Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Md Jamal Uddin and Version 2 by Vivi Li.
The global burden of chronic kidney disease (CKD) intertwined with cardiovascular disease has become a major health problem. Oxidative stress (OS) plays an important role in the pathophysiology of CKD. The nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant responsive element (ARE) antioxidant system plays a critical role in kidney protection by regulating antioxidants during OS. Heme oxygenase-1 (HO-1), one of the targets of Nrf2-ARE, plays an important role in regulating OS and is protective in a variety of human and animal models of kidney disease. Thus, activation of Nrf2-HO-1 signaling may offer a potential approach to the design of novel therapeutic agents for kidney diseases.
- chronic kidney diseases
- oxidative stress
- Nrf2
- HO-1
- small molecule natural products
1. Introduction
The incidence and prevalence of chronic kidney disease (CKD) patients is increasing worldwide. The prevalence of CKD between male and female patients is not constant between countries, however, kidney functions decline faster in males than females [1]. Importantly, CKD is not only a risk factor for increasing global mortality but it is also a critical factor involved in cardiovascular disease (CVD) [2]. The close link between CKD and CVD has been known for a long time [3][4][5][3,4,5]. Not only traditional risk factors such as hypertension, dyslipidemia, and diabetes, but also non-traditional risk factors such as disturbed minerals and vitamins in CKD may play important roles in the progression of CVD. The current treatment options for CKD are controlling blood pressure, serum glucose, and serum lipid profile [6], as well as a modification of lifestyle [7][8][7,8]. Since the efficacy of the current therapeutic strategy is still limited [9], there is a need to develop a more effective therapeutic option for treating CKD. Although the exact mechanism involved in the development of CKD is elusive, many lines of evidence strongly suggest that oxidative stress (OS) plays a critical role in the progression of CKD [10][11][12][13][10,11,12,13].
OS is an imbalance between cellular reactive oxygen species (ROS) levels and antioxidant enzymes, leading to a pathological condition. ROS regulates various signaling pathways, including the growth and differentiation of cells, mitogenesis, production, and breakdown of the extracellular matrix (ECM), inflammation, and apoptosis [14]. OS-mediated damaging effects of cells are controlled by activating the antioxidant defense system. OS has also been noticed to be affected by sex hormones in ischemic kidney injury [15]. Unfortunately, there is an impairment of antioxidative defense and a reduced activity of antioxidant enzymes in CKD [16]. Hence, promoting the endogenous antioxidants defense system may become an important strategy in inhibiting OS-mediated cellular damage in CKD.
Phytochemicals and other natural products are cytoprotective against OS by scavenging oxygen-free radicals and enhancing the level of antioxidants [17]. The literature on protective effects of antioxidant natural products against CKD has been reported [18][19][20][18,19,20]. Nuclear factor erythroid 2-related factor 2 (Nrf2) is the master regulator of the cellular antioxidant defense system [17]. Studies review that augmentation of Nrf2 activity prevents the progression of acute kidney injury (AKI) to CKD transition [21][22][21,22]. Natural bioactive compounds and their sources have been demonstrated to have kidney protective potential by activating Nrf2 in experimental CKD models [23][24][23,24]. In a recent review on clinical studies, bardoxolone methyl (CDDO-me), a semi-synthetic triterpenoid activating the Nrf2 pathway, has been reported as an effective therapeutic for diabetic kidney disease (DKD), although it has limitations in that it increases the risk of heart failure [25]. Heme oxygenase-1 (HO-1), one of the target molecules of Nrf2, attenuates the overall production of ROS through its ability to degrade heme and to produce carbon monoxide (CO), biliverdin/bilirubin, and the release of free iron. Induction of HO-1 mediates many beneficial effects in the cardiovascular system and kidney [26]. Also, the modulatory role of HO-1 has been reported in various kidney injury models including CKD [27][28][29][30][31][32][33][34][27,28,29,30,31,32,33,34]. Several natural HO-1 inducers and their therapeutic applications in various diseases, including CKD, have been reported [35].
