedent that forces to limit therapy only to exceptional situations [11][17]. At present, there is a notable decrease in the effect of the drugs used to combat fungi in the oral cavity due to the resistance of microorganisms to the drugs used, mainly because of the emergence of resistant yeasts, the appearance of new harmful species, over-prescription, and the increase in therapeutic doses, as mentioned in previous lines [4][10]. Species such as Candida are competent in understanding and preventing the long-term advantage of phenotypes related to virulence and drug resistance. On the contrary, the authors such as Costa C. et al. and Cuenca M. et al. define the term drug resistance, in this case regarding antifungals, as the simultaneous acquisition of tolerance to several drugs due to a genetic change, where the pathogenic microorganism is inhibited by a higher than normal concentration of the drug, observed in common strains [12][13][18,19].
Dental prostheses are a risk factor for subprosthetic candidiasis and can cause mortality in hospitalized older adults. Fungal resistance is mainly due to the ability to produce biofilms, and this can often lead to dental therapeutic failure and requires the investigation of alternatives for treatment [14][20].
A study by Cruz-Quintana et al. explained that it is important to carry out studies on the genome of oral fungi in order to determine the role played by genes as potential therapeutics, but it is necessary to deepen studies on the molecular mechanisms that produce drug resistance by these fungi. Thus, it was determined that the genome of C. albicans is of the utmost importance since it will allow the establishment of safe diagnostic protocols in the future, as well as the development of new antifungals that allow its control [15][21]. In the face of antifungals, it is mandatory to identify, with microbiological techniques based on various identification criteria, several species [16][22].
In 1994, a biochemical method was described based on revealing species-specific enzymatic alterations through hydrolysis using a substance with chromogenic attributes to reveal GCL (crevicular gingival fluid) based on a glow of colors that occurs when they develop colonies. Without a doubt, this will allow generating more effective treatments, as well as finding alternatives with greater viability to control the pathologies that significantly affect oral health [17][23].
When talking about resistance and its mechanisms, we can mention those generated against echinocandins and azoles could be mentioned in here, determining the need to investigate pharmacological options against the resistance generated toward traditional drugs [5][11].
5. Phytotherapy Alternatives to Address the Resistance of Antifungals in the Oral Cavity
Traditional herbal medicine known as phytotherapy currently occupies a very important place as a therapeutic alternative, although it is true that a disadvantage is the lack of phytotherapeutic evidence in some species, which does not allow to give the necessary endorsement for its use. However, it is necessary to pave the way for the discovery of new drugs following the ethnobotanical clues of medicinal plants of conventional use [18][24].
Martínez et al. state that in the presence of a problematic derivative of resistance to antifungal treatments in the dental field, plenty of investigations have been conducted in recent years, with the goal of controlling these pathologies and their effects on the health of the oral cavity. An alternative is the use of phytotherapy through indigenous or native plants [19][25]. A previous study on phytotherapy showed the in vitro antifungal effect of different hydroalcoholic concentrations of Uncaria Tomentosa (UT) on C. albicans (ATCC), highlighting that a hydroalcoholic extract of UT at 100%, such as nystatin, is more effective on fungi species because of its high sensitivity; however, concentrations of 50% and 75% result in a medium sensitivity, while resistance can be observed at a 25% concentration [20][26].
It is important to mention that Ayurvedic medicine has long been used to treat dental conditions dating back to 2000 BC; however, phytotherapy in dentistry is not well known and is hardly used. There is evidence of its effects on the antimicrobial, antiplaque, analgesic, healing, antioxidant, and anti-inflammatory spectrum, establishing its viability in the treatment and prevention of dental caries, periodontal disease, oral ulcers, and mucosal wounds [21][27].
