A universal infrastructural issue is wetting of surfaces; millions of dollars are invested annually for rehabilitation and maintenance of infrastructures including roadways and buildings to fix the damages caused by moisture and frost. The biomimicry of the lotus leaf can provide superhydrophobic surfaces that can repel water droplets, thus reducing the penetration of moisture, which is linked with many deterioration mechanisms in infrastructures, such as steel corrosion, sulfate attack, alkali-aggregate reactions, and freezing and thawing. In cold-region countries, the extent of frost damage due to freezing of moisture in many components of infrastructures will be decreased significantly if water penetration can be minimized. Consequently, it will greatly reduce the maintenance and rehabilitation costs of infrastructures.
- biomimetic coating
- durability
- infrastructures
- lotus leaf
- self-cleaning
- superhydrophobicity
1. Introduction
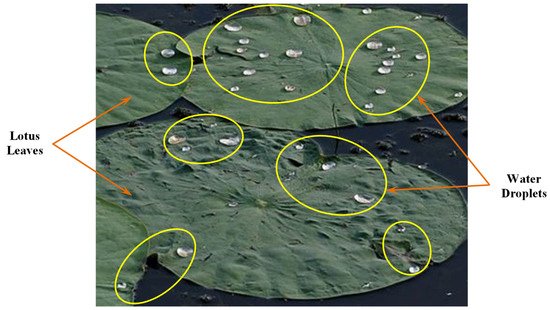
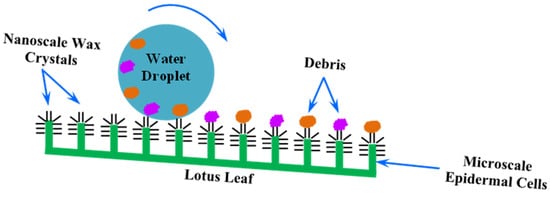
2. Surface Structure and Characteristics of Lotus Leaf

3. Lotus-Leaf-Inspired Biomimetic Coatings
| Coating Material | Key Characteristics | Specific Purposes | References | |||
|---|---|---|---|---|---|---|
| PDMS (Polydimethylsiloxane) | Intrinsic hydrophobic surface; remarkably high contact angle (close to 170°); sliding angle close to that of the lotus leaf; highly water-resistant; good self-cleaning property; chemically and thermally stable; stretchable. | Inverse-trapezoidal microstructures; microfabrication with micropillars/nanohairs. | [32,33,34,35] | [32][33][34][35] | ||
| UPC (Ultrafine powder coating) | At 3% PTFE (polytetrafluoroethylene): High contact angle (˃160°) and low sliding angle (˂5°); lower film thickness (controllable to 1 mil); reduced surface roughness; high-quality surface finishing. | Surface protection from moisture intervention | [13,36] | [13][36] | ||
| CNT (Carbon nanotube) film | Excellent anti-aging performance; effective to prevent the penetration of small water droplets; long-term durability after exposure to air and corrosive liquids. | Electrodes, biosensors, anti-fogging/icing and anti-aging materials. | [37,38] | [37][38] | ||
| Nickel (Ni), Ni/Nano-C, Ni/Nano-Cu | PFPE (perfluoropolyether) treated Ni: high contact angle (156°) and a rough surface; reduced friction coefficient; high hardness. Ni/Nano-C (Ni-C): better anti-corrosion performance. Ni/Nano-Cu (Ni-Cu): Large contact angle (155.5°) at optimal brush speed and a Cu concentration of 5 g/L; a sliding angle of 5°. |
Substrate protection; anti-corrosion surface coatings. | [39,40] | [39][40] | ||
| FOTS-TiO | 2 | (Fluoro-octyl-trichloro-silane-titanium) | Superamphiphobic (superhydro-oleophobic); high contact angle with peanut oil; liquid repellence with a surface tension as low as 23.8 mN/m; high thermal stability; self-cleaning; anti-fouling/anti-icing. | Surface treatment of materials and products (Zn plate, PU sponge, filter paper, cotton fibers, etc.); civil infrastructure maintenance; temperature sensitive nanotechnology applications. | [31,41] | [31][41] |
| Janus particles | Superhydrophobic performance with nanoscale roughness; covalent binding with substrate; tolerant to high water flushing speeds and organic solvents. | Nanoprobes, nanosensors, display systems, water-repellent textiles, drug delivery and control systems, functional coatings, etc. | [42,43] | [42][43] | ||
| Diamond-like carbon (DLC) | Balance of hardness and flexibility due to microstructures; high contact angle; low friction coefficient; greater corrosion resistance. | Bio-robotics, bio-medical devices, anti-corrosion surface coatings. | [44,45,46] | [44][45][46] | ||
