Actinomycetes are currently one of the major sources of bioactive secondary metabolites used for medicine development. Accumulating evidence has shown that Nocardiopsis, a key class of actinomycetes, has the ability to produce novel bioactive natural products. This review covers the sources, distribution, bioactivities, biosynthesis, and structural characteristics of compounds isolated from Nocardiopsis in the period between March 2018 and 2021. Our results reveal that 67% of Nocardiopsis-derived natural products are reported for the first time, and 73% of them are isolated from marine Nocardiopsis. The chemical structures of the Nocardiopsis-derived compounds have diverse skeletons, concentrating on the categories of polyketides, peptides, terphenyls, and alkaloids. Almost 50% of the natural products isolated from Nocardiopsis have been discovered to display various bioactivities. These results fully demonstrate the great potential of the genus Nocardiopsis to produce novel bioactive secondary metabolites that may serve as a structural foundation for the development of novel drugs.
- actinomycetes
- Nocardiopsis
- natural products
- bioactivities
- medicinal potentiality
1. Introduction
2. Polyketides
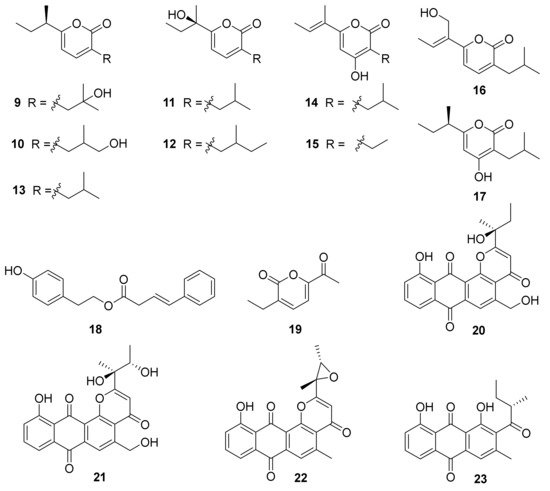
Three new angucyclines, nocardiopsistins A–C (1–3), were obtained from the deep-sea sponge-derived Nocardiopsis sp. strain HB-J378 (Figure 1) [48]. The antibacterial activities of compounds 1–3 were tested against MRSA (methicillin-resistant Staphylococcus aureus) and 1–3 exhibited antibacterial activity with MIC values of 3.12–12.5 μg/mL. Three core genes were identified through bioinformatic analysis of the sketch genome of the strain HB-J378 in a biosynthetic gene cluster encoding a typical aromatic or type II polyketide synthase (PKS) system, including acyl carrier protein (ACP), ketoacyl: ACP synthase α-subunit (KSα) and β-subunit (KSβ). The brief biosynthetic route for 1–3 was proposed according to the discovered oviedomycin pathway [49]. Compounds 1–3 were supposed to be biosynthesized by taking advantage of one molecule of isobutyral-CoA and nine molecules of malonyl-CoA, through complex enzymatic reactions to obtain the key angucycline biosynthetic intermediate UWM6, an analog of 1–3 [50], to acquire 1–3 (see Figure 1) [48].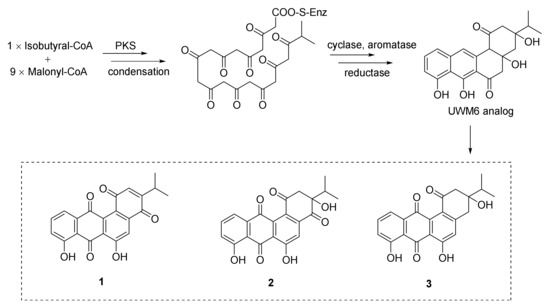
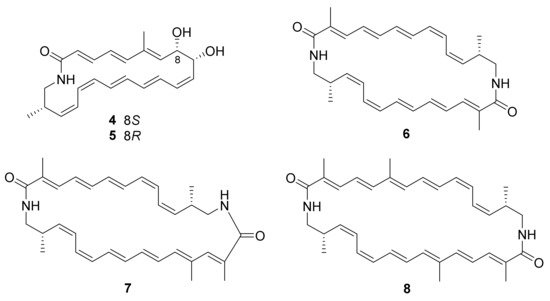
3. Alkaloids
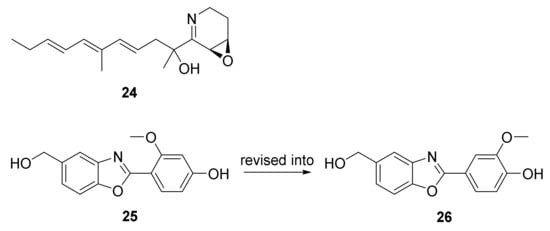
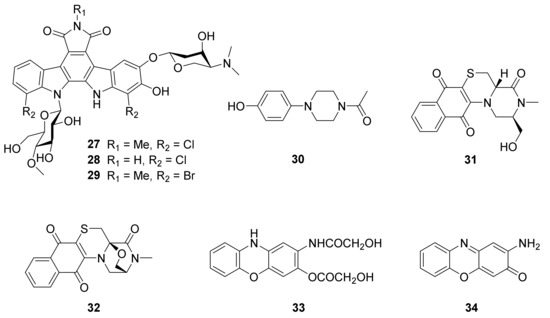
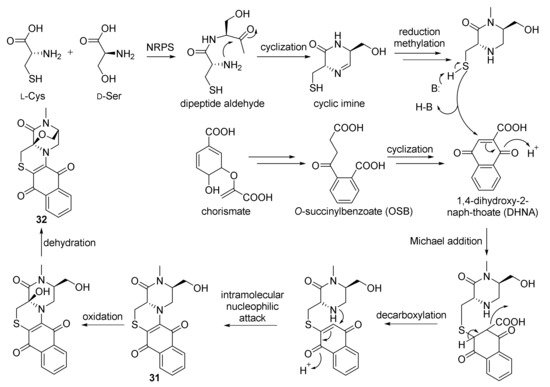
The approach of an optimized nitroso-based probe (dienophile probe), promoting the detection of compounds containing conjugated alkenes in crude broth extracts, was employed in the chemical investigation of a marine-derived Nocardiopsis sp. CNY-503 and gained the isolation of one new polyketide alkaloid, named nocarditriene (24), which contained an unprecedented epoxy-2,3,4,5-tetrahydropyridine structure (Figure 4) [58]. The structure of nocarbenzoxazole G (25), isolated from the marine-derived actinomycete N. lucentensis DSM 44048 in 2015 [60], was revised into the nocarbenzoxazole G (26) molecule by total synthesis in 2019. The benzoxazole skeleton was constructed with microwave assistance and continued by carbon–carbon bond formation with relevant aryl bromides [59]. Compound 26 was found to display moderate cytotoxicity against HepG2 and HeLa cell lines with IC50 values of 16 and 14 μM, respectively [60]. Chemical investigation of the strain N. flavescens NA01583, which was gained from marine sediment gathered at the coast near Hainan Island in 2016 through the genome mining of an indolocarbazole-type gene cluster, led to the isolation of three new indolocarbazole alkaloids, named loonamycins A−C (27−29) (Figure 5) [61]. Compound 29 was produced successfully by the allogenetic expression of the complete loo gene cluster in a vicarious host, Streptomyces lividans K4−114. The indolocarbazole skeleton of 27−29, belonging to the family of indolocarbazole alkaloids, is structurally similar to that of rebeccamycin and staurosporine with an additional rare modified tryptophan ring. The molecular bases of these modifications were investigated by carefully analyzing loo BGC for further genome mining and combinational biosynthetic research. The loo gene cluster sustains a ∼36 kb continuous DNA sequence, including 25 open reading frames which take charge of biosynthesis, regulation, and resistance (Figure 6A). A possible biosynthetic pathway for loonamycin was detected based on this bioinformatic analysis (Figure 6B). In particular, compound 27 showed potent cytotoxic activities toward eight cancer cell lines, including Sum1315 (breast cancer), SH-SY5Y (neuroblastoma), HCT116 (colorectal cancer), HT29 (colorectal cancer), HCC78 (lung cancer), HeLa (cervical cancer), SW620 (colorectal cancer), and SW872 (liposarcoma), with IC50 values of 41−283 nM [61].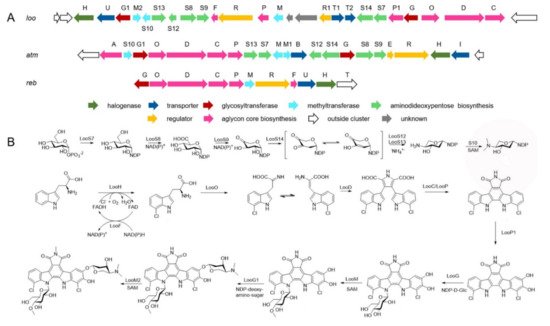
4. Peptides
The secondary metabolites research of the broth culture of Nocardiopsis sp. UR67A, derived from the marine sponge Callyspongia sp. collected from the Red Sea in 2018, resulted in the purification of one new cyclic hexapeptide, nocardiotide A (35) (Figure 8) [64]. Compound 35 was synthesized by combining solid-and solution-phase synthesis methods in 2021 [65]. Compound 35 exhibited obvious cytotoxic activities against HeLa, CT26 (murine colon carcinoma), and MM.1S (human multiple myeloma) cell lines with IC50 values ranging from 8 to 12 µM [64].
Figure 8. Structural formulas of compounds 35–45 [64,66–68].
