Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Saad Shaaban.
Organoselenium (OSe) compounds have recently gained considerable interest as a potential class of organic motifs due to their outstanding applications in synthetic organic and medicinal chemistry and their possible properties in materials science. These are attributed to the exceptional properties of the selenium (Se) element.
- polymerization
- organoselenium
- one-pot multicomponent
1. Introduction
Organoselenium (OSe) compounds have recently gained considerable interest as a potential class of organic motifs due to their outstanding applications in synthetic organic and medicinal chemistry and their possible properties in materials science [1,2,3,4][1][2][3][4]. These are attributed to the exceptional properties of the selenium (Se) element. The latter is a non-metal present in almost living organisms as part of selenoproteins (e.g., thioredoxin reductases and glutathione peroxidase antioxidants enzymes) [5,6,7,8,9][5][6][7][8][9]. Accordingly, Se is crucial for protecting human cells from oxidative damage and the immune system’s normal function [10,11,12][10][11][12]. Compared to sulfur (S), Se has a larger atomic radius (S: 1.02 Å vs. Se: 1.17 Å), lower electronegativity (S: 2.58 vs. Se: 2.55), higher polarizability (S: 2.9 Å vs. Se: 3.8 Å), and therefore Se is a likely better nucleophile than the S [5,8,13][5][8][13]. Accordingly, OSe compounds are known for their ability to react with O2-free radicals and thus prevent the progression of oxidative stress-related diseases [3,6,10,14][3][6][10][14]. Furthermore, OSe compounds were also used in material science due to their semiconductor potential and therefore used in sodium-ion batteries, solar cells, and H2 evolution catalysts [2,15,16,17][2][15][16][17]. Moreover, the Se center is present in many naturally occurring and bioactive interesting OSe compounds (Figure 1), such as the selenoaminoacids (e.g., selenocysteine (I), selenomethionine (II), and selenocystine (III)) [18,19,20][18][19][20]. Furthermore, ebselen (IV) is the most investigated Se compound with exciting GPX-like activity and has recently reached clinical phase III trials as a possible drug for Meniere’s disease [11,21,22][11][21][22] (Figure 1). Moreover, ethaselen (V) entered trial phase II for non-small lung cancer treatment [23,24,25,26][23][24][25][26]. On the other hand, different OSe compounds have also manifested efficient catalytic activity for various organic reactions, such as the palladium-based OSe complex VI for the Heck reaction (Figure 1) [27].
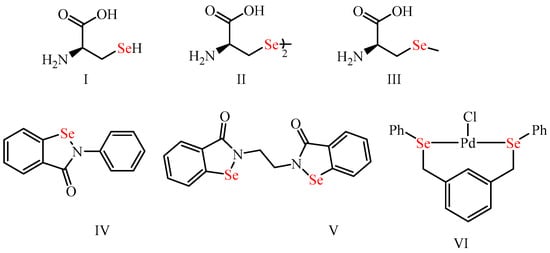
Figure 1.
Organoselenium compounds (
I
–
VI) with potential biological activities and diverse applications.
) with potential biological activities and diverse applications.
Given the exciting activities and the diverse applications of the OSe compounds, sustainable and efficient approaches for their preparation are in high demand. The synthesis of OSe compounds depends on their chemical structures (e.g., selenides, selenocyanates, diselenides) [26,28,29][26][28][29]. Standard methods include direct selenylation via reaction with proper Se reagents such as Na2Se2 and KSeCN. On the other hand, indirect selenylations include rearrangement of Se-containing precursors (e.g., isoselenocyanates) or multistep synthetic procedures using elemental Se together with organolithium or Grignard reagents and [2,13,24,30,31,32,33,34,35][2][13][24][30][31][32][33][34][35]. Despite being efficient, these classical strategies are relatively limited due to the challenges associated with the complicated synthetic procedure, regioselectivity, or harsh reaction conditions issues. Recently, various alternative reactions were developed as efficient and milder synthetic protocols within combinatorial chemistry (CC) [36,37,38,39,40,41,42,43][36][37][38][39][40][41][42][43]. The latter has emerged as a robust tool in medicinal chemistry [44,45][44][45]. It is now widely and consecutive covalent bonds formed between different building blocks [46]. Concurrently, drug candidates are discovered and selected by the screening of small molecule libraries for particular biological targets [47]. Despite the various strategies used in CC, multicomponent reactions (MCRs) are amongst the most investigated techniques for the efficient synthesis of chemical libraries [43,45][43][45]. MCRs have been known for over a century. They include generating skeletally diverse and complex molecular entities from more than two starting materials by straightforward chemical transformations employing comparatively mild conditions [37,48,49,50][37][48][49][50].