| Micro- and nanosized silica (SiO | 2 | ) particles | Strong liquid (e.g., water, brine, acidic solution) repellency with a high contact angle and a low sliding angle for droplets; strong binding adhesion with underlying substrate; high weathering resistance including UV (ultraviolet) protection; high transmission of light with low reflection; excellent wear and scratch resistance. | Anti-abrasion, anti-corrosion, and waterproofing applications; surface coatings for self-cleaning and energy harvesting. | [47,48] | [47][48] |
| Calcium hydroxide [Ca(OH) | 2 | ] microcapsules with polymeric shell | Regenerative lotus effect—controllable via sodium stearate solution; good resistance to water flushing; strong binding adhesion to substrate; superior corrosion resistance in chloride environment. | Substrate modifications, corrosion-resistant coatings. | [49,50] | [49][50] |
| Graphene oxide-silica (GO-SiO | 2 | ) | Highly hydrophilic; superior barrier performance and corrosion protection; good binding adhesion with substrate. | Electrode, capacitor, and biosensor fabrication; anti-corrosion composite coatings. | [51,52,53] | [51][52][53] |
| Photopolymer (PP) | Transparent; anti-reflective and self-cleaning abilities; increased solar light absorbance; UV- or electron-beam curable; resistant to acidic and basic conditions. | Harvesting of alternative energy—coating on solar cells; protective coatings and decorative finishes; surface modifications of fibers and films; coatings for biosensors and electrodes. | [54,55] | [54][55] | ||
| Copper (Cu) | Superhydrophobic/superolephobic; hierarchical flowerlike surface morphology; long-term chemical stability; high contact angle for pure water as well as under both acidic and basic conditions. | Protection of steel surfaces; self-cleaning steel structures; oil pipelines for anti-fouling and low fluid drag. | [56,57] | [56][57] | ||
| Zinc oxide (ZnO) film | Can be either superhydrophobic or superhydrophilic depending on surface morphology; superhydrophobicity with a contact angle of 155° to more than 170°; superhydrophilicity with a low contact angle of approximately 1–2.8°; UV-stable. |
Self-cleaning PV (Photovoltaic) and glazing applications | [58,59,60] | [58][59][60] | ||
| Acrylic polymer (AP) | High water repellency; delayed ice nucleation; reduced binding adhesion with ice; lower freezing point of water. | Anti-icing coatings for pavement/building protection from frost damage; anti/de-icing systems for cars and airplanes, telecommunication antennas, or wind turbines. | [30,61] | [30][61] | ||
| Antimony doped tin oxide/polyurethane (ATO/PU) film | Superhydrophobicity and high heat-insulation; water contact angle up to about 155°; high visible light transmittance (76%); low infrared transmission; high thermal stability. | Self-cleaning solar cells; heat-insulating glass. | [62,63,64] | [62][63][64] | ||
| PMMA (Polymethyl methacrylate) | Increased PV efficiency (up to 17% gains); high optical transparency (˃80%); low reflection; chemically resistant to aqueous alkalis and most acids; high moisture resistance; protected from oxygen; UV-durable; abrasion-resistant. | Natural light harvesting for alternative energy; roofing membranes; balcony and parking deck surfacing and waterproofing applications. | [65,66] | [65][66] | ||
| PPS/PTFE (Polyphenylene sulfide/polytetrafluoroethylene) | Superamphiphobic; high contact angle (151–172°); excellent impact and wear resistance; low coefficient of friction; high cohesion and thermal stability; high anti-scaling ability. | Lubricant surface coatings; anti-scaling coatings. | [67,68,69,70] | [67][68][69][70] |
References
- Tetra Tech. Brine Impact Study on Roadway Concrete; Tech Canada Inc.: Edmonton, AB, Canada, 2019.