2. Small Molecule Natural Products Activating Nrf2-HO-1 Signaling
A substantial quantity of natural products has been reported to confer renoprotection and improve disease outcomes of the various types of CKD, primarily through activating the Nrf2/HO-1 antioxidant defense systems and attenuating the proinflammatory signaling pathways. Here, rwesearchers reviewed the existing literature over the past decade to compile comprehensive information on the kidney protective potential of naturally occurring compounds. Experimental and disease models, the pathobiology involved, the research outcomes, and the molecular markers altered by these compounds are summarized in Table 1 and Table 2 and Figure 13. To facilitate the discussion, rwesearchers have categorized the kidney protective effects of these natural compounds into two distinct chemical groups: phenolic and non-phenolics. This categorization also highlights common bioactive compounds, belonging to phenolic group which represents the largest chemical class showing enormous bioactivity with the potential to be future drug candidates.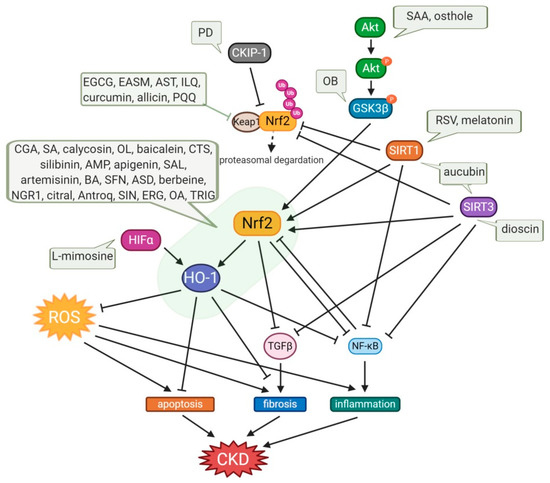
Figure 13. Protective effects of small-molecule natural products on OS in CKD. Osthole and SAA enhance the activation of the Akt/Nrf2/HO-1 signaling pathway with suppression of NF-kB and TGFβ1, consequently attenuating OS, inflammation, and fibrosis. OB induces the phosphorylation of GSK3β, which inhibits Fyn-mediated Nrf2 nuclear export, and activates the transcription of Nrf2-driven antioxidant genes. Expression of SIRT1, which inhibits NF-kB activity, and the activation of Nrf2 are enhanced by aucubin, melatonin, and RSV, which also upregulates SIRT3, resulting in amelioration of kidney injury. Dioscin upregulates SIRT3 level, promotes Nrf2, and suppresses Keap1 expression, resulting in inhibition of inflammation, lipid metabolism, OS, and kidney fibrosis. PD increases the CKIP-1 expression level and promotes the interaction of CKIP-1 with Nrf2, consequently activating the Nrf2-ARE antioxidative pathway. Allicin, AST, curcumin, EASM, EGCG, ILQ, and PQQ attenuate OS via the Nrf2/HO-1 signaling pathway with inhibition of Keap1, and they also reduce TGFβ-mediated fibrosis and NF-kB-induced inflammation. In the cases of an anti-fibrotic effect of apigenin, ASD, baicalein, BA, CGA, CTS, ERG, OL, and SFN, AMP, antroq, artemisinin, berbeine, calycosin, SA, SIN, and TRIG, they are mediated not only by upregulation of the Nrf2/HO-1 antioxidant signaling pathway and downregulation of NF-kB-induced inflammation, but also via TGFβ suppression. Treatments with citral, NGR1, OA, SAL, and silibinin have potency for anti-apoptotic effects with regulation of Bcl2/Bax and caspase3. The decrease in the NLRP3 inflammasome was also observed in treatments with baicalein, EGCG, and OL. L-mimosine activates HIF1α, which upregulates renoprotective HIF target genes, such as VEGF, HO-1, and GLUT1, and decreases fibrosis markers. AMP, ampelopsin; Antroq, antroquinonol; ASD, akebia saponin D; AST, astaxanthin; BA, betulinic acid; CGA, chlorogenic acid; CTS, cryptotanshinone; EASM, ethyl acetate extract of Salvia miltiorrhiza; EGCG, Epigallocatechin gallate; ERG, ergone; GSK3β, glycogen synthase kinase 3β; HIFα, hypoxia-inducible factor α; ILQ, isoliquiritin; NGR1, notoginsenoside R1; OA, oleanolic acid; OB, obacunone; OL, oleuropein; PD, polydatin; PQQ, pyrroloquinoline quinone; RSV, resveratrol; SA, sinapic acid; SAA, salvianolic acid A; SAL, salidroside; SFN, sulforaphane; SIN, sinomenine; TRIG, trigonelline.
Table 1.
Kidney protective effects provided by phenolic compounds of phytochemicals targeting the Nrf2-HO-1 signaling pathway.
| No. | Modulator | Chemical Class and Natural Sources | Experimental Model | Disease Model | Pathobiology Involved | Major Research Outcomes | Molecular Markers | Ref. |
|---|
AQP2, aquaporin 2; α-SMA, α-smooth muscle actin; BSA, bovine serum albumin; CAT, catalase; CKD, chronic kidney disease; COX2, cyclooxygenase; DHE, dihydroethidium; DKD, diabetic kidney disease; ECM, extracellular matrix; EMT, epithelial-to-mesenchymal transition; eNOS, endothelial nitric oxide synthase; FN, fibronectin; GMCs, glomerular mesangial cells; GPx, glutathione peroxidase; GSK3β, glycogen synthase kinase 3β; HFD, high fat diet; HG, high glucose; HO-1, Heme oxygenase-1; ICAM, intercellular adhesion molecule 1; iNOS, inducible nitric oxide synthase; LDH, lactate dehydrogenase; LN, lupus nephritis; LPS, lipopolysaccharide; MCP-1, monocyte chemoattractant protein-1; MDA, malondialdehyde; MDSCs, myeloid-derived suppressor cells; MGN, membranous glomerulonephritis; MMCs, mouse mesangial cells; NGAL, neutrophil gelatinase-associated lipocalin; NLRP3, NLR family pyrin domain containing 3; Nqo1, NADPH quinone oxidoreductase; Nrf2, nuclear factor erythroid 2-related factor 2; MPO, myeloperoxidase; NT, nitrotyrosine; OS, oxidative stress; PAI-1, plasminogen activator inhibitor-1; SOD, superoxide dismutase; STZ, streptozotocin; TBARS, thiobarbituric acid reactive substances; UUO, unilateral ureteral obstruction; VCAM, vascular cell adhesion molecule 1.