From an economical step and atom viewpoint, the MCR one-pot strategy would offer more robust access to OSe compounds. Though the MCRs have emerged as an efficient tool for constructing C-S bonds, similar approaches for synthesizing C-Se bonds have recently attracted more attention.
2. Metal-Catalyzed Synthesis of the OSe Compounds
In 2013, de Oliveira et al. reported the one-pot Cu-catalyzed (CuCl) synthesis of OSe propargylamines in excellent yields (up to 95%) via A3-coupling of trimethylsilyl Se-acetylene, p-methoxybenzaldehyde, and piperidine catalyzed in DCM as the solvent and in the presence of succinic acid additive at 50 °C (Scheme 1) [51].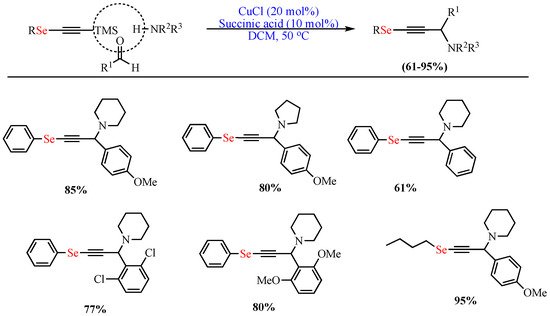
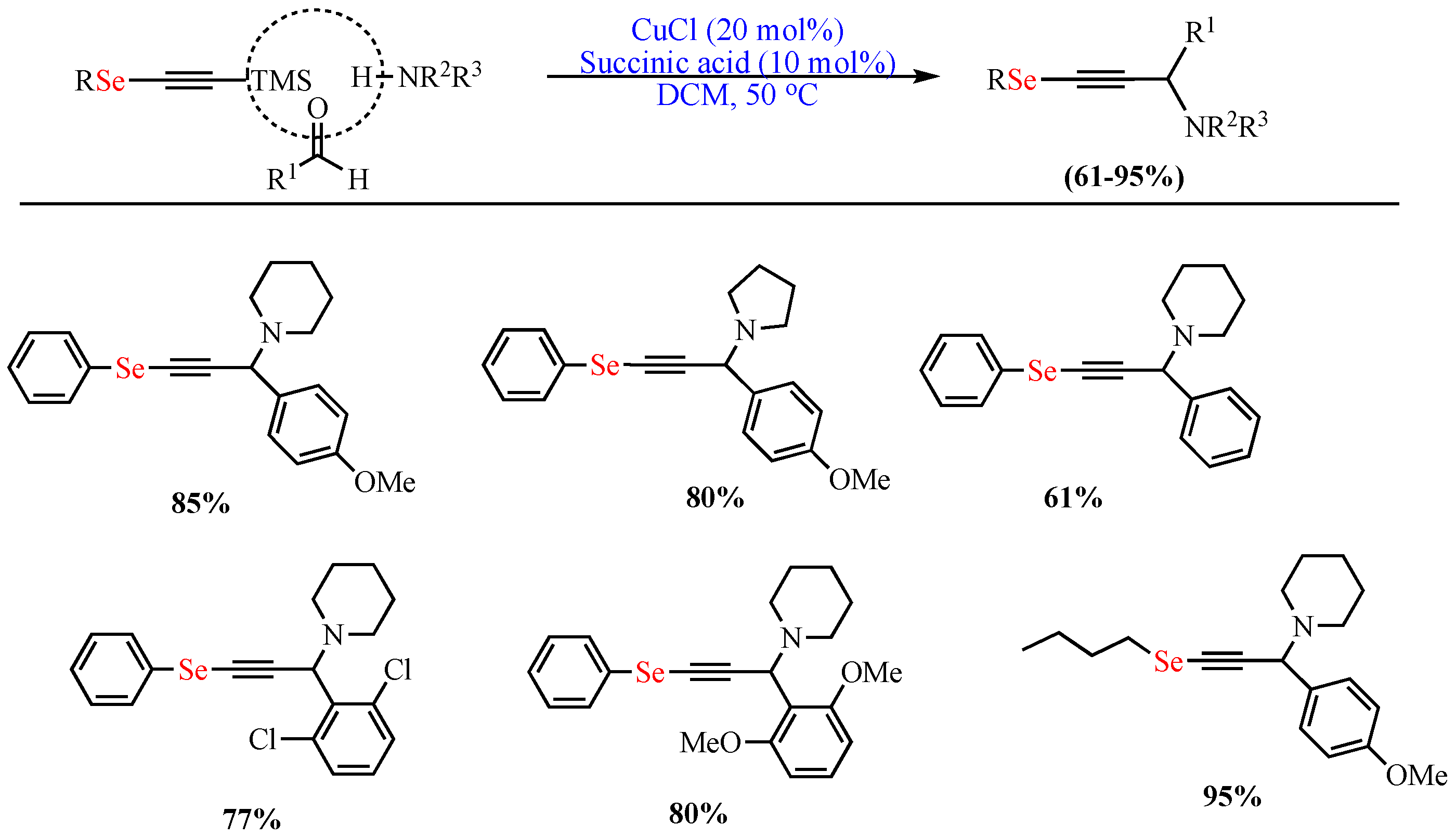
Scheme 1. Synthesis of OSe propargylamines.