- You, Z.; Gilbertson, C.; Dam, T.V. Identifying Best Practices in Pavement Design, Materials, Construction, and Maintenance in Wet Freeze Climates Similar to Michigan; Report No. SPR-1666; Michigan Department of Transportation: Bay City, MI, USA, 2018.
- Gadi, Z. Measuring the Impacts of Climatic Exposure to Pavement Surface Deterioration with Low Cost Technology. Master’s Thesis, Department of Building, Civil and Environmental Engineering, Concordia University, Montreal, QC, Canada, 2018.
- Lövqvist, L.; Balieu, R.; Kringos, N. Freeze-thaw damage in asphalt: A set of simplified simulations. In Proceedings of the 63rd Annual Conference of Canadian Technical Asphalt Association, Regina, SK, Canada, 11–14 November 2018.
- Liu, Y. Impact of Freeze-Thaw and De-icer on the Structural and Functional Performance of Canadian Airport Asphalt Materials. Ph.D. Thesis, Department of Civil and Environmental Engineering, University of Waterloo, Waterloo, ON, Canada, 2021.
- Guo, Z.; Liu, W.; Su, B.-L. Superhyrophobic surfaces: From natural to biomimetic to functional. J. Colloid Interface Surf. 2021, 353, 335–355.
- Samaha, M.A.; Gad-el-Hak, M. Polymeric slippery coatings: Nature and applications. Polymers 2014, 6, 1266–1311.
- Zhang, D.; Wang, L.; Qian, H.; Li, X. Superhydrophobic surfaces for corrosion protection: A review of recent progresses and future directions. J. Coat. Technol. Res. 2016, 13, 11–29.
- Dicks, H. A new way of valuing nature—articulating biomimicry and ecosystem services. Environ. Ethics 2017, 39, 281–299.
- Hwang, J.; Jeong, Y.; Park, J.M.; Lee, K.H.; Hong, J.W.; Choi, J. Biomimetics: Forecasting the future of science, engineering, and medicine. Int. J. Nanomed. 2015, 10, 5701–5713.
- Strauss, S.D. The Big Idea: How Business Innovators Get Great Ideas to Market; Kaplan Financial Series; Dearborn Trade Publishing: Chicago, IL, USA, 2002.
- McKeag, T. How One Engineer’s Birdwatching Made Japan’s Bullet Train Better. GreenBiz. 2012. Available online: https://www.greenbiz.com (accessed on 29 December 2020).
- Mozumder, M.S.; Zhang, H.; Zhu, J. Mimicking lotus leaf: Development of micro-nanostructured biomimetic superhydrophobic polymeric surfaces by ultrafine powder coating technology. Macromol. Mater. Eng. 2011, 296, 929–936.
- Latthe, S.S.; Terashima, C.; Nakata, K.; Fujishima, A. Superhydrophobic surfaces developed by mimicking hierarchical surface morphology of lotus leaf. Molecules 2014, 19, 4256–4283.
- Zorba, V.; Stratakis, E.; Barberoglou, M.; Spanakis, E.; Tzanetakis, P.; Anastasiadis, S.H.; Fotakis, C. Biomimetic artificial surfaces quantitatively reproduce the water repellency of a lotus leaf. Adv. Mater. 2008, 20, 4049–4054.
- Xu, Q.; Zhang, W.; Dong, C.; Sreeprasad, T.S.; Xia, Z. Biomimetic self-cleaning surfaces: Synthesis, mechanism and applications. J. R. Soc. Interface 2016, 13, 20160300.
- Gonzalez, M.; Safiuddin, M.; Cao, J.; Tighe, S.L. Sound absorption and friction responses of nanoconcrete for rigid pavements. Transp. Res. Rec. 2013, 2369, 87–94.
- Ashby, M.; Ferreira, P.; Schodek, D. Nanomaterials, Nanotechnologies and Design: An Introduction for Engineers and Architects, 1st ed.; Butterworth-Heinemann Elsevier Ltd.: Oxford, UK, 2009.