Table 2.
Kidney protective effects provided by non-phenolic compounds of phytochemicals targeting the Nrf2-HO-1 signaling pathway.
| No. | Modulator | Chemical Class and Natural Sources | Experimental Model | Disease Model | Pathobiology Involved | Major Research Outcomes | Molecular Markers | Ref. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic compounds | |||||||||||||||||
| 1 | Ampelopsin | Flavonoid; Ampelopsis grossedentata | HG-stimulated hGMCs | OS | OS, ECM accumulation | Amelioration of OS and ECM accumulation | ↓ROS, ↓MDA, ↑SOD, ↓Nox2, ↓Nox4, ↓NADPH, ↓FN, ↓Col IV, ↑n-Nrf2, ↑HO-1, |
[36][109] | |||||||||
| Non-phenolic compounds | |||||||||||||||||
| 1 | Akebia Saponin D | triterpenoid saponin; Dipsaci Radix | STZ-injected mice | DKD | OS, inflammation | Amelioration of kidney damage, inflammation, OS, and apoptosis | ↓TNFα, ↓IL-1β, ↓IL-6, ↓MCP-1, ↓ROS, ↓MDA, ↓LDH, ↑SOD, ↑Bcl2, ↓Bax, ↓cleaved caspase3/caspase3, ↓cleaved caspase9/caspase9, ↑n-Nrf2, ↓p-NF-kB/t-NF-kB, ↑HO-1, ↑Nqo1, ↓p-IkBα/t-IkBα |
[62][133] | |||||||||
| 2 | Apigenin | Flavonoid; common fruits and vegetables | HG-treated HK-2 cells | Oxidative damage | Oxidative damage | ||||||||||||
| HG-treated HK-2 cells | Decrease in apoptosis, inhibition of OS, and inflammatory response | ↓TNFα, ↓IL-1β, ↓IL-6, ↓MCP-1, ↓ROS, ↓MDA, ↓LDH, ↑SOD, ↑Bcl2, ↓Bax, ↓cleaved caspase3/caspase3, ↓cleaved caspase9/caspase9, ↑Nrf2, ↓p-NF-kB/t-NF-kB, ↑HO-1, ↑Nqo1, ↓p-IkBα/t-IkBα | ↓LDH, ↓MDA, ↑SOD, ↑CAT, ↓TNFα, ↓IL-1β, ↓IL-6, ↑Nrf2, ↑HO-1 | [ | 37 | ] | [ | 110] | |||||||||
| 3 | Astaxanthin | Xanthophyll carotenoid; algae, shrimp, lobster, crab, salmon, and other organisms | STZ-injected rat | DKD | ECM accumulation | Amelioration of kidney injury | ↓FN, ↓TGFβ1, ↓ICAM-1 | [38 | |||||||||
| 2 | Allicin | Diallyl thiosulfinate; garlic (Allium sativum L.) | ] | 5/6 nephrectomy Wistar rat | [ | CKD | Fibrosis, OS | 111 | Antihypertensive and antioxidant effects | ] | |||||||
| ↑AT1R, ↑AT2R, ↑Nrf2, ↓Keap1, | ↑CAT, ↑SOD, ↓HO-1, ↑eNOS | [ | 63 | ] | [ | 134 | ] | HG-treated GMCs | Kidney fibrosis | OS | |||||||
| 3 | Increase in antioxidative capacity | Antroquinonol | ↓FN, ↓TGFβ1, ↓ICAM-1, ↑SOD, | Enone; mushroom (Antrodia camphorate) ↓MDA, ↓ROS, ↓DHE, ↑n-Nrf2, ↓keap1, ↓SOD-1, ↓Nqo1, ↓HO-1 |
|||||||||||||
| Adriamycin -injected BALB/c mice | FSGS | OS | Decrease in kidney dysfunction, anti-OS, anti-inflammation | ↓desmin, ↓O | 2 | ●− | , (serum, urine ↓ O2●−, ↓NO), ↓DHE, ↓p47phox, ↑Nrf2, ↑GPx, ↓NF-kB p65, ↓MCP-1, ↓IL-6, ↓CD3, ↓F4/80, ↓Col I, ↓Col III, ↓Col IV, ↓TGFβ1 | [64][135] | Adriamycin-treated BALB/c mice | ||||||||
| 4 | FSGS | OS, inflammation | Anti-inflammation, antioxidation | ↓TGFβ1, ↓collagen1, ↓α-SMA, ↓MDA, ↑GSH, ↑SOD, ↑CAT, (serum: ↓IL-1 β, IL-18), ↑Nrf2, ↓NLRP3 | Artemisinin[39 | sesquiterpene lactones; Asteraceae Artemisia annua | STZ-injected rat] | DKD[112] | |||||||||
