Galectins are soluble β-galactoside binding proteins, which are involved in regulation of multiple cellular processes including cell growth, differentiation, apoptosis, and immune responses. Sixteen galectin genes have been identified in animals, 12 of which are expressed in humans. Out of all galectins, galectin-16 is poorly characterized yet and current knowledge suggest that this is a tissue-specific gene with functions and expression limited to placenta and brain among others.
- galectin
- LGALS16
- placenta
- brain tissues
- cell differentiation
- transcription factor
- miRNA
1. Molecular Characteristics of Galectin-16 Gene and Recombinant Protein
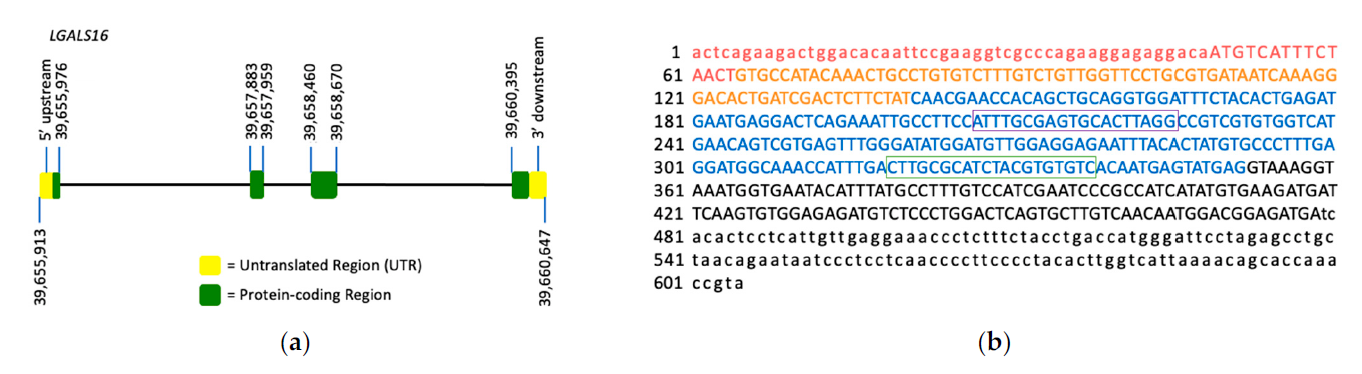
Figure 1. LGALS16 gene structure and the mRNA sequence. (a) LGALS16 (4,735 bp) is located on chromosomal band 19q13.2 and contains 4 exons (ENSG00000249861). (b) NCBI reference sequence of LGASL16 mRNA (NM_001190441.3). Each exon is highlighted with red, orange, blue, and black representing exons 1, 2, 3, and 4, respectively. The protein coding sequence (CDS) is indicated in capitals while UTRs in small characters. The oligonucleotide sequences for PCR amplification are boxed.
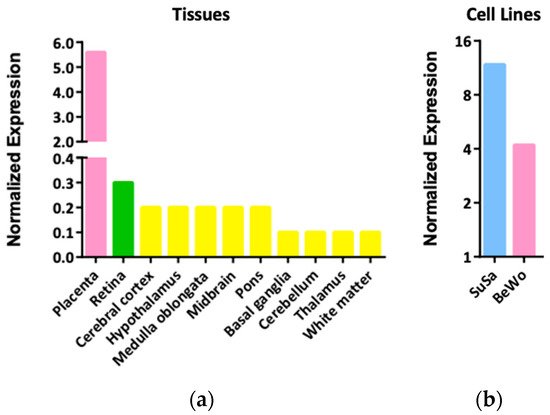
2. Expression Patterns and Functions of LGALS16 in Cells and Tissues
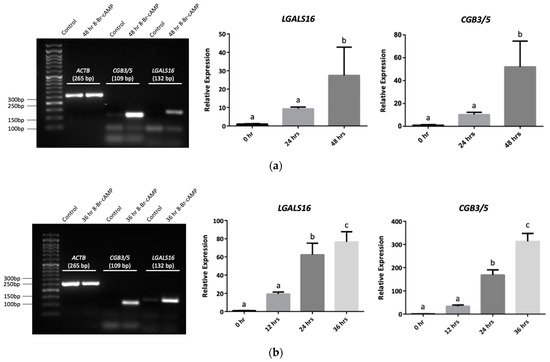
3. Transcriptional and Post-Transcriptional Regulation of LGALS16
3.1. Transcription Factors
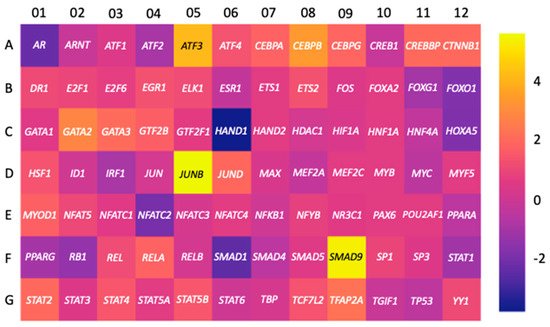
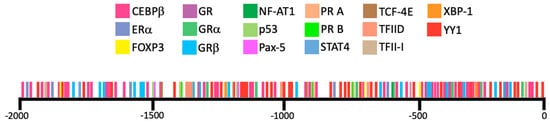
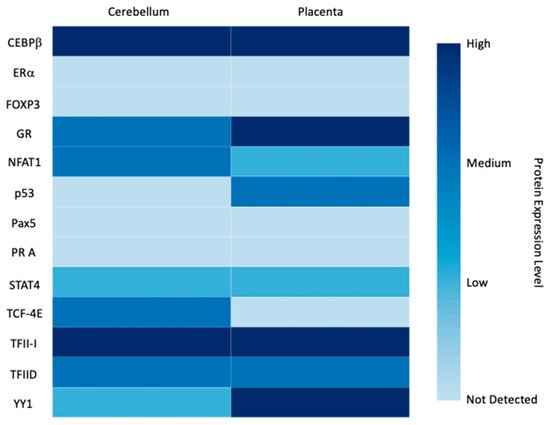
3.2. miRNAs
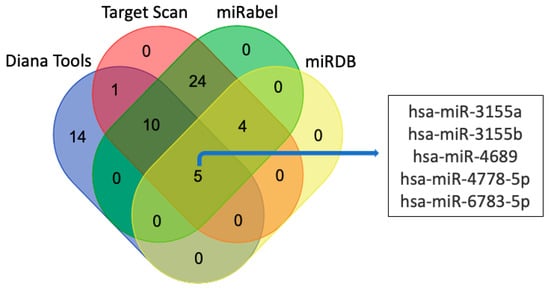
3.3. LGALS16 and Human Diseases
References