One new diketopiperazine, 1-demethylnocazine A (36) (Figure 8), was separated from the broth culture of the actinomycete Nocardiopsis sp. TRM20105, which was derived from a local cotton field in Tarim Basin, through the approach of antifungal activity tracking purification against Candida albicans. Compound 36 displayed weak antifungal activity against C. albicans, with an MIC value of 3.16 mM [66].
One new cyclic tetrapeptide, androsamide (37), was isolated from the actinobacterium Nocardiopsis sp. CNT-189 was gathered from the surf zone sediment of the Bahamas shore (Figure 8) [67]. Androsamide (37) displayed moderate cytotoxicity against Caco2 (human colorectal adenocarcinoma), AGS (human gastric adenocarcinoma), and HCT116 (human colorectal carcinoma) cell lines with the IC50 values of 13 μM, 18 μM and 21 μM, respectively. Compound 37 exhibited significant inhibition to the motility of the Caco2 cell line caused by epithelial−mesenchymal transition (EMT) [67]. The expression of cell motility-related genes in Caco2 cells was also examined through Human Cell Motility RT2 Profiler PCR Array. Ras-related C3 botulinum toxin substrate 2 (RAC2) and calpain 1 (CAPN1) have been proved to be the human cell motility-related genes [69–71]. The RT2 Profiler PCR Array results showed that 37 with the significant inhibition of the expression of RAC2 in nontoxic concentration 0.65 μM (1/20 of IC50), and decreased the expression of CAPN1 by 1.3 μM (1/10 of IC50) in Caco2 cells [67].
Secondary metabolites of Nocardiopsis sp. HT88, which was separated from the fresh stems of Mallotus nudiflorus L. (plant), was comprehensively studied for its crude extracts displayed significant antibacterial activities. Eight proline (or hydroxyproline, Hyp)-containing cyclic dipeptides: cyclo(L-Pro-L-Leu) (38), cyclo(Pro-Leu) (39), cyclo(L-trans-Hyp-L-Leu) (40), cyclo(D-trans-Hyp-D-Leu) (41), cyclo(D-Pro-L-Phe) (42), cyclo(L-Pro-L-Phe) (43), and cyclo(D-cis-Hyp-L-Phe) (44), and cyclo(L-trans-Hyp-L-Phe) (45) (Figure 8) were isolated from the broth culture of HT88 guided by antibacterial bioassay. Unfortunately, none of the purified DKPs (38–45) exhibited antibacterial activity against measured strains at 100 μg/disc [68].
Some of the peptides are hydrolyzed fragments of a precursor protein, called ribosomal posttranslational peptides (RiPPs) [73], and others are biosynthesized by non-ribosomal peptide synthetase (NRPS) assembly lines (Figure 9) [72,74].
Figure 9. Biosynthesis of ribosomal and non-ribosomal peptides [72].
There is no research on the biosynthesis of compounds 35 and 37. The cyclodipeptides’ (CDP) core structures are biosynthesized through NRPS or tRNA-dependent CDP synthase (CDPS) (Figure 10) pathways [75,76].
Figure 10. Biosynthesis of CDP through CDPS pathway [76].
Compound 36 is belonging to the family of nocazine and is proposed to be synthesized through a CDPS-dependent pathway (Figure 11) [76]. Compounds 38–45 are modified CDP and are also deduced to be synthesized by the CDPS assembly line.
Figure 11. Proposed biosynthesis of compound 36 [76].
5. Terphenyls
Chemical investigation of the actinobacterium strain Nocardiopsis sp. OUCMDZ-4936, purified from a sample gathered from Dongzhaigang Mangrove Reserve, Hainan Province, China, led to the isolation of three new p-terphenyl derivatives, nocarterphenyls A−C (46−48), together with three known analogs (49−51) (Figure 12) [77]. Compound 46 exhibited significant cytotoxicity against the HL60 and HCC1954 cancer cell lines, with IC50 values of 0.38 ± 0.01 and 0.10 ± 0.01 μM, respectively. Compounds 49 and 51 showed potent cytotoxic activities against K562 and A549 cancer cell lines with IC50 values ranging from 0.10 to 0.77 μM. Compound 51 also exhibited cytotoxicity against MV4-11 and MDA-MB-468 cancer cell lines, with IC50 values of 0.77 ± 0.02 and 0.67 ± 0.01 μM, respectively [77].
Figure 12. Structural formulas of compounds 46–56 [77,78].
Five new p-terphenyl derivatives—called nocarterphenyls D−H (52−56) (Figure 12)—were gained and identified from the broth culture of the actinobacterium Nocardiopsis sp. HDN154086, separated from a marine sediment sample gathered from the South China Sea. It is the first time to find a 2,2′-bithiazole scaffold in natural products. p-Terphenylquinones 53 and 54 contain the unusual substitutions of thioether-linked fatty acid methyl ester. Compounds 52 and 53 demonstrated potent activities against seven kinds of bacteria—E. coli, Proteus sp., M. phlei, V. parahemolyticus, B. subtilis, B. cereus, and MRSA—with MIC values of 1.5−12 μM. Notably, compound 53 displayed significant antibacterial activity against MRSA, exceeding the positive control ciprofloxacin [78].
6. Others
Three known compounds, tryptophan (57), kynurenic acid (58), and 4-amino-3-methoxy benzoic acid (59) (Figure 13), were isolated from the actinobacterium Nocardiopsis sp. UR67, which was derived from the marine sponge Callyspongia sp. gained at a depth of 10 m from the Red Sea [64].
Figure 13. Structural formulas of compounds 57–64 [64,79,80].
One new natural product phenolic acid derivative, 4-amino-6-methylsalicylic acid (60), along with two known compounds, 5-methylresorcinol (61) and linoleic acid (62), were isolated from the Nocardiopsis sp. AS23C strain, which was purified from the marine alga Sargassum arnaudianum derived from the Red Sea at the Hurghada coast, Egypt (Figure 13). The crude extracts of the strain AS23C displayed antibacterial activities against Gram-positive B. subtilis ATCC6051, S. viridochromogenes Tü 57, and S. aureus. Neither of the compounds 60 and 61 displayed cytotoxicity against KB-3-1 (human cervix carcinoma) cell line [79].
A rare actinomycete Nocardiopsis sp. SCA21, deriving from a marine sediment sample gathered from Havelock Island, Andaman, and the Nicobar Islands, India, exhibited the potential ability to produce bioactive secondary metabolites. Chemical investigation of the broth culture of the strain resulted in the purification of two known bioactive compounds—a bromophenol derivative, 4-bromophenol (63), and a phthalate ester, Bis (2-ethylhexyl) phthalate (64) (Figure 13). Compounds 63 and 64 displayed notable enzyme-inhibitory activities against α-glucosidase. However, there was no inhibited activity against α-amylase of compound 64. Both compounds 63 and 64 demonstrated potent free radical scavenging activities against DPPH and ABTS radicals. Additionally, 63 and 64 also revealed broad-spectrum inhibitory activities against MRSA ATCC-46071, MRSA ATCC NR-46171, S. aureus ATCC 12600, B. subtilis ATCC 6633, and Klebsiella pneumonia ATCC 13883 [80].
A novel sterol (65) with an unidentified substitution was isolated from marine actinomycete N. alba MCCB 110. Compound 65 was found to display antibacterial activity against the aquaculture pathogen V. harveyi, and did not exhibit toxicities to the VERO cell line and shrimp hemocytes, up to 1000 ppm [81].
7. Conclusions
From March 2018 to 2021, a total of 63 natural products have been isolated from the genus Nocardiopsis, and 67% of them are first-discovered compounds. These findings sufficiently demonstrate that actinomycetes Nocardiopsis have great potential to produce compounds with novel structures. The structures of the isolated compounds with diverse skeletons are mainly concentrated on the classes of polyketides, peptides, terphenyls, and alkaloids (Table 1 and Figure 14).
Table 1. Compounds isolated from Nocardiopsis during March 2018–2021.