| OS | Amelioration of kidney dysfunction and OS | ↓MDA, ↑t-SOD, ↑GPx, ↓TGFβ1, ↑t-Nrf2, ↑n-Nrf2, ↑HO-1, ↑Nqo1 | [ | 65 | ] | [ | 136 | ] | 4 | Baicalein | Flavonoid; roots of Scutellaria baicalensis Georgi | Pristine -injected BALB/c mice | LN | OS, inflammation | |||
| 5 | Aucubin | iridoid glycoside; leaf of | Attenuation of kidney dysfunction, antioxidation, anti-inflammation, inhibition of MDSC expansion | Eucommia ulmoides | ↓IL-1b, ↓IL-18, ↓O | 2 | ¯˙, ↑ GPx, ↑Nrf2, ↑HO-1, ↓ NLRP3, ↓Casp-1, ↓mIL-1 β, ↓p-NF-kB |
HFD-fed and STZ-injected mice | [ | DKD | 40 | OS, inflammation | ][113] | ||||
| Amelioration of kidney dysfunction, anti-inflammation, anti-OS | LPS-primed spleen-derived MDSCs | OS, inflammation | ↓ROS, ↓IL-1β, ↓IL-18, ↑Nrf2, ↑HO-1, ↓NLRP3, ↓mIL-1β/pro-IL-1β, ↓Casp-1-p20/pro-casp-1-p45, ↓p-NF-kB/NF-kB, ↓Ang-1, ↓p47phox, ↓GP91phox, ↓iNOS |
||||||||||||||
| ↓FN, ↓collagen IV, ↓MDA, | ↑SOD, ↑CAT, ↑GSH/T-GSH, ↓TNFα, ↓IL-6, ↓IL-1β, ↓p65, ↓IkBα, ↑Nrf2, ↑HO-1, ↑Nqo1, ↑FOXO3α, ↓p-FOXO3α/FOXO3α, ↑SIRT1, ↑SIRT3, | 5 | Calycosin | Isoflavone; root of Astragalus membranaceus | HFD-fed/ STZ-injected SD rat | DKD | Inflammation, OS, fibrosis | Inhibition of inflammatory, oxidative, and fibrotic events | ↓IL-33, ↓ST2, ↓NF-kB p65, ↓TNFα, ↓IL-1 β, ↓IL-6, ↑Nrf2, ↓MDA, ↓TGFβ | [41][114] | |||||||
| 6 | 7 | Chlorogenic acid | Cinnamate ester; coffee, fruits, and vegetables | STZ-injected and HFD-fed SD rat | DKD | Betulinic acid | pentacyclic triterpenoid; from the outer bark of white birch trees (Betula alba) | OS, inflammation | Relieve kidney injury, mitigation of OS, inflammation | ↓MDA, ↑SOD, ↑GSH-Px, ↑n-Nrf2, ↑HO-1, ↓IL-6, ↓TNFα, ↓IL-1 β, ↑c-NF-kB, ↓n-NF-kB, ↑IkBα, ↓p-IkBα, |
[42][115] | ||||||
| STZ-injected SD rat | DKD | OS | Anti-OS | ↓IL-1 β, ↓IL-6, ↓MDA, ↑SOD, ↑CAT, ↑p-AMPK/AMPK, ↓p-IkBα/IkBα, ↓p-NF-kB/NF-kB, ↑Nrf2, ↑HO-1 | [ | 68 | ] | [139] | HG-treated rat mesangial cell line (HBZY-1) | Mitigation of OS, inflammation, increase in cell proliferation | ↑n-Nrf2, ↑HO-1, ↑c-NF-kB, ↓n-NF-kB, ↑IkBα, ↓p-IkBα, ↓IL-6, ↓TNFα, ↓IL-1 β | ||||||
| 8 | Citral | Terpeonids; Litsea cubeba | Adriamycin -injected BALB/c mice | FSGS | 7 | Cryptotanshinone | Quinoid diterpene; Salvia miotiorrhiza bunge | UUO-operated mice | Kidney fibrosis | OS, inflammation | Attenuation of OS and inflammation | ↓collagen-1, ↓FN, ↓CD68, ↓CD3, ↑IkBα, ↓NF-kB p65, ↑SOD2, ↑CAT, ↑GSH, ↓MDA, ↑Nuclear Nrf2, ↓cytosolic Nrf2, ↑HO-1 |
[43][116] | ||||
| 8 | |||||||||||||||||
| ↑SOD, ↑CAT, ↑GSH-Px, ↓MPO, | ↓TNFα, ↓IL-6, ↓IL-1 β, ↑IkBα, ↓p-IkBα, ↓NF-kB, ↑n-Nrf2, ↑HO-1, ↑t-bilirubin | [ | 49 | ][121] | |||||||||||||
| ↓Ac-FOXO3α/FOXO3α | [ | 66 | ] | [ | 137 | ] | OS | Amelioration of kidney dysfunction, anti-OS, anti-inflammation, anti-apoptosis | ↓O2¯˙, (serum, urine ↓O2¯˙, ↓NO), ↓DHE, ↓p47phox, ↑Nrf2, ↑Nqo1, ↑HO-1, ↓desmin, ↓TUNEL, ↓Casp-3p17, ↓Casp-9p37, ↓Bax/Bcl2, ↓pNF-kB p65, ↓MCP-1, ↓ CD3, ↓F4/80 | [69][140] | |||||||
