The continuous research advances in the microbiome field is changing clinicians’ points of view about the involvement of the microbiome in human health and disease, including autoimmune diseases such as alopecia areata (AA). Both gut and cutaneous dysbiosis have been considered to play roles in alopecia areata. A new approach is currently possible owing also to the use of omic techniques for studying the role of the microbiome in the disease by the deep understanding of microorganisms involved in the dysbiosis as well as of the pathways involved. These findings suggest the possibility to adopt a topical approach using either cosmetics or medical devices, to modulate or control, for example, the growth of overexpressed species using specific bacteriocins or postbiotics or with pH control. This will favour at the same time the growth of beneficial bacteria which, in turn, can impact positively both the structure of the scalp ecosystem on the host’s response to internal and external offenders.
- alopecia areata
- microbiota
- omics
- postbiotics
1. Alopecia Areata and Microbiota
2. Studying the Microbiota by Omics Techniques
The study of the interaction between the host and the microbiota in terms of the specific genes, metabolites, and proteins they produced is presently feasible owing to the advent of “omics” techniques that allow delineating novel roles for microbes in health [56][42]. For many decades, the knowledge regarding microbiota was limited to culture-based techniques. Despite their limitations, however, they were fundamental for microbiota characterisation in the past and are still used today as the starting point for microbiome studies. The main limitations are that they are labour-intensive and not high-throughput, and they are unable to detect the virome and archaea. “Omics” techniques fall under the great hat of systems biology techniques; they include metataxonomic, metagenomic, metabolomic, metabonomic, transcriptomic, and proteomic approaches and allow the comprehension of the microbiota inhabiting a given ecosystem, not only in terms of populations but also in terms of its functionality. The metataxonomic approach is a high-throughput technology used to characterise the entire microbiota and create a metataxonomic tree, aiming to describe the relationships between all sequences obtained [57][43] (Figure 1a).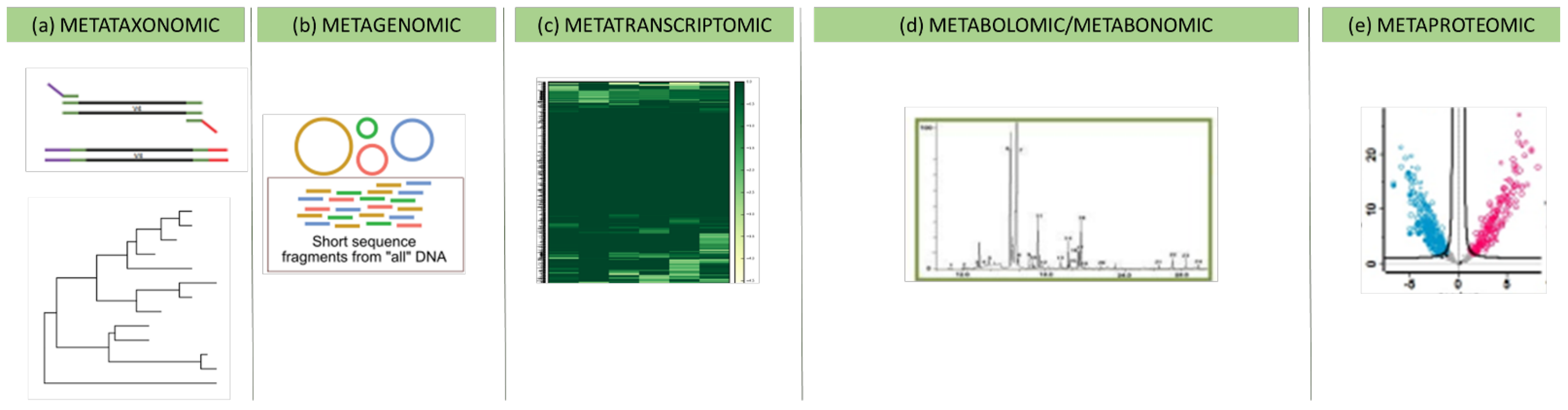
3. Modulation of Microbiota in Alopecia Areata
A link between AA and gut microbiome could be hypothesised following recent evidence on the involvement of short-chain fatty acids, mainly butyric acid, derived from microbial metabolism in the gut and the differentiation of peripheral Treg lymphocytes [90][62]. These last are the important immunological mediators of AA [91][63]. One of the main drivers of the gut microbiome is diet [97][64], and much research focused on establishing the role of diet in shaping the microbiome; this was also reported as regards the skin microbiome [98,99][65][66]. Indeed, diets have been reported to affect skin physiology and microbiome. Therefore, the existence of a gut–skin microbiome axis has been well-established for many dermatological diseases including atopic dermatitis [100][67]. The role of diet in AA can also be hypothesised [101,102][68][69]. Firstly, an unbalanced diet can lead to a lower intake of some macronutrients and micronutrients, and this can have an impact on gut microbial composition and functionality as well as the microbiome inhabiting the scalp up to the perifollicular region [101][68]. Hair is a fast-growing element, which needs a balanced supply of nutrients to grow correctly [9,102][9][69]. Under this assumption, targeted dietary approaches could represent a further therapeutic option or adjuvant therapy for AA subjects. Even though there is presently no scientific basis for the hypothesis that, for example, the syndrome of “leaky gut” may be one of the etiological factors of AA, the latter shares some genetic characteristics with other autoimmune diseases (rheumatoid arthritis, diabetes I, celiac disease, systemic lupus erythematosus (SLE), psoriasis, multiple sclerosis, etc.) in which the association of the disease with an altered intestinal permeability has been demonstrated [103][70]. Suggested therapies include, among others, diet, additional nutritional supplementation with probiotics or botanical extract, a gluten-free or low-FODMAP diet, a low-sugar diet, or an antifungal diet. Additionally, restoring the unbalanced gut microbiota with a healthy