Synthesis of OSe propargylamines.
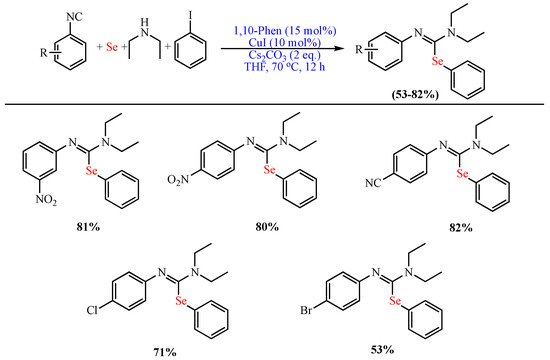
Scheme 2. Synthesis of functionalized isoselenoureas.
Synthesis of functionalized isoselenoureas.
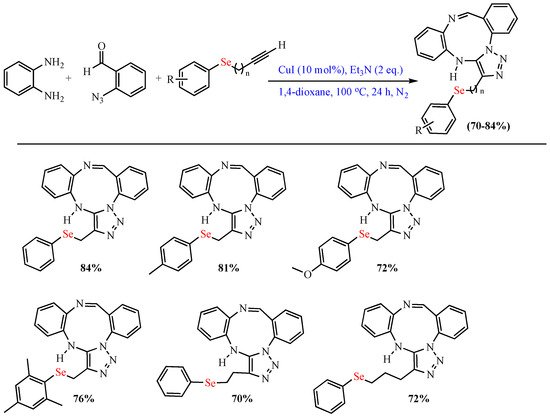
Scheme 3. Synthesis of (arylselanyl)-alkyl-1,2,3-triazolo-1,3,6-triazonines.