| LPS-treated RAW 264.7 macrophages | OS | ↓NO, ↓NF-kB, ↓IL-6, ↓TNFα, ↓IL-1β, ↓p-ERK1/2(10min), ↓p-JNK1/2(15,30min) | |||||||||||||||
| 9 | Dioscin | Steroid saponin; Dioscoreae rhizoma | 10% fructose -fed mice | CKD | Oxidative damage, lipid metabolism, fibrosis | Inhibition of inflammation, lipid metabolism, OS, kidney fibrosis | ↓MDA, ↑SOD, ↑GSH-Px, ↓α-SMA, ↑SIRT3, ↑SOD2, ↓IL-1β, ↓IL6, ↓TNFα, ↓NF-kB, ↓HMGB1, ↓COX2, ↓c-Jun, ↓c-Fos, ↓SREBP-1c, ↓SCD-1, ↓FASn, ↓p-Akt, ↓p-FoxO1A, ↓ACC, ↑CPT1, ↑Nrf2, ↓Keap1, ↑GST, ↓TGFβ1, ↓p-Smad3, ↑Smad7 |
10 | Ethyl acetate extract of Saliva miltiorrhiza | Diterpenoids, phenolic compounds, flavonoids, triterpenoids; dried root of Salvia miltiorrhiza Bunge | STZ-injected mice | DKD | Oxidative stress | Antioxidation, attenuation of kidney dysfunction | ↑Nrf2, ↑HO-1, ↑Nqo1, ↓Keap1 | [50][122] | |
| HG-treated SV40-MES-13 MMCs | |||||||||||||||||
| [ | |||||||||||||||||
| 59 | |||||||||||||||||
| 6 | Berberine | isoquinoline alkaloid; Coptidis Rhizoma and Cortex Phellodendri | STZ-injected mice | DKD | OS | Anti-fibrosis | ↓α-SMA, ↓collagen-1, ↑Nrf2, ↑NQO1, ↑HO-1 |
[67][138 | hyperglycemia | Antioxidation | ↓ROS, ↑Nrf2, ↑HO-1, ↑Nqo1, ↓Keap1 |
||||||
| ] | |||||||||||||||||
| HG-treated NRK 52E cells | EMT | ↓E-cadherin, ↓α-SMA, ↑n-Nrf2, ↑Nqo1, ↑HO-1, ↓p-Smad2, ↓p-Smad3 |
Curcumin | Curcuminoid; turmeric (Curcuma longa) | 5/6 nephrectomy Wistar rat | CKD | OS, inflammation | Protection of kidney function, antioxidant, anti-inflammation | ↓Nox4, ↑eNOS, ↓nitrotyrosine, ↓MCP-1, ↓Keap-1, ↑Nrf2, ↑GPx-1, ↑CAT, ↑SOD-1, ↓phospho serine D1R |
[44][117] | |||||||
| [ | 70 | ] | [ | 141 | ] | 0.25% Adenine -diet rat | CKD | OS, inflammation | Amelioration of kidney function and OS | ↓IL-1 β, ↓IL-6, ↓TNFα, ↑cycstatin C, ↓adiponecitn, ↑sclerostin, ↑SOD, ↑Nrf2, ↑GSH reductase. ↓ caspase3 |
|||||||
| 10 | Ergone (alisol B 23-acetate, pachymic acid B) | steroid; Polyporus umbellatus, surface layer of Poria cocos, Alisma orientale | AngII- treated HK-2 and conditionally immortalized MPC5 cells | [ | 45][118] | ||||||||||||
| CKD | OS, inflammation, impaired Nrf 2 activation | inhibition of the RAS/Wnt/b-catenin signaling cascade | (HK-2) ↓Snail1, ↓MMP-7, ↓Twist, | ↓FSP-1, ↓Col I, ↓Col III, ↓α-SMA, | ↓vimentin, ↑E-cadherin, ↓NF-kB, ↓MCP-1, ↓COX2, ↑Nrf2, ↑HO-1 (podocyte) ↓Snail1, ↓MMP-7, ↓Twist, ↓FSP-1, ↑podocin, ↑nephrin, ↑podocalyxin, ↑synaptopodin, ↓desmin, ↑WT1, ↓Akt2, ↓NF-kB, ↓MCP-1, ↓COX2, ↑Nrf2, ↑HO-1 |
[71][142] | HG-treated NRK-52E cells | OS | OS | Increase in cell viability, inhibition of EMT | |||||||
| 11 | L-mimosine | Amino acid; | ↑E-cadherin, ↓α-SMA, ↑Nrf2, ↑HO-1 | Mimosa pudica | [46][119] | ||||||||||||
| Rats with remnant kidneys after subtotal nephrectomy (5/6 nephrectomy) | CKD | Fibrosis | Improvement of kidney function, inhibition of fibrosis | ↑HIF-1α, ↑HIF-2α, ↑VEGF, ↑HO-1, | ↑GLUT-1, ↓α-SMA, ↓collagen III | [ | 72][143] | 9 | Epigallocatechin-3 -Gallate | Polyphenol; Dried leaves of tea plant (Camellia sinensis) | STZ-injected mice | DKD | Oxidative damage, inflammation, | Anti-OS | ↓TGFβ1, ↓PAI-1, ↓ICAM-1, ↓VCAM-1, ↓MDA, ↓iNOS, ↓3-NT, ↑Nqo1, ↑HO-1, ↑t-Nrf2, ↑c-Nrf2, ↑n-Nrf2, ↑n-Nrf2/t-Nrf2 | [ | |