one via FMT could represent useful therapeutic options, as reported above [101][68]. The rationale behind the usefulness of “rebalancing” the gut microbiome is linked to the improvement of the absorption and synthesis of nutrients (amino acid/proteins, biotin, SCFAs, and vitamin D), which are also essential for hair follicle tropism and immunomodulation, and this, ultimately, results in hair regrowth [104][71]. However, current legislative limitations and the scarcity of clinical trials pose the need for larger studies before implementing FMT in the panel of the treatments currently available and approved for AA. Indeed, gut dysbiosis should not be considered a localised phenomenon. Alteration in the gut microecology as in the microbiome functionality may have consequences on the general inflammatory and immunological state of the host up to involving the scalp ecosystem and physiology [98][65]. The evidence of the modification of fundamental pathways of the immune and inflammatory responses and pathways involved in the transport of micronutrients, such as vitamin D (VitD) on the scalp of subjects affected by AA, could be the first stage for an evaluation of etiological agents important in the knowledge of AA. Various autoimmune diseases have been associated with a deficiency in VitD [105][72]. Indeed, VitD is strictly linked with skin immunity since it can regulate lymphocyte functionality, dendritic cell maturation, and cytokine secretion [105][72]. In particular, it suppresses T-helper 1 and T-helper 17 cell formation, and this leads to a decrease in inflammatory cytokines [105][72]. A deficiency of this vitamin has also been reported in AA [106][73]. Therefore, topical calcipotriol has been successfully used for treating AA [107,108,109][74][75][76]. Using a meta-analysis, Lee et al. [110][77] reported a higher prevalence of VitD deficiency in AA subjects than in the control group. Most interestingly, according to several lines of evidence, the decrease in serum VitD levels significantly and inversely correlates with AA severity [111[78][79],112], also in children [113][80]. The production of IFN-γ by human peripheral blood mononuclear cells (PBMCs) and CD4+ T cells was significantly decreased by Vit D [114,115,116][81][82][83]. This suggested that VitD might probably counteract the IP collapse in AA by modulating the production of IFN-γ [106][73]. Therefore, the evidence of VitD deficiency in the AA could be a consequence of the decreased expression of some bacterial-related pathways. Indeed, human studies have reported significant associations between vitamin D and microbiome composition [117][84] Therefore, as stated by Thompson et al. [76][85], a deficiency in micronutrients might also contribute to AA through dysregulation of immune cell function, DNA synthesis, and oxidative stress induction. Indeed, the use of topically applied probiotics may be a natural, targeted treatment approach to several skin disorders in which a dysbiosis of the microbiome could be hypothesised, including AA. There is a growing amount of research reporting evidence of the health-promoting effect of probiotics on skin health [119][86]. Probiotics act primarily by increasing levels of beneficial bacteria or, indirectly, by influencing the immune system which, in turn, influences the host microbiome. However, the use of live bacteria on skin poses several challenges. For this reason, they are usually used in topical formulation in the form of non-viable microorganisms with the same probiotic activity and health benefits as viable microorganisms but safer than live probiotics [120][87]: They are the so-called “paraprobiotics” [120][87]. A new open perspective in the field is that represented by “postbiotics”. The term refers to molecules released by beneficial bacteria that are responsible for the beneficial effects of probiotics themselves [120,121][87][88]. They include peptides, enzymes, short-chain fatty acids (SCFAs), antimicrobial peptides (AMPs), polysaccharides, cell-surface proteins, vitamins, plasmalogens, and organic acids [121][88]. The mechanisms implicated in their health benefits are not fully elucidated, but a recent study reported different functional properties (e.g., antimicrobial, antioxidant, and immunomodulatory) [121][88].References
- Odom, R.B.; Davidsohn, I.J.; William, D.; Henry, J.B.; Berger, T.G. Clinical diagnosis by laboratory methods. In Andrews’ Diseases of the Skin: Clinical Dermatology; Elston, D.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2006.