- Than, N.G.; Romero, R.; Goodman, M.; Weckle, A.; Xing, J.; Dong, Z.; Xu, Y.; Tarquini, F.; Szilagyi, A.; Gal, P.; et al. A primate subfamily of galectins expressed at the maternal-fetal interface that promote immune cell death. Proc. Natl. Acad. Sci. USA 2009, 106, 9731–9736.
- Than, N.G.; Romero, R.; Xu, Y.; Erez, O.; Xu, Z.; Bhatti, G.; Leavitt, R.; Chung, T.H.; El-Azzamy, H.; LaJeunesse, C.; et al. Evolutionary origins of the placental expression of chromosome 19 cluster galectins and their complex dysregulation in preeclampsia. Placenta 2014, 35, 855–865.
- Blois, S.M.; Dveksler, G.; Vasta, G.R.; Freitag, N.; Blanchard, V.; Barrientos, G. Pregnancy galectinology: Insights into a complex network of glycan binding proteins. Front. Immunol. 2019, 10, 1166.
- Ely, A.Z.; Moon, J.M.; Sliwoski, G.R.; Sangha, A.K.; Shen, X.-X.; Labella, A.L.; Meiler, J.; Capra, J.A.; Rokas, A. The impact of natural selection on the evolution and function of placentally expressed galectins. Genome Biol. Evol. 2019, 11, 2574–2592.
- Singer, M.F. SINEs and LINEs: Highly repeated short and long interspersed sequences in mammalian genomes. Cell 1982, 28, 433–434.
- Weckselblatt, B.; Rudd, M.K. Human structural variation: Mechanisms of chromosome rearrangements. Trends Genet. 2015, 31, 587–599.
- Si, Y.; Yao, Y.; Ayala, G.J.; Li, X.; Han, Q.; Zhang, W.; Xu, X.; Tai, G.; Mayo, K.H.; Zhou, Y.; et al. Human galectin-16 has a pseudo ligand binding site and plays a role in regulating c-Rel mediated lymphocyte activity. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129755.
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, jcs208884.
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2013, 41, D991–D995.