| 12 | Melatonin | Endogenous indoleamine, coffee, walnut, etc. | Pristine -injected BALB/c mice | 47 | LN | OS, inflammation | Attenuation of OS, inflammation | ↑SIRT1, ↑Nrf2, ↓TNFα, ↓NF-kB, ↓iNOS, ↓NLRP3, ↑CD31 | ][120] | ||||||||
| [ | 73 | ] | [ | 144 | ] | HG-cultured MMC | ↑t-Nrf2, ↑c-Nrf2, ↑n-Nrf2, ↑Nqo1, ↑HO-1, ↓MDA, ↓iNOS, ↓VCAM-1, ↓ICAM-1, ↓COL4, ↓FN |
||||||||||
| 13 | Notoginsenoside R1 | Saponin; Panax notoginseng | db/db mice | DKD | OS | Anti-OS, decrease in apoptosis | ↓Collagen I, ↓TGFβ1, ↑Nrf2, ↑HO-1, ↓Bax/Bcl2, ↓Caspase-3, ↓Caspase-9 | [74][145] | NZB/W F1 lupus-prone mice | LN | OS | Antioxidant and anti-inflammation | |||||
| AGEs-treated HK-2 cells | Mitochondria injury | ↓LDH, ↓ROS, ↑n-Nrf2, ↑HO-1, | ↑Nrf2, ↓p47phox, ↑Nqo1, ↑HO-1, ↑GPx, ↓CD3, ↓F4/80, ↓NF-kB, | ↓NLRP3, ↓IL-1 β, ↓IL-18, ↓casp1-p20, |
[48][56] |
||||||||||||
| ↓Bax/Bcl2, ↓Cspase-3, ↓Caspase-9, ↓TGFβ1, ↓collagen I | UUO mice | CKD | OS, inflammation | ||||||||||||||
| 14 | Obacunone | Kidney function improvement, prevention of OS and inflammation | Triterpenoid limonoid; citrus and other plants of the Rutaceae family | HG-treated NRK-52E cells | OS | OS | Inhibition of OS, mitochondrial injury, and apoptosis | ↑SOD, ↑GSH, ↑CAT, ↓ROS, ↓JC-1 monomer/aggregate, ↑p-GSK3β/GSK3β, ↓n-Fyn, ↑n-Nrf2, ↑Nqo1, ↑HO-1, ↑SOD, ↑GSH, ↑CAT, ↓c-CytC/m-CytC, ↓cleaved caspase3 | [75][146] | ||||||||
| 15 | Oleanolic acid | Triterpenoid; olive oil, Phytolacca Americana, Syzygium spp, garlic, etc. | Cyclosporine -treated ICR mice | Chronic nephropathy | Inflammation, fibrosis | Antioxidation, anti-inflammation | ↓α-SMA, ↑HO-1, ↑nuclear/total Nrf2, ↑SOD1, ↓MDA, ↓urinary 8-iso-PGF2α, ↓urine 8-oxo-dG, ↓Bax/Bcl2, ↓active caspase-3 | [76][147] | |||||||||
| 16 | Pyrroloquinoline quinone | In soil and foods such as kiwifruit and human breast milk | HG-treated HK-2 cells | OS | OS | Decrease in OS, inflammation and cellular senescence | ↓IL-1β, ↓TNFα, ↓NF-kB, ↓p16, ↓p21, ↓ROS, ↑SOD2, ↑CAT, ↓keap1, ↑Nrf2, ↑HO-1, ↑Nqo1, ↑GST, ↑GPx3, |
[77][148] | 11 | Isoliquiritin | Flavonoid glycoside; Chinese licorice (Glycyrrhiza uralensis) | Cationic BSA-injected SD rat | MGN | Inflammation and OS | Antioxidative, anti-inflammatory activities | ↓Keap1, ↑Nrf2, ↓n-Nrf2, ↑c-Nrf2, ↑HO-1, ↑Nqo1, ↓MDA, ↓NO, ↑SOD, ↑CAT, ↑GPx, ↑GSH, ↓NF-kB p65, ↓nuclear NF-kB p65, ↑cyclic NF-kB, ↓IKKb, ↓p-IKKb, ↓TNFα, ↓IL-1 β, ↓COX2, ↓iNOS, ↓p38 MAPK, ↓p-p38 MAPK | [ |
| 17 | Sinomenine | Alkaloid; Sinomenium acutum | UUO-operated ICR mice | CKD | Fibrosis, OS | 51 | Anti-fibrosis, antioxidation | ↑E-cadherin, ↓α-SMA, ↓FN, ↑HO-1, ↑Nqo1, ↑Nrf2, ↑SOD, ↑GPx, ↑CAT, ↑SOD2, ↓p-Smad3, ↓β-catenin][54] |
|||||||||