- Dawber, R. Alopecia areata. Monogr. Dermatol. 1989, 2, 89–102.
- Syed, S.A.; Sandeep, S. Alopecia areata: A review. J. Saudi Soc. Dermatol. Dermatol. Surg. 2013, 17, 37–45.
- Borde, A.; Åstrand, A. Alopecia areata and the gut-the link opens up for novel therapeutic interventions. Expert Opin. Ther. Targets 2018, 22, 503–511.
- Brenner, W.; Diem, E.; Gschnait, F. Coincidence of vitiligo, alopecia areata, onychodystrophy, localized scleroderma and lichen planus. Dermatologica 1979, 159, 356–360.
- Hordinsky, M.; Ericson, M. Autoimmunity: Alopecia areata. J. Investig. Dermatol. Symp. Proc. 2004, 9, 73–78.
- Billingham, R.E.; Silvers, W.K. A biologist’s reflections on dermatology. J. Investig. Dermatol. 1971, 57, 227–240.
- Kang, H.; Wu, W.Y.; Lo, B.K.; Yu, M.; Leung, G.; Shapiro, J.; McElwee, K.J. Hair follicles from alopecia areata patients exhibit alterations in immune privilege-associated gene expression in advance of hair loss. J. Investig. Dermatol. 2010, 130, 2677–2680.
- Leung, M.C.; Sutton, C.W.; Fenton, D.A.; Tobin, D.J. Trichohyalin is a potential major autoantigen in human alopecia areata. J. Proteome Res. 2010, 9, 5153–5163.
- Wang, E.H.C.; Yu, M.; Breitkopf, T.; Akhoundsadegh, N.; Wang, X.; Shi, F.T.; Leung, G.; Dutz, J.P.; Shapiro, J.; McElwee, K.J. Identification of Autoantigen Epitopes in Alopecia Areata. J. Investig. Dermatol. 2016, 136, 1617–1626.
- Bertolini, M.; McElwee, K.; Gilhar, A.; Bulfone-Paus, S.; Paus, R. Hair follicle immune privilege and its collapse in alopecia areata. Exp. Dermatol. 2020, 29, 703–725.
- Elsner, L.; Flügge, P.F.; Lozano, J.; Muppala, V.; Eiz-Vesper, B.; Demiroglu, S.Y.; Malzahn, D.; Herrmann, T.; Brunner, E.; Bickeböller, H.; et al. The endogenous danger signals HSP70 and MICA cooperate in the activation of cytotoxic effector functions of NK cells. J. Cell. Mol. Med. 2010, 14, 992–1002.
- Ito, T.; Ito, N.; Saatoff, M.; Hashizume, H.; Fukamizu, H.; Nickoloff, B.J.; Takigawa, M.; Paus, R. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J. Investig. Dermatol. 2008, 128, 1196–1206.
- Ito, T.; Meyer, K.C.; Ito, N.; Paus, R. Immune privilege and the skin. Curr. Dir. Autoimmun. 2008, 10, 27–52.
- Petukhova, L.; Duvic, M.; Hordinsky, M.; Norris, D.; Price, V.; Shimomura, Y.; Kim, H.; Singh, P.; Lee, A.; Chen, W.V.; et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature 2010, 466, 113–117.
- Xing, L.; Dai, Z.; Jabbari, A.; Cerise, J.E.; Higgins, C.; Gong, W.; de Jong, A.; Harel, S.; DeStefano, G.M.; Rothman, L.; et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat. Med. 2014, 20, 1043–1049.