|
Types |
Comps. |
Sources |
Distribution |
Bioactivities |
Years |
Refs |
|
Polyketides |
1–3 |
Sponge Theonella sp. derived Nocardiopsis sp. HB-J378 (GenBank No. MH779065) |
|
Antibacterial activity |
2018 |
[48] |
|
|
4, 5 |
Saltpan-derived Nocardiopsis sp. CG3 (GenBank No. MG972881) |
Kenadsa, Algeria |
|
2019 |
[51] |
|
|
6−8 |
Saltpan-derived Nocardiopsis sp. CG3 (GenBank No. MG972881) |
Kenadsa, Algeria |
Cytotoxicity |
2019 |
[51] |
|
|
9−12, 14, 15, 17 |
Desert soil-derived Nocardiopsis spp. HDN154-146 (Genbank no. KY794927) and HDN154-168 (Genbank No. MF952649) |
Xinjiang, China |
|
2019 |
[52] |
|
|
13, 16 |
Desert soil-derived Nocardiopsis spp. HDN154-146 (Genbank no. KY794927) and HDN154-168 (Genbank No. MF952649) |
Xinjiang, China |
Cytoprotective activity |
2019 |
[52] |
|
|
18, 19 |
Deep-sea water-derived N. dassonvillei subsp. albirubida HDN 17-237 (Genbank No. MN416280) |
Mariana Trench |
|
2020 |
[55] |
|
|
20, 21 |
Marine animal-derived N. aegyptia HDN19-252 (GenBank No. MN822699) |
Antarctic |
Antibacterial activity |
2021 |
[56] |
|
|
22, 23 |
Marine animal derived strain N. aegyptia HDN19-252 (GenBank No. MN822699) |
Antarctic |
|
2021 |
[56] |
|
Alkaloids |
24 |
Marine-derived Nocardiopsis sp. CNY-503 |
|
|
2018 |
[58] |
|
|
27 |
Marine sediment-derived N. flavescens NA01583 (GenBank No. MT371575) |
Hainan China |
Cytotoxicity |
2020 |
[61] |
|
|
28, 29 |
Marine sediment-derived N. flavescens NA01583 (GenBank No. MT371575) |
Hainan China |
|
2020 |
[61] |
|
|
30 |
Marine sediment-derived Nocardiopsis sp. SCA30 (GenBank No. MT573349) |
Havelock Island |
Antibacterial and anticancer activities |
2021 |
[22] |
|
|
31, 32 |
Sponge Petrosia sp.-derived N. dassonvillei SCSIO 40065 (GenBank No. MW492395) |
South China Sea |
Antibacterial and cytotoxic activities |
2021 |
[62] |
|
|
33, 34 |
Marine sediment-derived N. dassonvillei JS106 (GenBank No. MN416229) |
Lianyungang, China, |
Antiquorum sensing activity |
2021 |
[63] |
|
Peptides |
35 |
Sponge Callyspongia sp.-derived Nocardiopsis sp. UR67 |
Red Sea |
Cytotoxicity |
2018 |
[64] |
|
|
36 |
Cotton field-derived Nocardiopsis sp. TRM20105 |
Tarim Basin |
Antifungal activity |
2019 |
[66] |
|
|
37 |
Shore sediment-derived Nocardiopsis sp. CNT-189 (GenBank No. KY111725.1) |
Bahamas |
Cytotoxicity and colorectal cancer motility inhibitor |
2020 |
[67] |
|
|
38−45 |
Stems of Mallotus nudiflorus L-derived Nocardiopsis sp. HT88 (Genbank No. MH817156) |
|
|
2020 |
[68] |
|
Terphenyls |
46, 49−51 |
Mangrove-derived Nocardiopsis sp. OUCMDZ-4936 (Genbank No. MK129184) |
Hainan China |
Cytotoxicity |
2019 |
[77] |
|
|
47, 48 |
Mangrove-derived Nocardiopsis sp. OUCMDZ-4936 (Genbank No. MK129184) |
Hainan China |
|
2019 |
[77] |
|
|
52, 53 |
Marine sediment-derived Nocardiopsis sp. HDN154086 (GenBank No. MK129184) |
South China Sea |
Antibacterial activity |
2021 |
[78] |
|
|
54−56 |
Marine sediment-derived Nocardiopsis sp. HDN154086 (GenBank No. MK129184) |
South China Sea |
|
2021 |
[78] |
|
Others |
57−59 |
Sponge Callyspongia sp.-derived Nocardiopsis sp. UR67 |
Red Sea |
|
2018 |
[64] |
|
|
60−62 |
Alga Sargassum arnaudianum-derived Nocardiopsis sp. AS23C (GenBank No. MH144210) |
Red Sea |
|
2019 |
[79] |
|
|
63, 64 |
Marine sediment-derived Nocardiopsis sp. SCA21 (GenBank No. MH105056) |
Havelock Island |
Enzyme inhibitory, antibacterial, and free radical scavenging activities |
2019 |
[80] |
|
|
65 |
Marine-derived N. alba MCCB110 |
|
Antibacterial activity |
2021 |
[81] |
Figure 14. Structural types of compounds isolated from Nocardiopsis from March 2018 to 2021.
The sources of the genus Nocardiopsis are distributed throughout diverse ecological systems, including desert, marine, mangrove, and saltpan areas (Figure 15). More than two out of three natural products were isolated from marine-derived Nocardiopsis, demonstrating that the ocean is a vast treasure house with abundant microbial sources to produce various novel natural products. From March 2018 to 2021, the percentage of the marine-derived compounds increased by 10%, compared with the period 2015−February 2018 [5], which further demonstrates that more and more attention has been concentrated on the development of the marine resources. Among the marine-derived natural products, the sources of Nocardiopsis strains are mainly collected from marine sediment and sponges (Figure 15).
Figure 15. Sources of natural products from Nocardiopsis from March 2018 to 2021.
The genus Nocardiopsis has the potential ability to produce a great diversity of bioactive secondary metabolites, including antibacterial, cytotoxic, cytoprotective, enzyme inhibitory, free radical scavenging, antiquorum sensing, colorectal cancer motility inhibitory, and antifungal compounds (Figure 16). Almost 50% of the Nocardiopsis-derived natural products have been discovered to exhibit various bioactivities. The activities of the bioactive compounds are mainly focused on the categories of antibacterial and cytotoxic activities (Figure 16), which serves as a reminder that there is huge potential for new antibiotics and anticancer compounds to be developed from Nocardiopsis.
Figure 16. Bioactivities of natural products from Nocardiopsis discovered from March 2018 to 2021.
There were 13 antibacterial natural products isolated from Nocardiopsis in the period March 2018 to 2021 (Table 2). Among them, compounds 2, 30, and 53 exhibit strong antibacterial activity against MRSA, and compounds 20 and 21 display significant antibacterial activity against MRCNS (Table 2). These five compounds might be developed into new antibiotics to respond to the challenge of increasing antibacterial resistance. Compound 52 shows significant antibacterial activities against a series of strains, which has the potential to be exploited into novel antibiotics with a broad spectrum of antibacterial activity (Table 2). Compound 65 with antibacterial activity displays no toxic against VERO cell line and shrimp hemocytes up to 1000 ppm, could be developed as an environmentally friendly antibiotic (Table 2). All of the isolated antibacterial secondary metabolites provide the structural basis for novel antibiotic research.
Table 2. Antibacterial activities of compounds isolated from Nocardiopsis from March 2018 to 2021.
|
Strains |
Comps. |
Values (MIC) |
Pros |
Cons |
|
MRSA |
1, 3 |
12.5 μg/mL |
Specific inhibition of MRSA |
Moderate activity [48] |
|
2 |
3.12 μg/mL |
Strong activity; Specific inhibition of MRSA |
[48] |
|
|
MRCNS |
20/21 (μM) |
6.2/6.2 |
Broad-spectrum antibacterial activity; Strong activity against MRCNS compared with positive control |
Moderate activity against B. subtilis and Proteus sp. compared with positive control [56] |
|
B. subtilis |
6.2/6.2 |
|||
|
Proteus sp. |
12.5/6.2 |
|||
|
B. cereus |
6.2/6.2 |
|||
|
E. coli |
6.2/6.2 |
|||
|
M. Phlei |
6.2/3.1 |
|||
|
MRSA ATCC NR-46071 |
30 (μg/mL) |
15.6 |
Strong activity |
[22] |
|
MRSA ATCC NR-46171 |
7.8 |
|||
|
B. subtilis 1064 |
31/32 (μg/mL) |
8/16 |
Broad-spectrum antibacterial activity |
Weak activity [62] |
|
M. Luteus SCSIO ML01 |
16/32 |
|||
|
S. aureus ATCC 29213 |
64/64 |
|||
|
MRSA shhsA1 |
32/32 |
|||
|
E. faecalis ATCC 29212 |
32/- |
|||
|
V. alginolyticus 13214 |
32/64 |
|||
|
Proteus sp. |
52/53 (μM) |
3.1/12 |
52 showed strong and broad-spectrum activity, and 53 displayed strong activity of MRSA |
53 displayed moderate activity against Proteus sp. and B. subtilis [78] |
|
B. cereus |
1.5/- |
|||
|
M. phlei |
6.2/- |
|||
|
B. subtilis |
3.1/12 |
|||
|
MRSA |
-/6.2 |
|||
|
V. parahemolyticus |
6.2/- |
|||
|
E. coli |
3.1/- |
|||
|
K. pneumoniae ATCC 13883 |
63/64 (μg/mL) |
125/250 |
Broad-spectrum antibacterial activity |
Weak activity [80] |
|
Listeria cytogens ATCC 13932 |
62.5/- |
|||
|
S. aureus ATCC 12600 |
62.5/125 |
|||
|
B. subtilis ATCC 6633 |
7.81/7.81 |
|||
|
MRSA ATCC NR-46171 |
15.62/7.81 |
|||
|
MRSA ATCC NR-46071 |
125/15.62 |
|||
|
V. harveyi MCCB 111 |
65 |
20 mm (zone of inhibition) |
Not toxic against VERO cell line and shrimp hemocytes up to 1000 ppm |
[81] |
There were 13 cytotoxic natural products isolated from Nocardiopsis in the period March 2018 to 2021 (Table 3). Compound 27 shows extremely significant and broad-spectrum cytotoxicity against a series of cell lines with the IC50 values at the nM level, and compounds 46 and 49−51 display potent and broad-spectrum cytotoxicity (Table 3). All four compounds have the potential to be developed as new anticancer drugs. Compounds 8, 31, 32, 35, and 37 with moderate cytotoxicity provide structural inspiration for the research and development of new anticancer drugs (Table 3).
Eight secondary metabolites isolated from Nocardiopsis have various bioactivities, including cytoprotective, antiquorum sensing, colorectal cancer motility and enzyme inhibitory, and free radical scavenging activities (Table 3).