| [ | 78 | ] | [ | 149 | ] | 12 | |||||||||||
| TGFβ-treated/H | Oleuropein, peracetylatedoleuropein | 2Secoiridoid; olive leaves, roots, and unprocessed olive drupes | O2Pristine -injected BALB/c mice | -treated HEK293 cells, TGFβ-treated RAW264.7 cells | ↑E-cadherin, ↓α-SMA, ↓FN, ↑HO-1, ↑Nqo1, ↑Nrf2, ↑SOD,LN |
Inflammation and OS | Amelioration of kidney abnormalities, inhibition of proinflammation, antioxidation | ↓MMP-3, ↓iNOS, ↓mPGEs-1, ↓PGE2, ↑Nrf2, ↑HO-1, ↓pSTAT3, ↓NF-kB-p65, ↑IkBα, ↓pp38, ↓pJNK, ↓pERK1/2 ↓NLRP3, ↓ASC, ↓IL-18, ↓ IL-1β, ↓cleaved caspase-1, ↓cleaved caspase 11 |
[52][123] | ||||||||
| ↑GPx, ↑CAT, ↑SOD2, ↓p-Smad3, ↓β-catenin | 13 | Osthole | Coumarin; Fructus Cnidii | ||||||||||||||
| 18 | 2% adenine suspension -received rat | CKD | Sulforaphane | Inflammation | Protection of kidney function, antiinflammation | ↓TNFα, ↓IL-6, ↓IL-8, ↓NF-kB/p65, | Isothiocyanate (organosulfur compound); Cruciferous vegetables such as broccoli, brussels sprouts, and cabbages | STZ-injected and meglumine diatrizoate-injected Wistar rats | DKD, CIN | OS | ↓TGFβ1, ↓MCP-1, ↑p-Akt/Akt, ↑Nrf2 |
Renoprotective | ↓MDA, ↓8-oxo-dG, ↑Nrf2, ↑HO-1, ↓IL6, ↑Caspase3[53][124] |
||||
| [ | 79 | ] | [ | 80 | ] | [ | 150 | ,151] | 14 | Polydatin | Stilbenoid glucoside; Polygonum cuspidatum Sieb.et Zucc | STZ-injected diabetic mice | DKD | OS | Improvement of antioxidative effect and kidney dysfunction | ↑CKIP-1, ↑Nrf2, ↑HO-1, ↑SOD1, ↓FN, ↓ICAM-1, ↓MDA, ↑t-SOD |
|
| Meglumine diatrizoate-treated NRK-52E cells | Cell viability | ↑Nrf2, ↑HO-1, ↓IL6 | HG-treated rat GMCs | ↑Nrf2, ↓Keap1, ↑n-Nrf2, ↓n-CKIP-1, ↑ARE binding activity, ↑HO-1, ↑SOD1, ↓DHE, ↓H2O2, ↓FN, ↓ICAM-1 | |||||||||||||
| F344 rat kidneys transplanted Lewis rat | CRAD | OS | OS alleviation, kidney functional and morphological improvements | ↓MDA, ↓8-isoprostane, ↓ox-LDL, ↓8-oxo-dG, ↑SOD, ↑CAT, ↑GPx, ↑GR, ↑ γ-GCS, ↑Nrf2, ↑HO-1, ↑Nqo-1 | [80][151] | 15 | Resveratrol | Phytoalexin; red grapes (Vitis vinifera L.), peanuts (Arachis spp.), berries (Vaccinium spp.) | STZ-induced Wistar rat | DKD | OS | Anti-inflammation, Anti-OS | ↓iNOS, ↓NF-kB, ↓Nrf2, ↓NGAL, ↓IL-1β, ↓IL-6, ↓IL-8, ↓TNFα | [54 | |||
| 19 | Trigonelline | ] | [ | 125 | ] | ||||||||||||
| Alkaloid; traditional herbs (especially fenugreek), coffee bean, soybean, and other edible food plants | Oxalate-induced MDCK cells | EMT | Fibrosis | Attenuation of EMT, prevention of cell migration and ROS overproduction, | ↓FN, ↓vimentin, ↓α-SMA, | ↑ E-cadherin, ↑ZO-1, ↓MMP9, ↓ROS, ↑Nrf2 |
[81][152 | 4-hydroxy-2-hexenal-treated mouse cortical collecting duct cells (M1) | OS | ↓nuclear p65, ↑cytosol IkB, ↑SIRT1, ↓Nox4, ↓COX2, ↑AQP2, ↓pERK/ERK, ↓pJNK/JNK, ↓pP38/P38, ↓Nrf2, ↑Keap1 |
[55][126] | ||||||
| 16 | Rotenone | Isoflavonone; seeds and stems of jicama vine plant, the roots of Fabaceae, etc. | UUO-operated mice | Kidney fibrosis | Mitochondrial abnormality | Anti-OS, anti-inflammation, anti-fibrosis | ↓TBARS, ↓HO-1, ↓TNFα, ↓IL-1β, ↓ICAM1, ↓collagen I, ↓FN, ↓α-SMA, ↓PAI-1, ↓collagen III, ↓TGFβ, ↑mtDNA, ↑mtNd1 |