- Scharschmidt, T.C.; Vasquez, K.S.; Pauli, M.L.; Leitner, E.G.; Chu, K.; Truong, H.A.; Lowe, M.M.; Rodriguez, R.S.; Ali, N.; Laszik, Z.G.; et al. Commensal Microbes and Hair Follicle Morphogenesis Coordinately Drive Treg Migration into Neonatal Skin. Cell Host Microbe 2017, 21, 467–477.e5.
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155.
- Jaspers, C.; Fraune, S.; Arnold, E.A.; Miller, D.J.; Bosch, T.C.G.; Voolstra, C.R. Resolving structure and function of metaorganisms through a holistic framework combining reductionist and integrative approaches. Zoology 2019, 133, 81–87.
- Migacz-Gruszka, K.; Branicki, W.; Obtulowicz, A.; Pirowska, M.; Gruszka, K.; Wojas-Pelc, A. What’s New in the Pathophysiology of Alopecia Areata? The Possible Contribution of Skin and Gut Microbiome in the Pathogenesis of Alopecia—Big Opportunities, Big Challenges, and Novel Perspectives. Int. J. Trichology 2020, 11, 185–188.
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141.
- Malys, M.K.; Campbell, L.; Malys, N. Symbiotic and antibiotic interactions between gut commensal microbiota and host immune system. Medicina 2015, 51, 69–75.
- Belkaid, Y.; Harrison, O.J. Homeostatic Immunity and the Microbiota. Immunity 2017, 46, 562–576.
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14.
- Gallo, R.L.; Nakatsuji, T. Microbial symbiosis with the innate immune defense system of the skin. J. Investig. Dermatol. 2011, 131, 1974–1980.
- Naik, S.; Bouladoux, N.; Wilhelm, C.; Molloy, M.J.; Salcedo, R.; Kastenmuller, W.; Deming, C.; Quinones, M.; Koo, L.; Conlan, S.; et al. Compartmentalized control of skin immunity by resident commensals. Science 2012, 337, 1115–1119.
- Lee, Y.B.; Byun, E.J.; Kim, H.S. Potential Role of the Microbiome in Acne: A Comprehensive Review. J. Clin. Med. 2019, 8, 987.
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; FitzGerald, M.G.; Fulton, R.S.; et al. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214.
- Cogen, A.L.; Nizet, V.; Gallo, R.L. Skin microbiota: A source of disease or defence? Br. J. Dermatol. 2009, 158, 442–455.
- Zeeuwen, P.L.; Kleerebezem, M.; Timmerman, H.M.; Schalkwijk, J. Microbiome and skin diseases. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 514–520.
- Edslev, S.M.; Agner, T.; Andersen, P.S. Skin Microbiome in Atopic Dermatitis. Acta Derm. Venereol. 2020, 100, adv00164.
- Thio, H.B. The Microbiome in Psoriasis and Psoriatic Arthritis: The Skin Perspective. J. Rheumatol. Suppl. 2018, 94, 30–31.
- Rocha, M.A.; Bagatin, E. Skin barrier and microbiome in acne. Arch. Dermatol. Res. 2018, 310, 181–185.
- Dréno, B.; Dagnelie, M.A.; Khammari, A.; Corvec, S. The Skin Microbiome: A New Actor in Inflammatory Acne. Am. J. Clin. Dermatol. 2020, 21 (Suppl. 1), 18–24.
- Fitz-Gibbon, S.; Tomida, S.; Chiu, B.H.; Nguyen, L.; Du, C.; Liu, M.; Elashoff, D.; Erfe, M.C.; Loncaric, A.; Kim, J.; et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J. Investig. Dermatol. 2013, 133, 2152–2160.
- Dreno, B.; Martin, R.; Moyal, D.; Henley, J.B.; Khammari, A.; Seité, S. Skin microbiome and acne vulgaris: Staphylococcus, a new actor in acne. Exp. Dermatol. 2017, 26, 798–803.
- Claudel, J.P.; Auffret, N.; Leccia, M.T.; Poli, F.; Corvec, S.; Dréno, B. Staphylococcus epidermidis: A Potential New Player in the Physiopathology of Acne? Dermatology 2019, 235, 287–294.
- Tajran, J.; Gosman, A.A. Anatomy, Head and Neck, Scalp. . In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551565/ (accessed on 31 March 2022).
- Rinaldi, F.; Pinto, D.; Marzani, B.; Rucco, M.; Sorbellini, E.; Giuliani, G. Human microbiome: What’s new in scalp diseases. J. Transl. Sci. 2018, 4, 1–4.