- Gonzalez, M.; Safiuddin, M.; Cao, J.; Tighe, S. Sound absorption and friction properties of nano-lotus leaf coated concrete for rigid pavement. Mater. Sci. 2016, 22, 445–450.
- Koch, K.; Bhushan, B.; Jung, Y.C.; Barthlott, W. Fabrication of artificial lotus leaves and significance of hierarchical structure for superhydrophobicity and low adhesion. Soft Matter 2009, 5, 1386–1393.
- Gu, Y.; Zhang, W.; Mou, J.; Zheng, S.; Jiang, L.; Sun, Z.; Wang, E. Research progress of biomimetic superhydrophobic surface characteristics, fabrication, and application. Adv. Mech. Eng. 2017, 9, 1–13.
- Hossain, M.M.; Bhalum, Dhamrai, Dhaka, Bangladesh. Personal communication, 2021.
- Cheng, Y.-T.; Rodak, D.E.; Wong, C.A.; Hayden, C.A. Effects of micro- and nano-structures on the self-cleaning behaviour of lotus leaves. Nanotechnology 2006, 17, 1359–1362.
- Kim, W.; Kim, D.; Park, S.; Lee, D.; Hyun, H.; Kim, J. Engineering lotus leaf-inspired micro- and nanostructures for the manipulation of functional engineering platforms. J. Ind. Eng. Chem. 2018, 61, 39–52.
- Yamamoto, M.; Nishikawa, N.; Mayama, H.; Nonomura, Y.; Yokojima, S.; Nakamura, S.; Uchida, K. Theoretical explanation of the lotus effect: Superhydrophobic property changes by removal of nanostructures from the surface of a lotus leaf. Langmuir 2015, 31, 7355–7363.
- Safiuddin, M.; Hossain, K.; Collins, C.M. Potential applications of self-cleansing nano lotus leaf biomimicked coating in different construction sectors. In Proceedings of the 6th International Materials Specialty Conference, CSCE 2018 Annual Conference, Fredericton, NB, Canada, 13–16 June 2018.
- Poole, B. Biomimetics: Borrowing from Biology. Science Features. The Naked Scientists, 2007. Available online: https://www.thenakedscientists.com/articles/science-features/biomimetics-borrowing-biology (accessed on 29 December 2020).
- Ensikat, H.J.; Ditsche, P.; Neinhuis, C.; Barthlott, W. Superhydrophobicity in perfection: The outstanding properties of the lotus leaf. Beilstein J. Nanotechnol. 2011, 2, 152–161.
- Thistlewaite, J.; Henstra, D.; Peddle, S.; Scott, D. Canadian Voices on Changing Flood Risk: Findings from a National Survey; Faculty of Environment, Interdisciplinary Centre on Climate Change, and Partners for Action; University of Waterloo: Waterloo, ON, Canada, 2017.
- Cao, L.; Jones, A.K.; Sikka, V.K.; Wu, J.; Gao, D. Anti-icing superhydrophobic coatings. Langmuir 2009, 25, 12444–12448.
- Chen, L.; Guo, Z.; Liu, W. Biomimetic multi-functional superamphiphobic FOTS-TiO2 particles beyond lotus leaf. ACS Appl. Mater. Interfaces 2016, 8, 27188–27198.
- Cong, H.; Pan, T. Photopatternable conductive PDMS materials for microfabrication. Adv. Funct. Mater. 2008, 18, 1912–1921.
- Lee, S.M.; Kwon, T.H. Effects of intrinsic hydrophobicity on wettability of polymer replicas of a superhydrophobic lotus leaf. J. Micromech. Microeng. 2007, 14, 687–692.
- Pan, Z. Bio-inspired Oleophobic/Conductive Micro/Nano Structures and Their Applications in Frozen Oil Adhesion Reduction. Ph.D. Thesis, Department of Chemical Engineering, University of Waterloo, Waterloo, ON, Canada, 2016.
- Wu, Y.; Wang, J.; Zhang, D.; Li, L.; Zhu, Y. Preparation and characterization of superhydrophobic surface based on polydimethylsiloxane (PDMS). J. Adhes. Sci. Technol. 2019, 33, 1870–1881.