[56][127] | |||||||||
| 17 | Salidroside | phenylpropanoid glycoside; plant Rhodiola rosea | HG-treated mouse podocytes | Apoptosis | Apoptosis | Improvement of cell viability | ↓Caspase-9, ↓caspase-3, ↑HO-1, ↑p-ILK/ILK, ↑p-Akt/Akt, ↑p-ERK/ERK, ↑p-JNK/JNK, ↓p-p38/p38, ↑Nrf2 | [57][128] | |||||||||
| 18 | Salvianolic acid A | Polyphenol derivative; root of Salvia miltiorrhiza | STZ-injected mice | DKD | OS | Anti-OS | ↓VCAM-1, ↑HO-1, ↓α-SMA, ↓NT, ↓DHE, ↑GPx-1 |
[58][129] | |||||||||
| HG-treated HK-2 cells | ↑HO-1, ↓α-SMA, ↓p65, ↓ROS | ||||||||||||||||
| ] | 5/6 nephrectomized SD rats | CKD | OS | OS attenuation, | ↑t-SOD, ↑GPx, ↑CAT, ↓MDA, ↓ROS, ↓Nox4, ↑p-Akt/Akt, ↑p-GSK3β/GSK3β, ↑p-Nrf2/Nrf2, ↑HO-1 | ][130] | |||||||||||
| H2O2-treated/LPS-treated HK-2 cells | Cell viability improvement, decrease in OS | ↑t-SOD, ↑GPx, ↑CAT, ↓MDA, ↓ROS, ↓Nox4, ↑p-Akt/Akt, ↑p-GSK3β/GSK3β, ↑n-Nrf2, ↑HO-1, ↓p-NF-kB p65/NF-kB p65, ↓ICAM-1, ↓p-NF-kB p65, ↓ICAM-1, ↑n-Nrf2, ↑HO-1 | |||||||||||||||
| 19 | Silibinin | Flavonoliganas: milk thistle seeds | Arsenic -induced rat | CKD | Inflammation | Attenuation of OS, inflammation, and apoptosis | ↓TNFα, ↓iNOS, ↓NO, ↓NF-kB, ↓Caspase-3, ↓NADPH oxidase, ↑Nrf2 |
[60][131] | |||||||||
| 20 | Sinapnic acid | Hydroxycinnamic acid; wine, vinegar | STZ-injected rat | DKD | OS, inflammation | Amelioration of OS and inflammation | ↑CAT, ↑GPx, ↑SOD, ↓TNFα, ↓IL-6, ↓NO2, ↓MDA, ↓TFGβ, ↑HO-1, ↑Nrf2, ↓NF-kB, ↑IkBα, ↑Bcl2, ↓Caspase3, ↓Bax |
[61][132] | |||||||||
AGEs, advanced glycation end products; AngII, angiotensin II; α-SMA, α-smooth muscle actin; AT1/2R, angiotensin II receptor type 1/2; CAT, catalase; CIN, contrast induced nephropathy; CKD, chronic kidney disease; COX2, cyclooxygenase 2; CRAD, chronic renal allograft dysfunction; DHE, dihydroethidium; DKD, diabetic kidney disease; EMT, epithelial-to-mesenchymal transition; eNOS, endothelial nitric oxide synthase; FSGS, focal segmental glomerulosclerosis; γ-GCS, γ-glutamine cysteine synthase; GPx, glutathione peroxidase; GR, glutathione reductase; GSK3β, glycogen synthase kinase 3β; GST, Glutathione-S-transferase; HFD, high fat diet; HG, high glucose; HIF, hypoxia-inducible factor; HMGB1, high-mobility group box 1; HO-1, Heme oxygenase-1; iNOS, inducible nitric oxide synthase; LDH, lactate dehydrogenase; LN, lupus nephritis; LPS, lipopolysaccharide; MCP-1, monocyte chemoattractant protein-1; MDA, malondialdehyde; MDCK, Madin-Darby canine kidney; MMP, matrix metalloproteinase; NLRP3, NLR family pyrin domain containing 3; Nqo1, NADPH quinone oxidoreductase; Nrf2, nuclear factor erythroid 2-related factor 2; OS, oxidative stress; ox-LDL, oxidized low-density lipoprotein; RAS, renin-angiotensin system; SOD, superoxide dismutase; STZ, Streptozotocin; UUO, unilateral ureteral obstruction.