- Pinto, D.; Sorbellini, E.; Marzani, B.; Rucco, M.; Giuliani, G.; Rinaldi, F. Scalp bacterial shift in Alopecia areata. PLoS ONE 2019, 14, e0215206.
- Pinto, D.; Calabrese, F.M.; De Angelis, M.; Celano, G.; Giuliani, G.; Gobbetti, M.; Rinaldi, F. Predictive Metagenomic Profiling, Urine Metabolomics, and Human Marker Gene Expression as an Integrated Approach to Study Alopecia Areata. Front. Cell. Infect. Microbiol. 2020, 10, 146.
- Sharon, G.; Garg, N.; Debelius, J.; Knight, R.; Dorrestein, P.C.; Mazmanian, S.K. Specialized metabolites from the microbiome in health and disease. Cell Metab. 2014, 20, 719–730.
- Marchesi, J.R.; Ravel, J. The vocabulary of microbiome research: A proposal. Microbiome 2015, 3, 31.
- Handelsman, J.; Rondon, M.R.; Brady, S.F.; Clardy, J.; Goodman, R.M. Molecular biological access to the chemistry of unknown soil microbes: A new frontier for natural products. Chem. Biol. 1998, 5, R245–R249.
- Oliver, S.G.; Winson, M.K.; Kell, D.B.; Baganz, F. Systematic functional analysis of the yeast genome. Trends Biotechnol. 1998, 16, 373–378.
- Nicholson, J.K. Global systems biology, personalized medicine and molecular epidemiology. Mol. Syst. Biol. 2006, 2, 52.
- Rodriguez-Valera, F. Environmental genomics, the big picture? FEMS Microbiol. Lett. 2004, 231, 153–158.
- Kitano, H. Systems biology: A brief overview. Science 2002, 295, 1662–1664.
- Liu, S.; Si, C.; Yu, Y.; Zhao, G.; Chen, L.; Zhao, Y.; Zhang, Z.; Li, H.; Chen, Y.; Min, L.; et al. Multi-omics Analysis of Gut Microbiota and Metabolites in Rats with Irritable Bowel Syndrome. Front. Cell. Infect. Microbiol. 2019, 9, 178.
- Malla, M.A.; Dubey, A.; Kumar, A.; Yadav, S.; Hashem, A.; Abd Allah, E.F. Exploring the Human Microbiome: The Potential Future Role of Next-Generation Sequencing in Disease Diagnosis and Treatment. Front. Immunol. 2019, 9, 2868.
- Mullish, B.H.; Osborne, L.S.; Marchesi, J.R.; McDonald, J.A. The implementation of omics technologies in cancer microbiome research. Ecancermedicalscience 2018, 12, 864.
- Blumenberg, M. SKINOMICS: Transcriptional Profiling in Dermatology and Skin Biology. Curr. Genom. 2012, 13, 363–368.
- Oliva, M.; Renert-Yuval, Y.; Guttman-Yassky, E. The ‘omics’ revolution: Redefining the understanding and treatment of allergic skin diseases. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 469–476.
- He, J.; Jia, Y. Application of omics technologies in dermatological research and skin management. J. Cosmet. Dermatol. 2022, 21, 451–460.
- Jansma, J.; El Aidy, S. Understanding the host-microbe interactions using metabolic modeling. Microbiome 2021, 9, 16.
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270.
- Oh, J.; Byrd, A.L.; Park, M.; NISC Comparative Sequencing Program; Kong, H.H.; Segre, J.A. Temporal Stability of the Human Skin Microbiome. Cell 2016, 165, 854–866.
- Li, Z.; Xia, J.; Jiang, L.; Tan, Y.; An, Y.; Zhu, X.; Ruan, J.; Chen, Z.; Zhen, H.; Ma, Y.; et al. Characterization of the human skin resistome and identification of two microbiota cutotypes. Microbiome 2021, 9, 47.
- Barnard, E.; Shi, B.; Kang, D.; Craft, N.; Li, H. The balance of metagenomic elements shapes the skin microbiome in acne and health. Sci. Rep. 2016, 6, 39491.
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32 (Suppl. 1), D277–D280.
- Langille, M.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821.
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450.
- Han, Y.M.; Sheng, Y.Y.; Xu, F.; Qi, S.-S.; Liu, X.-J.; Hu, R.-M.; Miao, Y.; Huang, G.-Q.; Yang, Q.-P. Imbalance of T-helper 17 and regulatory T cells in patients with alopecia areata. J. Dermatol. 2015, 42, 981–988.
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73.
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353.