Although the Nocardiopsis-derived natural products exhibit excellent diverse bioactivities, further research on their bioactive mechanisms is deficient. Only three compounds, 13, 16, and 37, have been studied for their active mechanisms between March 2018 and 2021. To the best of our knowledge, all the activity tests were evaluated in vitro, and bioactive evaluations in vivo are also lacking between March 2018 and 2021. In terms of the current situation, it is still a long way from the drugs being approved for clinical use.
Table 3. Cytotoxicity and other activities of compounds isolated from Nocardiopsis from March 2018 to 2021.
|
Bioactivities |
Cells/stains/enzyme |
Comps. |
Values |
Pros |
Cons |
|
Cytotoxicity (IC50) |
Hela cells KB3.1 |
6/7/8 (μM) |
6.8/5.4/2.4 |
Compound 8 with broad-spectrum cytotoxicity |
Moderate activity [51] |
|
PC-3 |
6.3/5.0/2.1 |
||||
|
A549 |
-/-/6.5 |
||||
|
SKOV-3 |
-/10.0/5.5 |
||||
|
SH-SY5Y |
27 (nM) |
283.6 |
Extremely potent and broad-spectrum cytotoxicity |
[61] |
|
|
Sum1315 |
121.3 |
||||
|
HT29 |
81.3 |
||||
|
SW620 |
90.5 |
||||
|
HCT116 |
31.4 |
||||
|
HeLa |
100.1 |
||||
|
SW872 |
92.3 |
||||
|
HCC78 |
41.5 |
||||
|
|
30 |
|
Broad-spectrum cytotoxicity |
IC50 untested [22] |
|
|
SF-268 |
31/32 (μM) |
17.0/11.9 |
Broad-spectrum cytotoxicity |
Moderate activity [62] |
|
|
MCF-7 |
25.7/20.7 |
||||
|
HepG2 |
31.2/12.0 |
||||
|
A549 |
34.4/13.5 |
||||
|
MM. 1S |
35 (μM) |
8 |
Broad-spectrum cytotoxicity |
Moderate activity [64] |
|
|
HeLa |
11 |
||||
|
CT26 |
12 |
||||
|
AGS |
37 (μM) |
13 |
Broad-spectrum cytotoxicity |
Moderate activity [67] |
|
|
Caco2 |
18 |
||||
|
HCT116 |
21 |
||||
|
L-02 |
49 (μM) |
17 |
Strong and broad-spectrum cytotoxicity |
[77] |
|
|
A549 |
5.1 |
||||
|
K562 |
0.77 |
||||
|
MCF-7 |
6.0 |
||||
|
P6C |
9.4 |
||||
|
N87 |
46/50/51 (μM) |
-/1.0/- |
|||
|
A673 |
-/0.76/8.9 |
||||
|
MV4-11 |
4.0/0.16/0.77 |
||||
|
K562 |
9.0/4.8/8.9 |
||||
|
A549 |
7.8/0.48/9.7 |
||||
|
BT474 |
6.0/3.6/- |
||||
|
H1229 |
-/0.72/- |
||||
|
HUCCT1 |
-/0.20/- |
||||
|
B16F10 |
-/0.76/- |
||||
|
MDA-MB-468 |
2.8/1.1/0.67 |
||||
|
H1975 |
-/3.1/4.4 |
||||
|
HL60 |
0.38/0.17/5.0 |
||||
|
A431 |
4.6/-/- |
||||
|
U251 |
-/4.7/- |
||||
|
HCC1954 |
0.10/0.48/2.0 |
||||
|
MCF-7 |
18/-/17 |
||||
|
MKN-45 |
-/0.49/12 |
||||
|
DU-145 |
-/0.52/1.0 |
||||
|
SPC-A1 |
-/2.0/9.8 |
||||
|
HCT116 |
-/1.9/- |
||||
|
143B |
5.5/5.0/7.7 |
||||
|
H2228 |
1.7/0.94/5.0 |
||||
|
MDA-MB-231 |
-/2.0/2.0 |
||||
|
Cytoprotective activity |
HaCaT cells |
13, 16 |
|
|
[52] |
|
Antiquorum sensing activity (IC50) |
C. violaceum 12472 |
33/34 (μg/mL) |
23.59/6.82 |
|
[63] |
|
Antifungal activity (MIC) |
C. albicans |
36 |
3.16 mM |
|
Weak activity [66] |
|
Colorectal cancer motility inhibition |
Caco2 |
37 |
|
Strong activity |
[67] |
|
Enzyme-inhibitory activity (IC50) |
α-glucosidase |
63/64 |
94.61/202.33 |
strong activity against α-glucosidase |
[80] |
|
α-amylase |
103.23/- |
||||
|
Free radical scavenging activity |
|
63/64 |
|
Strong activity |
[80] |
In this review, we summarized the secondary metabolites isolated from Nocardiopsis in the period between March 2018 and 2021. The literature survey comprehensively indicates that actinomycetes Nocardiopsis have great potential as producers to generate abundant and diverse novel bioactive secondary metabolites. Some potent antibacterial and cytotoxic compounds isolated from Nocardiopsis have the potential to be developed into new drugs. Additionally, all the natural products isolated from Nocardiopsis provide a structural foundation for drug design.
References
- Jose, P.A.; Maharshi, A.; Jha, B. Actinobacteria in natural products research: Progress and prospects. Res. 2021, 246, 126708.
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Clement, C.; Meier-Kolthoff, J.P.; Klenk, H.-P.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of actinobacteria. Mol. Biol. Rev. 2016, 80, 1–43.
- Demain, A.L.; Sanchez, S. Microbial drug discovery: 80 years of progress. Antibiot. 2009, 62, 5–16.
- Mahajan, G. B.; Balachandran, L., Antibacterial agents from actinomycetes–a review. Biosci., Elite Ed. 2012, E4, 240–253.
- Ibrahim, A. H.; Desoukey, S. Y.; Fouad, M. A.; Abdelmohsen, U. R.; Kamel, M. S.; Gulder, T. A. M., Natural product potential of the genus Nocardiopsis. Drugs 2018, 16.
- Li, J. W. H.; Vederas, J. C., Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165.
- Manivasagan, P.; Kang, K.-H.; Sivakumar, K.; Li-Chan, E. C. Y.; Oh, H.-M.; Kim, S.-K., Marine actinobacteria: An important source of bioactive natural products. Toxicol. Pharmacol. 2014, 38, 172–188.
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.-K., Pharmaceutically active secondary metabolites of marine actinobacteria. Res. 2014, 169, 262–278.
- AbdElgawad, H.; Zinta, G.; Abuelsoud, W.; Hassan, Y. M.; Alkhalifah, D. H. M.; Hozzein, W. N.; Zrieq, R.; Beemster, G. T.; Schoenaers, S., An actinomycete strain of Nocardiopsis lucentensis reduces arsenic toxicity in barley and maize. Hazard. Mater. 2021, 417, 126055.
- Adenan, N. H.; Lim, Y. Y.; Ting, A. S. Y., Nocardiopsis for the removal of triphenylmethane dyes: Decolorization and optimization studies. Water, Air, Soil Pollut. 2021, 232, 1–17.
- Patel, G. B.; Rakholiya, P.; Shindhal, T.; Varjani, S.; Tabhani, N.; Shah, K. R., Lipolytic Nocardiopsis for reduction of pollution load in textile industry effluent and SWISS model for structural study of lipase. Technol. 2021, 341, 125673.
- Bennur, T.; Ravi Kumar, A.; Zinjarde, S. S.; Javdekar, V., Nocardiopsis species: a potential source of bioactive compounds. Appl. Microbiol. 2016, 120, 1–16.
- Chen, J.; Xu, L.; Zhou, Y.; Han, B., Natural products from actinomycetes associated with marine organisms. Drugs 2021, 19, 629.
- Kumar, S.; Solanki, D. S.; Parihar, K.; Tak, A.; Gehlot, P.; Pathak, R.; Singh, S. K., Actinomycetes isolates of arid zone of Indian Thar Desert and efficacy of their bioactive compounds against human pathogenic bacteria. Futura 2021, 72, 431–440.
- Pinto-Almeida, A.; Bauermeister, A.; Luppino, L.; Grilo, I. R.; Oliveira, J.; Sousa, J. R.; Petras, D.; Rodrigues, C. F.; Prieto-Davo, A.; Tasdemir, D.; Sobral, R. G.; Gaudencio, S. P., The diversity, metabolomics profiling, and the pharmacological potential of actinomycetes isolated from the Estremadura Spur Pockmarks (Portugal). Drugs 2022, 20, 21.
- Chen, L.; Wang, Z.; Du, S.; Wang, G., Antimicrobial activity and functional genes of actinobacteria from coastal wetland. Microbiol. 2021, 78, 3058–3067.
- Tatar, D., Isolation, phylogenetic analysis and antimicrobial activity of halophilic actinomycetes from different saline environments located near Corum province. Biologia (Cham, Switz.) 2021, 76, 773–780.
- Li, H.-W.; Zhi, X.-Y.; Yao, J.-C.; Zhou, Y.; Tang, S.-K.; Klenk, H.-P.; Zhao, J.; Li, W.-J., Comparative genomic analysis of the genus Nocardiopsis provides new insights into its genetic mechanisms of environmental adaptability. PLoS One 2013, 8, e61528.
- Bennur, T.; Kumar, A. R.; Zinjarde, S.; Javdekar, V., Nocardiopsis species as potential sources of diverse and novel extracellular enzymes. Microbiol. Biotechnol. 2014, 98, 9173–9185.