- Zhu, J.; Zhang, H. Ultrafine powder coatings: An innovation. Powder Coat. 2005, 16, 39–47.
- Li, H.; Cheng, K.; Zhang, Z.; Zhao, L.; Zhou, H.; Wang, H.; Li, Z. Effect of carbon nanotubes on aging properties of hydrogenated nitrile rubber in the dilute oxygen medium. J. Macromol. Sci. Part A 2021.
- Wang, P.; Zhao, T.; Bian, R.; Wang, G.; Liu, H. Robust superhydrophobic carbon nanotube film with lotus leaf mimetic multiscale hierarchical structures. ACS Nano 2017, 11, 12385–12391.
- Shafiei, M.; Alpas, A.T. Nanocrystalline nickel films with lotus leaf texture for superhydrophobic and low friction surfaces. Appl. Surf. Sci. 2009, 256, 710–719.
- Liu, H.; Wang, X.; Ji, H. Fabrication of lotus-leaf-like superhydrophobic surfaces via Ni-based nano-composite electro-brush plating. Appl. Surf. Sci. 2014, 288, 341–348.
- Prakash, P.; Satheesh, U.; Devaprakasam, D. Study of High Temperature Thermal Behavior of Alkyl and Perfluoroalkylsilane Molecules Self-Assembled on Titanium Oxide Nanoparticles. arXiv 2014, arXiv:1409.6823.
- Hu, J.; Zhou, S.; Sun, Y.; Fang, X.; Wu, L. Fabrication, properties and applications of Janus particles. Chem. Soc. Rev. 2012, 41, 4356–4378.
- Yang, H.; Liang, F.; Chen, Y.; Wang, Q.; Qu, X.; Yang, Z. Lotus leaf inspired robust superhydrophobic coating from strawberry-like Janus particles. NPG Asia Mater. 2015, 7, e176.
- Horiuchi, T.; Yoshida, K.; Kano, M.; Kumagai, M.; Suzuki, T. Evaluation of adhesion and wear resistance of DLC films deposited by various methods. Plasma Process. Polym. 2009, 6, 410–416.
- Sharma, R.; Barhai, P.K.; Kumari, N. Corrosion resistant behaviour of DLC films. Thin Solid Film. 2008, 516, 5397–5403.
- Wang, Y.; Wang, L.; Wang, S.; Wood, R.J.K.; Xue, Q. From natural lotus leaf to highly hard-flexible diamond-like carbon surface with superhydrophobic and good tribological performance. Surf. Coat. Technol. 2012, 206, 2258–2264.
- Ebert, D.; Bhushan, B. Durable lotus-effect surfaces with hierarchical structure using micro- and nanosized hydrophobic silica particles. J. Colloid Interface Sci. 2012, 368, 584–591.
- Li, J.; Zhou, L.; Yang, N.; Gao, C.; Zheng, Y. Robust superhydrophobic coatings with micro- and nano-composite morphology. RSC Adv. 2017, 7, 44234–44238.
- Rasitha, T.P.; Vanithakumari, S.C.; George, R.P.; Philip, J. Porous microcapsule-based regenerating superhydrophobic coating on 304L SS and its corrosion properties. J. Mater. Eng. Perform. 2019, 28, 7047–7057.
- Wang, Q.; Li, J.; Zhang, C.; Qu, X.; Liu, J.; Yang, Z. Regenerative superhydrophobic coating from microcapsules. J. Mater. Chem. 2010, 20, 3211–3215.
- Bai, X.L.; Wu, B.; Jiang, K.; Liu, C.J.; Zhang, H.; Zhang, F.; Huang, X.; Cai, M.J.; Wang, P.F. Preparation and properties of GO-SiO2 anticorrosive coatings. IOP Conf. Ser. Mater. Sci. Eng. 2019, 668, 012019.
- Kou, L.; Gao, C. Making silicananoparticle-covered graphene oxide nanohybrids as general building blocks for large-area superhydrophilic coatings. Nanoscale 2011, 3, 519–528.