Menezes, A. et al. confirm what was said in the previous paragraph since when evaluating the chemical effects of the essential oil of Bauhinia rufa flower as an antifungal against Candida spp isolates, it showed an excellent content of essential oil, and the effects derived from its antifungal action place it as a possible alternative to develop a new antifungal; as a result, it obtained a yield of 0. 067%, 0.045%, 0.098%, and 0.065%, with relative densities of 0.907, 0.905, 0.908, and 0.904 g/mL−1, respectively, in the study sites, exposing a chemical profile with the presence of 39 mixtures—six with the highest proportions of β-pinene, elemol, globulol, trans-verbenol, viridiflorol, and oplopanone; this shows that the antifungal incidence against Candida was excellent in all tests performed [22][28]. Mansourian A. et al., in their experimental study, identified C. albicans in immunosuppressed patients resistant to antifungal drugs, which led these researchers to turn their attention to the medicinal herbs Syzygium aromaticum and Punica granatum. Thus, they worked with 21 oral C. albicans isolates from patients with prosthetic stomatitis at the Department of Prosthodontics, Faculty of Dentistry, Tehran University of Medical Sciences; S. aromaticum species showed better activity against Candida than conventionally used antifungals (p < 0.001) [22][23][28,29].
In a study by Thamburan et al., patient samples and the standard strain 5027ATCC (PTCC10231) of C. albicans yeast were investigated using the well diffusion method. Nystatin and methanol were obtained as positive and negative controls, respectively. The results were conclusive: Both S. aromaticum and P. granatum showed important antifungal activity using the well method. S. aromaticum showed high anti-Candida activity in relation to nystatin, generating statistical significance (p < 0.001) [24][30].
Thamburan et al., in their research, also identified a population with clinically significant oropharyngeal candidiasis, which did not allow the provision of oral medications, also hindering food intake. Azole antifungals are generally used in this type of pathology; however, a limitation is the high resistance in the general population and also a number of toxic effects. Therefore, the evaluation of two native South African species called Tulbaghia alliacea and Tulbaghia violacea was proposed in this research. The researchers compared the in vitro antifungal activity on Candida species of natural extracts of T. alliacea and T. violacea, obtaining important data since it was demonstrated that the extract of T. alliacea was a natural fungicide. This activity could be due to an active component called marasmicin, concluding that the extracts of T. alliacea showed anti-infective activity against Candida species in vitro [24][30].
All of this shows that studies related to phytotherapy provide favorable results in terms of antifungal treatments in the oral cavity against the resistance generated by fungi according to conventional pharmacological treatments. Even in the case of hepatotoxicity generated by antifungal drugs, phytotherapy shows an outstanding advantage. The following is a compilation of several studies on plant alternatives to overcome drug resistance in the oral cavity as a therapeutic option, describing the plant family, plant common name, botanical name, bioactive compounds and type of extract, fungus on which it acts, and main findings.
The limited access to antifungal drugs for the specific treatment of C. albicans in the oral cavity has promoted research on products of natural origin for the discovery of new therapeutic possibilities. Based on this context, tropical countries are pioneers in the production of these natural products with potential antimicrobial activity. Walicyranison Plinio Silva-Rocha et al., in their research on the effect of E. uniflorain on C. albicans, found that more than 80% of A549 cells (human alveolar epithelial cell line) remained viable even when exposed to four times the concentration of E. uniflora EC (8000 μg/mL; unpublished data), and E. uniflora EC inhibited hyphal formation in both the liquid and solid media tested. It also impaired hydrolytic enzyme production. This is one of the first studies to describe the interaction of a natural product with the full expression of three different factors in C. albicans. Therefore, E. uniflora may be a therapeutic alternative for oral candidiasis in the future. Thus, phytotherapy could become a future option, as previous studies have shown [25][87].
In some parts of the world, such as in the city of Blumenau, several practices such as acupuncture, homeopathy, phytotherapy, hydrotherapy, and anthroposophical medicine are offered by health professionals, including doctors, nurses, dentists, oral health technicians, and nurses. In the study proposed by Mattos et al., it was reported that most health professionals (96.2%) know and believe in the therapeutic effects of medicinal plants but do not prescribe them in their practice. A large percentage of professionals (98.7%) believe that it is a priority to include this practice to complement health services, with the aim of reducing the resistance and side effects produced by the drugs commonly used. These investigations show the importance of the use of phytotherapy; the idea is to advance studies and standardize protocols for the use and management of these active components through experimental studies [26][88].