- Bennur, T.; Kumar, A. R.; Zinjarde, S.; Javdekar, V., Nocardiopsis species: Incidence, ecological roles and adaptations. Res. 2015, 174, 33–47.
- Shady, N. H.; Tawfike, A. F.; Yahia, R.; Fouad, M. A.; Brachmann, A. O.; Piel, J.; Abdelmohsen, U. R.; Kamel, M. S., Cytotoxic activity of actinomycetes Nocardia and Nocardiopsis sp. associated with marine sponge Amphimedon sp. Nat. Prod. Res. 2021, 2021, 1–6.
- Siddharth, S.; Aswathanarayan, J. B.; Kuruburu, M. G.; Madhunapantula, S. R. V.; Vittal, R. R., Diketopiperazine derivative from marine actinomycetes Nocardiopsis SCA30 with antimicrobial activity against MRSA. Arch. Microbiol. 2021, 203, 6173–6181.
- Trivedi, N.; Thumar, J., Chemical profiling of antimicrobial metabolites from halophilic actinomycete Nocardiopsis Al-H10-1 (KF384482) isolated from Alang, Gulf of Khambhat, India. bioRxiv 2021.
- Goel, N.; Fatima, S. W.; Kumar, S.; Sinha, R.; Khare, S. K., Antimicrobial resistance in biofilms: Exploring marine actinobacteria as a potential source of antibiotics and biofilm inhibitors. Rep. 2021, 30, e00613.
- Choi, G.; Kim, G. J.; Choi, H.; Choi, I.-W.; Lee, D.-S., Anti-inflammatory and anti-fibrotic activities of Nocardiopsis 13G027 in lipopolysaccharides-induced RAW 264.7 macrophages and transforming growth factor beta-1-stimulated nasal polyp-derived fibroblasts. Microbiol. Biotechnol. Lett. 2021, 49, 543–551.
- Sarmiento-Vizcaíno, A.; Martín, J.; Reyes, F.; García, L. A.; Blanco, G., Bioactive natural products in actinobacteria isolated in rainwater from storm clouds transported by western winds in Spain. Microbiol. 2021, 12, 773095.
- Gamaleldin, N. M.; Bakeer, W.; El-Gendy, A. O.; Sayed, A. M.; Shamikh, Y. I.; Hassan, H. M.; Horn, H.; Abdelmohsen, U. R.; Hozzein, W. N., Exploration of chemical diversity and antitrypanosomal activity of some Red Sea-derived actinomycetes using the OSMAC approach supported by LC-MS-based metabolomics and molecular modelling. Antibiotics 2020,
- Widada, J.; Damayanti, E.; Alhakim, M. R.; Yuwono, T.; Mustofa, M., Two strains of airborne Nocardiopsis alba producing different volatile organic compounds (VOCs) as biofungicide for Ganoderma boninense. FEMS Microbiol. Lett. 2021, 368, fnab138.
- Wang, Z.; Fu, P.; Liu, P.; Wang, P.; Hou, J.; Li, W.; Zhu, W., New pyran-2-ones from alkalophilic actinomycete, Nocardiopsis alkaliphila nov. YIM-80379. Chem. Biodiversity 2013, 10, 281–287.
- Lu, C.; Li, Y.; Wang, H.; Wang, B.; Shen, Y., A new phenoxazine derivative isolated from marine sediment actinomycetes, Nocardiopsis 236. Drug Discoveries Ther. 2013, 7, 101–104.
- Tian, S.; Yang, Y.; Liu, K.; Xiong, Z.; Xu, L.; Zhao, L., Antimicrobial metabolites from a novel halophilic actinomycete Nocardiopsis terrae YIM 90022. Prod. Res. 2014, 28, 344–346.
- Yamashita, T.; Imoto, M.; Isshiki, K.; Sawa, T.; Naganawa, H.; Kurasawa, S.; Zhu, B.; Umezawa, K., Isolation of a new indole alkaloid, pendolmycin, from Nocardiopsis. Nat. Prod. 1988, 51, 1184–1187.
- Lin, Z.; Torres, J. P.; Ammon, M. A.; Marett, L.; Teichert, R. W.; Reilly, C. A.; Kwan, J. C.; Hughen, R. W.; Flores, M.; Tianero, M. D.; Peraud, O.; Cox, J. E.; Light, A. R.; Villaraza, A. J. L.; Haygood, M. G.; Concepcion, G. P.; Olivera, B. M.; Schmidt, E. W., A Bacterial source for mollusk pyrone polyketides. Biol. 2013, 20, 73–81.
- Gao, X.; Lu, Y.; Xing, Y.; Ma, Y.; Lu, J.; Bao, W.; Wang, Y.; Xi, T., A novel anticancer and antifungus phenazine derivative from a marine actinomycete BM-17. Res. 2012, 167, 616–622.
- Kase, H.; Iwahashi, K.; Matsuda, Y., K-252a, a potent inhibitor of protein kinase C from microbial origin. Antibiot. 1986, 39, 1059–1065.
- Raju, R.; Piggott, A. M.; Huang, X.-C.; Capon, R. J., Nocardioazines: A novel bridged diketopiperazine scaffold from a marine-derived bacterium inhibits P-glycoprotein. Lett. 2011, 13, 2770–2773.
- Kim, M. C.; Kwon, O.-W.; Park, J.-S.; Kim, S. Y.; Kwon, H. C., Nocapyrones H–J, 3,6-disubstituted α-pyrones from the marine actinomycete Nocardiopsis KMF-001. Chem. Pharm. Bull. 2013, 61, 511–515.
- Wu, Z.; Xie, L.; Xia, G.; Zhang, J.; Nie, Y.; Hu, J.; Wang, S.; Zhang, R., A new tetrodotoxin-producing actinomycete, Nocardiopsis dassonvillei, isolated from the ovaries of puffer fish Fugu rubripes. Toxicon 2005, 45, 851–859.
- Peltola, J. S. P.; Andersson, M. A.; Kampfer, P.; Auling, G.; Kroppenstedt, R. M.; Busse, H.-J.; Salkinoja-Salonen, M. S.; Rainey, F. A., Isolation of toxigenic Nocardiopsis strains from indoor environments and description of two new Nocardiopsis species, exhalans sp. nov. and N. umidischolae sp. nov. Appl. Environ. Microbiol. 2001, 67, 4293–4304.
- Kim, Y.; Ogura, H.; Akasaka, K.; Oikawa, T.; Matsuura, N.; Imada, C.; Yasuda, H.; Igarashi, Y., Nocapyrones: α- and γ-pyrones from a marine-derived Nocardiopsis Mar. Drugs 2014, 12, 4110–4125.
- Sun, M.-W.; Guo, Z.-X.; Lu, C.-H., Two new polyketides from Nocardiopsis lucentensis DSM 44048. Prod. Res. 2016, 30, 1036–1041.
- Zhang, H.; Saurav, K.; Yu, Z.; Mandi, A.; Kurtan, T.; Li, J.; Tian, X.; Zhang, Q.; Zhang, W.; Zhang, C., α-Pyrones with diverse hydroxy substitutions from three marine-derived Nocardiopsis J. Nat. Prod. 2016, 79, 1610–1618.
- Kim, J.; Shin, D.; Kim, S.-H.; Park, W.; Shin, Y.; Kim, W. K.; Lee, S. K.; Oh, K.-B.; Shin, J.; Oh, D.-C., Borrelidins C–E: new antibacterial macrolides from a saltern-derived halophilic Nocardiopsis Mar. Drugs 2017, 15, 166/1–166/11.
- Zhang, X.; He, H.; Ma, R.; Ji, Z.; Wei, Q.; Dai, H.; Zhang, L.; Song, F., Madurastatin B3, a rare aziridine derivative from actinomycete Nocardiopsis LS150010 with potent anti-tuberculosis activity. J. Ind. Microbiol. Biotechnol. 2017, 44, 589–594.
- Eliwa, E. M.; Abdel-Razek, A. S.; Frese, M.; Wibberg, D.; Halawa, A. H.; El-Agrody, A. M.; Bedair, A. H.; Kalinowski, J.; Sewald, N.; Shaaban, M., New bioactive compounds from the marine-derived actinomycete Nocardiopsis lucentensis ASMR2. Z. Naturforsch., B: J. Chem. Sci. 2017, 72, 351–360.
- Sun, M.-W.; Zhang, X.-M.; Bi, H.-L.; Li, W.-J.; Lu, C.-H., Two new sesquiterpenoids produced by halophilic Nocardiopsis chromatogenes YIM 90109. Prod. Res. 2017, 31, 77–83.
- Hamed, A.; Abdel-Razek, A. S.; Frese, M.; Stammler, H. G.; El-Haddad, A. F.; Ibrahim, T. M. A.; Sewald, N.; Shaaban, M., Terretonin N: A new meroterpenoid from Nocardiopsis Molecules 2018, 23, 299/1–299/12.
- Xu, D.; Nepal, K. K.; Harmody, D.; McCarthy, P. J.; Wright, A. E.; Wang, G.; Chen, J.; Zhu, H., Nocardiopsistins A–C: New angucyclines with anti-MRSA activity isolated from a marine sponge-derived Nocardiopsis HB-J378. Synth. Syst. Biotechnol. 2018, 3, 246–251.
- Lombo, F.; Brana, A. F.; Salas, J. A.; Mendez, C., Genetic organization of the biosynthetic gene cluster for the antitumor angucycline oviedomycin in Streptomyces antibioticus ATCC 11891. ChemBioChem 2004, 5, 1181–1187.