- Rosenfeld, C.S. The placenta-brain-axis. J. Neurosci. Res. 2021, 99, 271–283.
- Inamochi, Y.; Mochizuki, K.; Goda, T. Histone code of genes induced by co-treatment with a glucocorticoid hormone agonist and a p44/42 MAPK inhibitor in human small intestinal Caco-2 cells. Biochim. Biophys. Acta 2014, 1840, 693–700.
- Park, M.H.; Hong, J.T. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells 2016, 5, 15.
- Hayden, M.S.; Ghosh, S. NF-κB in immunobiology. Cell Res. 2011, 21, 223–244.
- Balsa, E.; Perry, E.A.; Bennett, C.F.; Jedrychowski, M.; Gygi, S.P.; Doench, J.G.; Puigserver, P. Defective NADPH production in mitochondrial disease complex I causes inflammation and cell death. Nat. Commun. 2020, 11, 2714.
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjöstedt, E.; Asplund, A. Tissue-based map of the human proteome. Science 2015, 347, 1260419.
- Nuzzo, A.M.; Giuffrida, D.; Zenerino, C.; Piazzese, A.; Olearo, E.; Todros, T.; Rolfo, A. JunB/Cyclin-D1 imbalance in placental mesenchymal stromal cells derived from preeclamptic pregnancies with fetal-placental compromise. Placenta 2014, 35, 483–490.
- Han, Y.M.; Romero, R.; Kim, J.S.; Tarca, A.L.; Kim, S.K.; Draghici, S.; Kusanovic, J.P.; Gotsch, F.; Mittal, P.; Hassan, S.S.; et al. Region-specific gene expression profiling: Novel evidence for biological heterogeneity of the human amnion. Biol. Reprod. 2008, 79, 954–961.
- Knyazev, E.N.; Zakharova, G.S.; Astakhova, L.A.; Tsypina, I.M.; Tonevitsky, A.G.; Sukhikh, G.T. Metabolic reprogramming of trophoblast cells in response to hypoxia. Bull. Exp. Biol. Med. 2019, 166, 321–325.
- Walcott, B.P.; Winkler, E.A.; Zhou, S.; Birk, H.; Guo, D.; Koch, M.J.; Stapleton, C.J.; Spiegelman, D.; Dionne-Laporte, A.; Dion, P.A.; et al. Identification of a rare BMP pathway mutation in a non-syndromic human brain arteriovenous malformation via exome sequencing. Hum. Genome Var. 2018, 5, 18001.
- Ma, S.; Pang, C.; Song, L.; Guo, F.; Sun, H. Activating transcription factor 3 is overexpressed in human glioma and its knockdown in glioblastoma cells causes growth inhibition both in vitro and in vivo. Int. J. Mol. Med. 2015, 35, 1561–1573.
- Garces de Los Favos Alonso, I.; Liang, H.C.; Turner, S.D.; Lagger, S.; Merkel, O.; Kenner, L. The role of activator protein-1 (AP-1) family members in CD30-positive lymphomas. Cancers 2018, 10, 93.
- Shankar, K.; Kang, P.; Zhong, Y.; Borengasser, S.J.; Wingfield, C.; Saben, J.; Gomez-Acevedo, H.; Thakali, K.M. Transcriptomic and epigenomic landscapes during cell fusion in BeWo trophoblast cells. Placenta 2015, 36, 1342–1351.
- Cheng, Y.-H.; Richardson, B.D.; Hubert, M.A.; Handwerger, S. Isolation and characterization of the human syncytin gene promoter. Biol. Reprod. 2004, 70, 694–701.
- Wang, Z.; Liu, Y.; Liu, J.; Kong, N.; Jiang, Y.; Jiang, R.; Zhen, X.; Zhou, J.; Li, C.; Sun, H.; et al. ATF3 deficiency impairs the proliferative-secretory phase transition and decidualization in RIF patients. Cell Death Dis. 2021, 12, 387.
- Jadhav, K.; Zhang, Y. Activating transcription factor 3 in immune response and metabolic regulation. Liver Res. 2017, 1, 96–102.
- Moslehi, R.; Mills, J.L.; Signore, C.; Kumar, A.; Ambroggio, X.; Dzutsev, A. Integrative transcriptome analysis reveals dysregulation of canonical cancer molecular pathways in placenta leading to preeclampsia. Sci. Rep. 2013, 3, 2407.