- Ramezanzadeh, B.; Haeri, Z.; Ramezanzadeh, M. A facile route of making silica nanoparticles-covered graphene oxide nanohybrids (SiO2-GO); fabrication of SiO2-GO/epoxy composite coating with superior barrier and corrosion protection performance. Chem. Eng. J. 2016, 303, 511–528.
- Huang, Z.; Cai, C.; Kuai, L.; Li, T.; Huttula, M.; Cao, W. Leaf-structure patterning for antireflective and self-cleaning surfaces on Si-based solar cells. Sol. Energy 2018, 159, 733–741.
- Peiffer, R.W. Photopolymerization; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1997.
- Guo, F.; Su, X.; Hou, G.; Li, P. Bioinspired fabrication of stable and robust superhydrophobic steel surface with hierarchical flowerlike structure. Colloids Surf. A Physicochem. Eng. Asp. 2012, 401, 61–67.
- Tam, J.; Palumbo, G.; Erb, U. Recent advances in superhydrophobic electrodeposits. Materials 2016, 9, 151.
- Nundy, S.; Ghosh, A.; Mallick, T.K. Hydrophilic and superhydrophilic self-cleaning coatings by morphologically varying ZnO microstructures for photovoltaic and glazing applications. ACS Omega 2020, 5, 1033–1039.
- Upadhaya, D.; Purkayastha, D.D. Robust superhydrophobicity of ZnO thin films for self-cleaning applications. Mater. Today Proc. 2021, 46, 6339–6343.
- Wu, J.; Xia, J.; Lei, W.; Wang, B.P. A one-step method to fabricate lotus leaves-like ZnO film. Mater. Lett. 2011, 65, 477–479.
- Antonini, C.; Innocenti, M.; Horn, T.; Marengo, M.; Amirfazli, A. Understanding the effect of superhydrophobic coatings on energy reduction in anti-icing systems. Cold Reg. Sci. Technol. 2011, 67, 58–67.
- Dai, Z.; Li, Z.; Li, L.; Xu, G. Synthesis and thermal properties of antimony doped tin oxide/waterborne polyurethane nanocomposite film as heat-insulating materials. Polym. Adv. Technol. 2011, 22, 1905–1911.
- Feng, J.; Huang, B.; Zhong, M. Fabrication of superhydrophobic and heat-insulating antimony doped tin oxide/polyurethane films by cast replica micromolding. J. Colloid Interface Sci. 2009, 336, 268–272.
- Zhou, H.; Wang, H.; Tian, X.; Zheng, K.; Cheng, Q. Preparation and properties of waterborne polyurethane/antimony doped tin oxide nanocomposite coatings via sol-gel reactions. Polym. Compos. 2013, 35, 1169–1175.
- Goodrum, K. The PMMA revolution. Interface 2016, 12–15.
- Huang, Z.; Yang, S.; Zhang, H.; Zhang, M.; Cao, W. Replication of leaf surface structures for light harvesting. Sci. Rep. 2015, 5, 1–10.
- Qian, H.; Zhu, Y.; Wang, H.; Song, H.; Wang, C.; Liu, Z.; Li, H. Preparation and antiscaling performance of superhydrophobic poly (phenylene sulfide)/polytetrafluoroethylene composite coating. Ind. Eng. Chem. Res. 2017, 56, 12663–12671.
- Sun, N.; Qin, S.; Wu, J.; Cong, C.; Qiao, Y.; Zhou, Q. Bio-inspired superhydrophobic polyphenylene sulfide/polytetrafluoroethylene coatings with high performance. J. Nanosci. Nanotechnol. 2012, 12, 7222–7225.
- Wang, H.; Zhao, J.; Zhu, Y.; Meng, Y.; Zhu, Y. The fabrication, nano/micro-structure, heat- and wear-resistance of the superhydrophobic PPS/PTFE composite coatings. J. Colloid Interface Sci. 2013, 402, 253–258.
- Wang, H.; Yan, L.; Gao, D.; Liu, D.; Wang, C.; Sun, L.; Zhu, Y. Tribological properties of superamphiphobic PPS/PTFE composite coating in the oilfield produced water. Wear 2014, 319, 62–68.