- Kharel, M. K.; Pahari, P.; Shepherd, M. D.; Tibrewal, N.; Nybo, S. E.; Shaaban, K. A.; Rohr, J., Angucyclines: Biosynthesis, mode-of-action, new natural products, and synthesis. Prod. Rep. 2012, 29, 264–325.
- Messaoudi, O.; Sudarman, E.; Bendahou, M.; Jansen, R.; Stadler, M.; Wink, J., Kenalactams A–E, polyene macrolactams isolated from Nocardiopsis J. Nat. Prod. 2019, 82, 1081–1088.
- Zhao, T.; Chang, Y.; Zhu, T.; Li, J.; Gu, Q.; Li, D.; Che, Q.; Zhang, G., α-Pyrone derivatives with cyto-protective activity from two Takla Makan desert soil derived actinomycete Nocardiopsis strains recovered in seawater based medium. Prod. Res. 2019, 33, 2498–2506.
- Bolling, B. W.; Parkin, K. L., Limited contribution of isoflavones to hepatocellular phase II enzyme-inducing activity of soybean (Glycine max) extracts. Food Chem. 2009, 113, 1069–
- Park, J. S.; Jung, J. S.; Jeong, Y. H.; Hyun, J. W.; Le, T. K. V.; Kim, D. H.; Choi, E. C.; Kim, H. S., Antioxidant mechanism of isoflavone metabolites in hydrogen peroxide‐stimulated rat primary astrocytes: critical role of hemeoxygenase‐1 and NQO1 expression. Neurochem. 2011, 119, 909–919.
- Wang, J.-X.; Sun, C.-X.; Shah, M.; Zhang, G.-J.; Gu, Q.-Q.; Zhu, T.-J.; Che, Q.; Li, D.-H., New metabolites from a Mariana Trench-derived actinomycete Nocardiopsis HDN 17-237. J. Asian Nat. Prod. Res. 2020, 22, 1031–1036.
- Zhou, L.; Chen, X.; Sun, C.; Chang, Y.; Huang, X.; Zhu, T.; Zhang, G.; Che, Q.; Li, D., Saliniquinone derivatives, saliniquinones G−I and heraclemycin E, from the marine animal-derived Nocardiopsis aegyptia HDN19-252. Drugs 2021, 19, 575.
- Shin, J.; Yang, S.-H.; Du, Y. E.; Park, K.; Kim, D.; Shin, D.; Kim, J.; Kim, S.-H.; Kim, Y. K.; Shin, J., Borrelidin from saltern-derived halophilic Nocardiopsis dissociates amyloid-β and tau fibrils. J. Alzheimer's Dis. Rep. 2021, 5, 7–13.
- Castro-Falcon, G.; Millan-Aguinaga, N.; Roullier, C.; Jensen, P. R.; Hughes, C. C., Nitrosopyridine probe to detect polyketide natural products with conjugated alkenes: Discovery of novodaryamide and nocarditriene. ACS Chem. Biol. 2018, 13, 3097–3106.
- Kim, T.; Lee, S.-A.; Noh, T.; Choi, P.; Choi, S.-J.; Song, B. G.; Kim, Y.; Park, Y.-T.; Huh, G.; Kim, Y.-J.; Ham, J., Synthesis, structure revision, and cytotoxicity of nocarbenzoxazole G. Nat. Prod. 2019, 82, 1325–1330.
- Sun, M.; Zhang, X.; Hao, H.; Li, W.; Lu, C., Nocarbenzoxazoles A–G, benzoxazoles produced by halophilic Nocardiopsis lucentensis DSM 44048. Nat. Prod. 2015, 78, 2123–2127.
- Yang, C. L.; Zhang, B.; Xue, W. W.; Li, W.; Xu, Z. F.; Shi, J.; Shen, Y.; Jiao, R. H.; Tan, R. X.; Ge, H. M., Discovery, biosynthesis, and heterologous production of loonamycin, a potent anticancer indolocarbazole alkaloid. Lett. 2020, 22, (12), 4665–4669.
- Zhang, X.; Chen, S.; Zhang, L.; Zhang, Q.; Zhang, W.; Chen, Y.; Zhang, W.; Zhang, H.; Zhang, C., Dassonmycins A and B, polycyclic thioalkaloids from a marine sponge-derived Nocardiopsis dassonvillei SCSIO 40065. Lett. 2021, 23, (8), 2858–2862.
- Miao, L.; Qian, S.; Qi, S.; Jiang, W.; Dong, K., Culture medium optimization and active compounds investigation of an anti-quorum sensing marine actinobacterium Nocardiopsis dassonvillei Microbiology 2021, 90, 112–123.
- Ibrahim, A. H.; Attia, E. Z.; Desoukey, S. Y.; Fouad, M. A.; Abdelmohsen, U. R.; Hajjar, D.; Anany, M. A.; Wajant, H.; Kamel, M. S.; Gulder, T. A. M., New cytotoxic cyclic peptide from the marine sponge-associated Nocardiopsis UR67. Mar. Drugs 2018, 16.
- Muhajir, M. I.; Hardianto, A.; Al‐Anshori, J.; Sumiarsa, D.; Mayanti, T.; Harneti, D.; Hidayat, A. T.; Supratman, U.; Maharani, R., Total synthesis of nocardiotide A by using a combination of solid‐and solution‐phase methods. ChemistrySelect 2021, 6, 12941–
- Chen, H.; Wan, C.; Zhang, L., A new diketopiperazine isolated from a Nocardiopsis strain TRM20105 guided by bioassay against Candida albicans. Prod. Res. 2019, 33, 3421–3425.
- Lee, J.; Gamage, C. D. B.; Kim, G. J.; Hillman, P. F.; Lee, C.; Lee, E. Y.; Choi, H.; Kim, H.; Nam, S.-J.; Fenical, W., Androsamide, a cyclic tetrapeptide from a marine Nocardiopsis, suppresses motility of colorectal cancer cells. J. Nat. Prod. 2020, 83, 3166–3172.
- Xiang, W.-X.; Liu, Q.; Li, X.-M.; Lu, C.-H.; Shen, Y.-M., Four pairs of proline-containing cyclic dipeptides from Nocardiopsis HT88, an endophytic bacterium of Mallotus nudiflorus L. Nat. Prod. Res. 2020, 34, 2219–2224.
- Storr, S. J.; Carragher, N. O.; Frame, M. C.; Parr, T.; Martin, S. G., The calpain system and cancer. Rev. Cancer 2011, 11, 364–374.
- Joshi, S.; Singh, A. R.; Zulcic, M.; Bao, L.; Messer, K.; Ideker, T.; Dutkowski, J.; Durden, D. L., Rac2 controls tumor growth, metastasis and M1-M2 macrophage differentiation in vivo. PLoS One 2014, 9, e95893.
- Wertheimer, E.; Gutierrez-Uzquiza, A.; Rosemblit, C.; Lopez-Haber, C.; Sosa, M. S.; Kazanietz, M. G., Rac signaling in breast cancer: a tale of GEFs and GAPs. Signalling 2012, 24, 353–362.
- Walsh, C. T.; O'Brien, R. V.; Khosla, C., Nonproteinogenic amino acid building blocks for nonribosomal peptide and hybrid polyketide scaffolds. Chem. Int. Ed. 2013, 52, 7098–7124.
- Arnison, P. G.; Bibb, M. J.; Bierbaum, G.; Bowers, A. A.; Bugni, T. S.; Bulaj, G.; Camarero, J. A.; Campopiano, D. J.; Challis, G. L.; Clardy, J., Ribosomally synthesized and post-translationally modified peptide natural products: Overview and recommendations for a universal nomenclature. Prod. Rep. 2013, 30, 108–160.
- Walsh, C. T.; Tang, Y., Natural product biosynthesis. Soc. Chem.: 2017.
- Mishra, A. K.; Choi, J.; Choi, S.-J.; Baek, K.-H., Cyclodipeptides: An overview of their biosynthesis and biological activity. Molecules 2017, 22, 1796.
- Giessen, T. W.; Marahiel, M. A., The tRNA-dependent biosynthesis of modified cyclic dipeptides. J. Mol. Sci. 2014, 15, 14610–14631.
- Wang, D.; Wang, Y.; Ouyang, Y.; Fu, P.; Zhu, W., Cytotoxic p-terphenyls from a marine-derived Nocardiopsis J. Nat. Prod. 2019, 82, 3504–3508.
- Chang, Y.; Che, Q.; Xing, L.; Ma, C.; Han, Y.; Zhu, T.; Pfeifer, B. A.; Peng, J.; Zhang, G.; Li, D., Antibacterial p-terphenyl with a rare 2,2′-bithiazole substructure and related compounds isolated from the marine-derived actinomycete Nocardiopsis HDN154086. J. Nat. Prod. 2021, 84, (4), 1226–1231.
- Eliwa, E. M.; Abdel-Razek, A. S.; Frese, M.; Halawa, A. H.; El-Agrody, A. M.; Bedair, A. H.; Sewald, N.; Shaaban, M., New naturally occurring phenolic derivatives from marine Nocardiopsis AS23C: Structural elucidation and in silico computational studies. Vietnam J. Chem. 2019, 57, 164–174.
- Siddharth, S.; Rai V, R., Isolation and characterization of bioactive compounds with antibacterial, antioxidant and enzyme inhibitory activities from marine-derived rare actinobacteria, Nocardiopsis SCA21. Microb. Pathog. 2019, 137, 103775.