- Tsukamoto, S.; Mizuta, T.; Fujimoto, M.; Ohte, S.; Osawa, K.; Miyamoto, A.; Yoneyama, K.; Murata, E.; Machiya, A.; Jimi, E.; et al. Smad9 is a new type of transcriptional regulator in bone morphogenetic protein signaling. Sci. Rep. 2014, 4, 7596.
- Heo, K.S.; Fujiwara, K.; Abe, J. Disturbed-flow-mediated vascular reactive oxygen species induce endothelial dysfunction. Circ. J. 2011, 75, 2722–2730.
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379.
- Paraskevopoulou, M.D.; Georgakilas, G.; Kostoulas, N.; Vlachos, I.S.; Vergoulis, T.; Reczko, M.; Filippidis, C.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-microT web server v5.0: Service integration into miRNA functional analysis workflows. Nucleic Acids Res. 2013, 41, W169–W173.
- Quillet, A.; Saad, C.; Ferry, G.; Anouar, Y.; Vergne, N.; Lecroq, T.; Dubessy, C. Improving bioinformatics prediction of microRNA targets by ranks aggregation. Front. Genet. 2020, 10, 1330.
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131.
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005.
- Menon, R.; Debnath, C.; Lai, A.; Guanzon, D.; Bhatnagar, S.; Kshetrapal, P.K.; Sheller-Miller, S.; Salomon, C.; Garbhini Study Team. Circulating exosomal miRNA profile during term and preterm birth pregnancies: A longitudinal study. Endocrinology 2019, 160, 249–275.
- Yoshino, Y.; Roy, B.; Dwivedi, Y. Altered miRNA landscape of the anterior cingulate cortex is associated with potential loss of key neuronal functions in depressed brain. Eur. Neuropsychopharmacol. 2020, 40, 70–84.
- Yan, S.; Zhang, H.; Xie, W.; Meng, F.; Zhang, K.; Jiang, Y.; Zhang, X.; Zhang, J. Altered microRNA profiles in plasma exosomes from mesial temporal lobe epilepsy with hippocampal sclerosis. Oncotarget 2017, 8, 4136–4146.
- Liao, B.; Zhou, M.X.; Zhou, F.K.; Luo, X.M.; Zhong, S.X.; Zhou, Y.F.; Qin, Y.S.; Li, P.P.; Qin, C. Exosome-derived miRNAs as biomarkers of the development and progression of intracranial aneurysms. J. Atheroscler. Thromb. 2020, 27, 545–610.
- Ludwig, N.; Leidinger, P.; Becker, K.; Backes, C.; Fehlmann, T.; Pallasch, C.; Rheinheimer, S.; Meder, B.; Stähler, C.; Meese, E.; et al. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016, 44, 3865–3877.
- Gong, S.; Gaccioli, F.; Dopierala, J.; Sovio, U.; Cook, E.; Volders, P.J.; Martens, L.; Kirk, P.D.W.; Richardson, S.; Smith, G.C.S.; et al. The RNA landscape of the human placenta in health and disease. Nat. Commun. 2021, 12, 2639.
- Braun, A.E.; Muench, K.L.; Robinson, B.G.; Wang, A.; Palmer, T.D.; Winn, V.D. Examining Sex Differences in the Human Placental Transcriptome During the First Fetal Androgen Peak. Reprod Sci. 2021, 28, 801–818.
- Santos, J.X.; Rasga, C.; Marques, A.R.; Martiniano, H.F.M.C.; Asif, M.; Vilela, J.; Oliveira, G.; Vicente, A.M. A role for geneenvironment interactions in Autism Spectrum Disorder is suggested by variants in genes regulating exposure to environmental factors. BioRxiv 2019, 520544.
- Vastrad, B.; Vastrad, C. Bioinformatics analyses of significant genes, related pathways and candidate prognostic biomarkers in Alzheimer’s disease. BioRxiv 2021.
- Zhao, B.; Shan, Y.; Yang, Y.; Zhaolong, Y.; Li, T.; Wang, X.; Luo, T.; Zhu, Z.; Sullivan, P.; Zhao, H.; et al. Transcriptome-wide association analysis of brain structures yields insights into pleiotropy with complex neuropsychiatric traits. Nat. Commun. 2021, 12, 2878.
- Cheng, P.; Feng, F.; Yang, H.; Jin, S.; Lai, C.;Wang, Y.; Bi, J. Detection and significance of exosomal mRNA expression profiles in the cerebrospinal fluid of patients with meningeal carcinomatosis. J. Mol. Neurosci. 2021, 71, 790–803.