- Bowe, W.P.; Joshi, S.S.; Shalita, A.R. Diet and acne. J. Am. Acad. Dermatol. 2010, 63, 124–141.
- Lee, S.Y.; Lee, E.; Park, Y.M.; Hong, S.J. Microbiome in the Gut-Skin Axis in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 354–362.
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. Bioessays 2016, 38, 1167–1176.
- Rinaldi, F.; Pinto, D.; Giuliani, G.; Sorbellini, E. Diet and Microbiome Influence on Alopecia Areata: Experience from Case Reports. J. Nutr. Med. Diet Care 2019, 5, 037.
- Mu, Q.; Kirby, J.; Reilly, C.M.; Luo, X.M. Leaky Gut as a Danger Signal for Autoimmune Diseases. Front. Immunol. 2017, 8, 598.
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.J.; Zhang, W. Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients 2020, 12, 381.
- Samantam, S. Vitamin D and immunomodulation in the skin: A useful affirmative nexus. Explor. Immunol. 2021, 1, 90–111.
- Lin, X.; Meng, X.; Song, Z. Vitamin D and alopecia areata: Possible roles in pathogenesis and potential implications for therapy. Am. J. Transl. Res. 2019, 11, 5285–5300.
- Kim, D.H.; Lee, J.W.; Kim, I.S.; Choi, S.Y.; Lim, Y.Y.; Kim, H.M.; Kim, B.J.; Kim, M.N. Successful treatment of alopecia areata with topical calcipotriol. Ann. Dermatol. 2012, 24, 341–344.
- Çerman, A.A.; Solak, S.S.; Altunay, İ.; Küçükünal, N.A. Topical calcipotriol therapy for mild-to-moderate alopecia areata: A retrospective study. J. Drugs Dermatol. 2015, 14, 616–620.
- Narang, T.; Daroach, M.; Kumaran, M.S. Efficacy and safety of topical calcipotriol in management of alopecia areata: A pilot study. Dermatol. Ther. 2017, 30, e12464.
- Lee, S.; Kim, B.J.; Lee, C.H.; Lee, W.S. Increased prevalence of vitamin D deficiency in patients with alopecia areata: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1214–1221.
- Gade, V.K.V.; Mony, A.; Munisamy, M.; Chandrashekar, L.; Rajappa, M. An investigation of vitamin D status in alopecia areata. Clin. Exp. Med. 2018, 18, 577–584.
- Daroach, M.; Narang, T.; Saikia, U.N.; Sachdeva, N.; Sendhil Kumaran, M. Correlation of vitamin D and vitamin D receptor expression in patients with alopecia areata: A clinical paradigm. Int. J. Dermatol. 2018, 57, 217–222.
- Unal, M.; Gonulalan, G. Serum vitamin D level is related to disease severity in pediatric alopecia areata. J. Cosmet. Dermatol. 2018, 17, 101–104.
- Ragab, D.; Soliman, D.; Samaha, D.; Yassin, A. Vitamin D status and its modulatory effect on interferon gamma and interleukin-10 production by peripheral blood mononuclear cells in culture. Cytokine 2016, 85, 5–10.
- Fawaz, L.; Mrad, M.F.; Kazan, J.M.; Sayegh, S.; Akika, R.; Khoury, S.J. Comparative effect of 25(OH)D3 and 1,25(OH)2D3 on Th17 cell differentiation. Clin. Immunol. 2016, 166–167, 59–71.
- Sheikh, V.; Kasapoglu, P.; Zamani, A.; Basiri, Z.; Tahamoli-Roudsari, A.; Alahgholi-Hajibehzad, M. Vitamin D3 inhibits the proliferation of T helper cells, downregulate CD4(+) T cell cytokines and upregulate inhibitory markers. Hum. Immunol. 2018, 79, 439–445.
- Yamamoto, E.A.; Jørgensen, T.N. Relationships Between Vitamin D, Gut Microbiome, and Systemic Autoimmunity. Front Immunol. 2020, 10, 3141.
- Thompson, J.M.; Mirza, M.A.; Park, M.K.; Qureshi, A.A.; Cho, E. The Role of Micronutrients in Alopecia Areata: A Review. Am. J. Clin. Dermatol. 2017, 18, 663–679.
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021.
- Toalaa, J.E.; Garcia Varelab, R.; Garciac, H.S.; Mata-Harod, V. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114.
- Tsilingiri, K.; Rescigno, M. Postbiotics: What else? Benef. Microbes 2013, 4, 101–107.