- Sunish, K. S.; Sreedharan, P.; Daniel, S.; Biji, M.; Rosamma, P.; Sukumaran, V.; Mohandas, A.; Singh, I. S. B., A novel substituted derivative of sterol from marine actinomycetes Nocardiopsis alba MCCB 110 antagonistic to the aquaculture pathogen Vibrio harveyi. Pathog. 2021, 157, 104967.
References
- Jose, P.A.; Maharshi, A.; Jha, B. Actinobacteria in natural products research: Progress and prospects. Microbiol. Res. 2021, 246, 126708.
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Clement, C.; Meier-Kolthoff, J.P.; Klenk, H.-P.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43.
- Demain, A.L.; Sanchez, S. Microbial drug discovery: 80 years of progress. J. Antibiot. 2009, 62, 5–16.
- Mahajan, G.B.; Balachandran, L. Antibacterial agents from actinomycetes—A review. Front. Biosci. Elite Ed. 2012, E4, 240–253.
- Ibrahim, A.H.; Desoukey, S.Y.; Fouad, M.A.; Abdelmohsen, U.R.; Kamel, M.S.; Gulder, T.A.M. Natural product potential of the genus Nocardiopsis. Mar. Drugs 2018, 16, 147.
- Li, J.W.H.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165.
- Manivasagan, P.; Kang, K.-H.; Sivakumar, K.; Li-Chan, E.C.Y.; Oh, H.-M.; Kim, S.-K. Marine actinobacteria: An important source of bioactive natural products. Environ. Toxicol. Pharmacol. 2014, 38, 172–188.
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.-K. Pharmaceutically active secondary metabolites of marine actinobacteria. Microbiol. Res. 2014, 169, 262–278.
- AbdElgawad, H.; Zinta, G.; Abuelsoud, W.; Hassan, Y.M.; Alkhalifah, D.H.M.; Hozzein, W.N.; Zrieq, R.; Beemster, G.T.; Schoenaers, S. An actinomycete strain of Nocardiopsis lucentensis reduces arsenic toxicity in barley and maize. J. Hazard. Mater. 2021, 417, 126055.
- Adenan, N.H.; Lim, Y.Y.; Ting, A.S.Y. Nocardiopsis sp. for the removal of triphenylmethane dyes: Decolorization and optimization studies. Water Air Soil Pollut. 2021, 232, 414.
- Patel, G.B.; Rakholiya, P.; Shindhal, T.; Varjani, S.; Tabhani, N.; Shah, K.R. Lipolytic Nocardiopsis for reduction of pollution load in textile industry effluent and SWISS model for structural study of lipase. Bioresour. Technol. 2021, 341, 125673.
- Bennur, T.; Ravi Kumar, A.; Zinjarde, S.S.; Javdekar, V. Nocardiopsis species: A potential source of bioactive compounds. J. Appl. Microbiol. 2016, 120, 1–16.
- Chen, J.; Xu, L.; Zhou, Y.; Han, B. Natural products from actinomycetes associated with marine organisms. Mar. Drugs 2021, 19, 629.
- Kumar, S.; Solanki, D.S.; Parihar, K.; Tak, A.; Gehlot, P.; Pathak, R.; Singh, S.K. Actinomycetes isolates of arid zone of Indian Thar Desert and efficacy of their bioactive compounds against human pathogenic bacteria. Biol. Futur. 2021, 72, 431–440.
- Pinto-Almeida, A.; Bauermeister, A.; Luppino, L.; Grilo, I.R.; Oliveira, J.; Sousa, J.R.; Petras, D.; Rodrigues, C.F.; Prieto-Davo, A.; Tasdemir, D.; et al. The diversity, metabolomics profiling, and the pharmacological potential of actinomycetes isolated from the Estremadura Spur Pockmarks (Portugal). Mar. Drugs 2022, 20, 21.
- Chen, L.; Wang, Z.; Du, S.; Wang, G. Antimicrobial activity and functional genes of actinobacteria from coastal wetland. Curr. Microbiol. 2021, 78, 3058–3067.
- Tatar, D. Isolation, phylogenetic analysis and antimicrobial activity of halophilic actinomycetes from different saline environments located near Corum province. Biologia 2021, 76, 773–780.
- Li, H.-W.; Zhi, X.-Y.; Yao, J.-C.; Zhou, Y.; Tang, S.-K.; Klenk, H.-P.; Zhao, J.; Li, W.-J. Comparative genomic analysis of the genus Nocardiopsis provides new insights into its genetic mechanisms of environmental adaptability. PLoS ONE 2013, 8, e61528.
- Bennur, T.; Kumar, A.R.; Zinjarde, S.; Javdekar, V. Nocardiopsis species as potential sources of diverse and novel extracellular enzymes. Appl. Microbiol. Biotechnol. 2014, 98, 9173–9185.
- Bennur, T.; Kumar, A.R.; Zinjarde, S.; Javdekar, V. Nocardiopsis species: Incidence, ecological roles and adaptations. Microbiol. Res. 2015, 174, 33–47.
- Shady, N.H.; Tawfike, A.F.; Yahia, R.; Fouad, M.A.; Brachmann, A.O.; Piel, J.; Abdelmohsen, U.R.; Kamel, M.S. Cytotoxic activity of actinomycetes Nocardia sp. and Nocardiopsis sp. associated with marine sponge Amphimedon sp. Nat. Prod. Res. 2021, 1–6.
- Siddharth, S.; Aswathanarayan, J.B.; Kuruburu, M.G.; Madhunapantula, S.R.V.; Vittal, R.R. Diketopiperazine derivative from marine actinomycetes Nocardiopsis sp. SCA30 with antimicrobial activity against MRSA. Arch. Microbiol. 2021, 203, 6173–6181.
- Trivedi, N.; Thumar, J. Chemical profiling of antimicrobial metabolites from halophilic actinomycete Nocardiopsis sp. Al-H10-1 (KF384482) isolated from Alang, Gulf of Khambhat, India. bioRxiv 2021.
- Goel, N.; Fatima, S.W.; Kumar, S.; Sinha, R.; Khare, S.K. Antimicrobial resistance in biofilms: Exploring marine actinobacteria as a potential source of antibiotics and biofilm inhibitors. Biotechnol. Rep. 2021, 30, e00613.
- Choi, G.; Kim, G.J.; Choi, H.; Choi, I.-W.; Lee, D.-S. Anti-inflammatory and anti-fibrotic activities of Nocardiopsis sp. 13G027 in lipopolysaccharides-induced RAW 264.7 macrophages and transforming growth factor beta-1-stimulated nasal polyp-derived fibroblasts. Microbiol. Biotechnol. Lett. 2021, 49, 543–551.
- Sarmiento-Vizcaíno, A.; Martín, J.; Reyes, F.; García, L.A.; Blanco, G. Bioactive natural products in actinobacteria isolated in rainwater from storm clouds transported by western winds in Spain. Front. Microbiol. 2021, 12, 773095.
- Gamaleldin, N.M.; Bakeer, W.; El-Gendy, A.O.; Sayed, A.M.; Shamikh, Y.I.; Hassan, H.M.; Horn, H.; Abdelmohsen, U.R.; Hozzein, W.N. Exploration of chemical diversity and antitrypanosomal activity of some Red Sea-derived actinomycetes using the OSMAC approach supported by LC-MS-based metabolomics and molecular modelling. Antibiotics 2020, 9, 629.
- Widada, J.; Damayanti, E.; Alhakim, M.R.; Yuwono, T.; Mustofa, M. Two strains of airborne Nocardiopsis alba producing different volatile organic compounds (VOCs) as biofungicide for Ganoderma boninense. FEMS Microbiol. Lett. 2021, 368, fnab138.
- Wang, Z.; Fu, P.; Liu, P.; Wang, P.; Hou, J.; Li, W.; Zhu, W. New pyran-2-ones from alkalophilic actinomycete, Nocardiopsis alkaliphila sp. nov. YIM-80379. Chem. Biodivers. 2013, 10, 281–287.
- Lu, C.; Li, Y.; Wang, H.; Wang, B.; Shen, Y. A new phenoxazine derivative isolated from marine sediment actinomycetes, Nocardiopsis sp. 236. Drug Discov. Ther. 2013, 7, 101–104.
- Tian, S.; Yang, Y.; Liu, K.; Xiong, Z.; Xu, L.; Zhao, L. Antimicrobial metabolites from a novel halophilic actinomycete Nocardiopsis terrae YIM 90022. Nat. Prod. Res. 2014, 28, 344–346.
- Yamashita, T.; Imoto, M.; Isshiki, K.; Sawa, T.; Naganawa, H.; Kurasawa, S.; Zhu, B.; Umezawa, K. Isolation of a new indole alkaloid, pendolmycin, from Nocardiopsis. J. Nat. Prod. 1988, 51, 1184–1187.
- Lin, Z.; Torres, J.P.; Ammon, M.A.; Marett, L.; Teichert, R.W.; Reilly, C.A.; Kwan, J.C.; Hughen, R.W.; Flores, M.; Tianero, M.D.; et al. A Bacterial source for mollusk pyrone polyketides. Chem. Biol. 2013, 20, 73–81.
- Gao, X.; Lu, Y.; Xing, Y.; Ma, Y.; Lu, J.; Bao, W.; Wang, Y.; Xi, T. A novel anticancer and antifungus phenazine derivative from a marine actinomycete BM-17. Microbiol. Res. 2012, 167, 616–622.
- Kase, H.; Iwahashi, K.; Matsuda, Y. K-252a, a potent inhibitor of protein kinase C from microbial origin. J. Antibiot. 1986, 39, 1059–1065.
- Raju, R.; Piggott, A.M.; Huang, X.-C.; Capon, R.J. Nocardioazines: A novel bridged diketopiperazine scaffold from a marine-derived bacterium inhibits P-glycoprotein. Org. Lett. 2011, 13, 2770–2773.
- Kim, M.C.; Kwon, O.-W.; Park, J.-S.; Kim, S.Y.; Kwon, H.C. Nocapyrones H–J, 3,6-disubstituted α-pyrones from the marine actinomycete Nocardiopsis sp. KMF-001. Chem. Pharm. Bull. 2013, 61, 511–515.
- Wu, Z.; Xie, L.; Xia, G.; Zhang, J.; Nie, Y.; Hu, J.; Wang, S.; Zhang, R. A new tetrodotoxin-producing actinomycete, Nocardiopsis dassonvillei, isolated from the ovaries of puffer fish Fugu rubripes. Toxicon 2005, 45, 851–859.
- Peltola, J.S.P.; Andersson, M.A.; Kampfer, P.; Auling, G.; Kroppenstedt, R.M.; Busse, H.-J.; Salkinoja-Salonen, M.S.; Rainey, F.A. Isolation of toxigenic Nocardiopsis strains from indoor environments and description of two new Nocardiopsis species, N. exhalans sp. nov. and N. umidischolae sp. nov. Appl. Environ. Microbiol. 2001, 67, 4293–4304.
- Kim, Y.; Ogura, H.; Akasaka, K.; Oikawa, T.; Matsuura, N.; Imada, C.; Yasuda, H.; Igarashi, Y. Nocapyrones: α- and γ-pyrones from a marine-derived Nocardiopsis sp. Mar. Drugs 2014, 12, 4110–4125.
- Sun, M.-W.; Guo, Z.-X.; Lu, C.-H. Two new polyketides from Nocardiopsis lucentensis DSM 44048. Nat. Prod. Res. 2016, 30, 1036–1041.
- Zhang, H.; Saurav, K.; Yu, Z.; Mandi, A.; Kurtan, T.; Li, J.; Tian, X.; Zhang, Q.; Zhang, W.; Zhang, C. α-Pyrones with diverse hydroxy substitutions from three marine-derived Nocardiopsis strains. J. Nat. Prod. 2016, 79, 1610–1618.
- Kim, J.; Shin, D.; Kim, S.-H.; Park, W.; Shin, Y.; Kim, W.K.; Lee, S.K.; Oh, K.-B.; Shin, J.; Oh, D.-C. Borrelidins C.–E: New antibacterial macrolides from a saltern-derived halophilic Nocardiopsis sp. Mar. Drugs 2017, 15, 166.
- Zhang, X.; He, H.; Ma, R.; Ji, Z.; Wei, Q.; Dai, H.; Zhang, L.; Song, F. Madurastatin B3, a rare aziridine derivative from actinomycete Nocardiopsis sp. LS150010 with potent anti-tuberculosis activity. J. Ind. Microbiol. Biotechnol. 2017, 44, 589–594.
- Eliwa, E.M.; Abdel-Razek, A.S.; Frese, M.; Wibberg, D.; Halawa, A.H.; El-Agrody, A.M.; Bedair, A.H.; Kalinowski, J.; Sewald, N.; Shaaban, M. New bioactive compounds from the marine-derived actinomycete Nocardiopsis lucentensis sp. ASMR2. Z. Naturforsch. B J. Chem. Sci. 2017, 72, 351–360.
- Sun, M.-W.; Zhang, X.-M.; Bi, H.-L.; Li, W.-J.; Lu, C.-H. Two new sesquiterpenoids produced by halophilic Nocardiopsis chromatogenes YIM 90109. Nat. Prod. Res. 2017, 31, 77–83.
- Hamed, A.; Abdel-Razek, A.S.; Frese, M.; Stammler, H.G.; El-Haddad, A.F.; Ibrahim, T.M.A.; Sewald, N.; Shaaban, M. Terretonin N: A new meroterpenoid from Nocardiopsis sp. Molecules 2018, 23, 299.
- Xu, D.; Nepal, K.K.; Harmody, D.; McCarthy, P.J.; Wright, A.E.; Wang, G.; Chen, J.; Zhu, H. Nocardiopsistins A–C: New angucyclines with anti-MRSA activity isolated from a marine sponge-derived Nocardiopsis sp. HB-J378. Synth. Syst. Biotechnol. 2018, 3, 246–251.
- Lombo, F.; Brana, A.F.; Salas, J.A.; Mendez, C. Genetic organization of the biosynthetic gene cluster for the antitumor angucycline oviedomycin in Streptomyces antibioticus ATCC 11891. ChemBioChem 2004, 5, 1181–1187.
- Kharel, M.K.; Pahari, P.; Shepherd, M.D.; Tibrewal, N.; Nybo, S.E.; Shaaban, K.A.; Rohr, J. Angucyclines: Biosynthesis, mode-of-action, new natural products, and synthesis. Nat. Prod. Rep. 2012, 29, 264–325.
- Messaoudi, O.; Sudarman, E.; Bendahou, M.; Jansen, R.; Stadler, M.; Wink, J. Kenalactams A–E, polyene macrolactams isolated from Nocardiopsis CG3. J. Nat. Prod. 2019, 82, 1081–1088.
- Zhao, T.; Chang, Y.; Zhu, T.; Li, J.; Gu, Q.; Li, D.; Che, Q.; Zhang, G. α-Pyrone derivatives with cyto-protective activity from two Takla Makan desert soil derived actinomycete Nocardiopsis strains recovered in seawater based medium. Nat. Prod. Res. 2019, 33, 2498–2506.
- Bolling, B.W.; Parkin, K.L. Limited contribution of isoflavones to hepatocellular phase II enzyme-inducing activity of soybean (Glycine max) extracts. Food Chem. 2009, 113, 1069–1075.
- Park, J.S.; Jung, J.S.; Jeong, Y.H.; Hyun, J.W.; Le, T.K.V.; Kim, D.H.; Choi, E.C.; Kim, H.S. Antioxidant mechanism of isoflavone metabolites in hydrogen peroxide-stimulated rat primary astrocytes: Critical role of hemeoxygenase-1 and NQO1 expression. J. Neurochem. 2011, 119, 909–919.
- Wang, J.-X.; Sun, C.-X.; Shah, M.; Zhang, G.-J.; Gu, Q.-Q.; Zhu, T.-J.; Che, Q.; Li, D.-H. New metabolites from a Mariana Trench-derived actinomycete Nocardiopsis sp. HDN 17-237. J. Asian Nat. Prod. Res. 2020, 22, 1031–1036.
- Zhou, L.; Chen, X.; Sun, C.; Chang, Y.; Huang, X.; Zhu, T.; Zhang, G.; Che, Q.; Li, D. Saliniquinone derivatives, saliniquinones G−I and heraclemycin E, from the marine animal-derived Nocardiopsis aegyptia HDN19-252. Mar. Drugs 2021, 19, 575.
- Shin, J.; Yang, S.-H.; Du, Y.E.; Park, K.; Kim, D.; Shin, D.; Kim, J.; Kim, S.-H.; Kim, Y.K.; Shin, J. Borrelidin from saltern-derived halophilic Nocardiopsis sp. dissociates amyloid-β and tau fibrils. J. Alzheimer’s Dis. Rep. 2021, 5, 7–13.
- Castro-Falcon, G.; Millan-Aguinaga, N.; Roullier, C.; Jensen, P.R.; Hughes, C.C. Nitrosopyridine probe to detect polyketide natural products with conjugated alkenes: Discovery of novodaryamide and nocarditriene. ACS Chem. Biol. 2018, 13, 3097–3106.
- Kim, T.; Lee, S.-A.; Noh, T.; Choi, P.; Choi, S.-J.; Song, B.G.; Kim, Y.; Park, Y.-T.; Huh, G.; Kim, Y.-J.; et al. Synthesis, structure revision, and cytotoxicity of nocarbenzoxazole G. J. Nat. Prod. 2019, 82, 1325–1330.
- Sun, M.; Zhang, X.; Hao, H.; Li, W.; Lu, C. Nocarbenzoxazoles A–G, benzoxazoles produced by halophilic Nocardiopsis lucentensis DSM 44048. J. Nat. Prod. 2015, 78, 2123–2127.
- Yang, C.L.; Zhang, B.; Xue, W.W.; Li, W.; Xu, Z.F.; Shi, J.; Shen, Y.; Jiao, R.H.; Tan, R.X.; Ge, H.M. Discovery, biosynthesis, and heterologous production of loonamycin, a potent anticancer indolocarbazole alkaloid. Org. Lett. 2020, 22, 4665–4669.
- Zhang, X.; Chen, S.; Zhang, L.; Zhang, Q.; Zhang, W.; Chen, Y.; Zhang, W.; Zhang, H.; Zhang, C. Dassonmycins A and B, polycyclic thioalkaloids from a marine sponge-derived Nocardiopsis dassonvillei SCSIO 40065. Org. Lett. 2021, 23, 2858–2862.
- Miao, L.; Qian, S.; Qi, S.; Jiang, W.; Dong, K. Culture medium optimization and active compounds investigation of an anti-quorum sensing marine actinobacterium Nocardiopsis dassonvillei JS106. Microbiology 2021, 90, 112–123.