Synthesis of (arylselanyl)-alkyl-1,2,3-triazolo-1,3,6-triazonines.
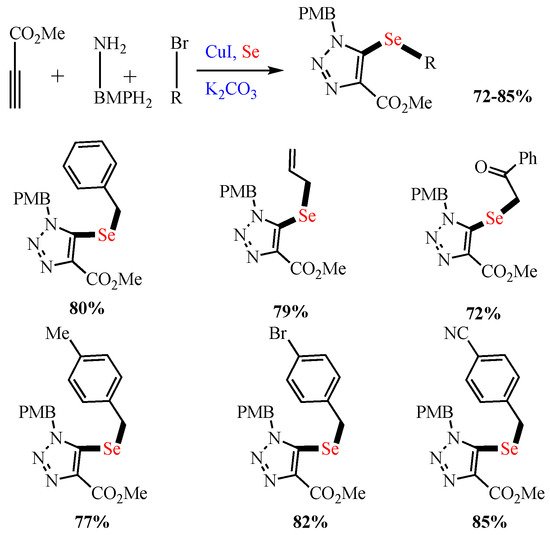
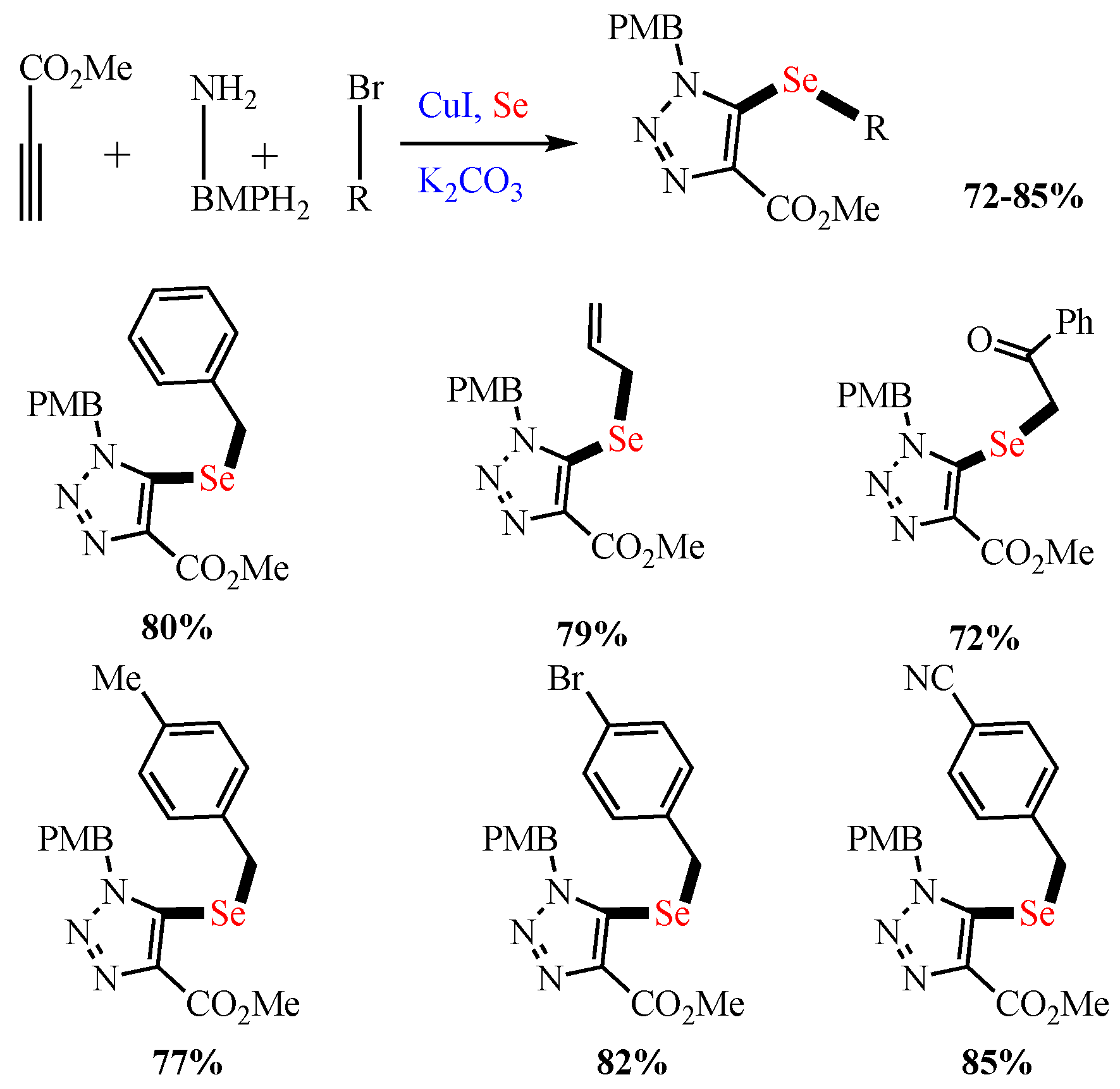
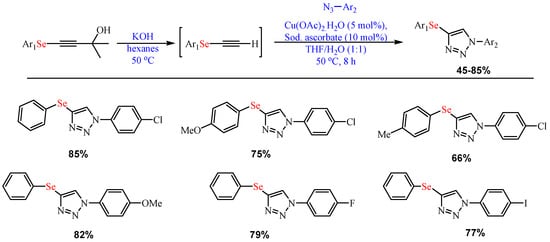
Scheme 4. Synthesis of 5-selenotriazoles.
Synthesis of 5-selenotriazoles.
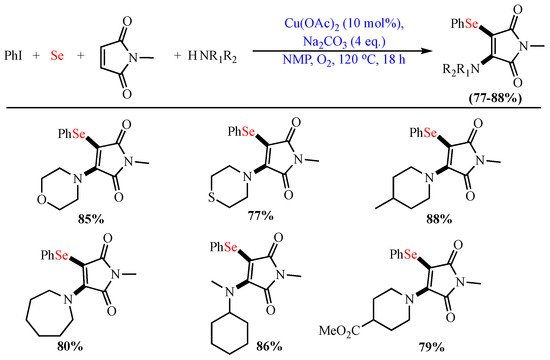
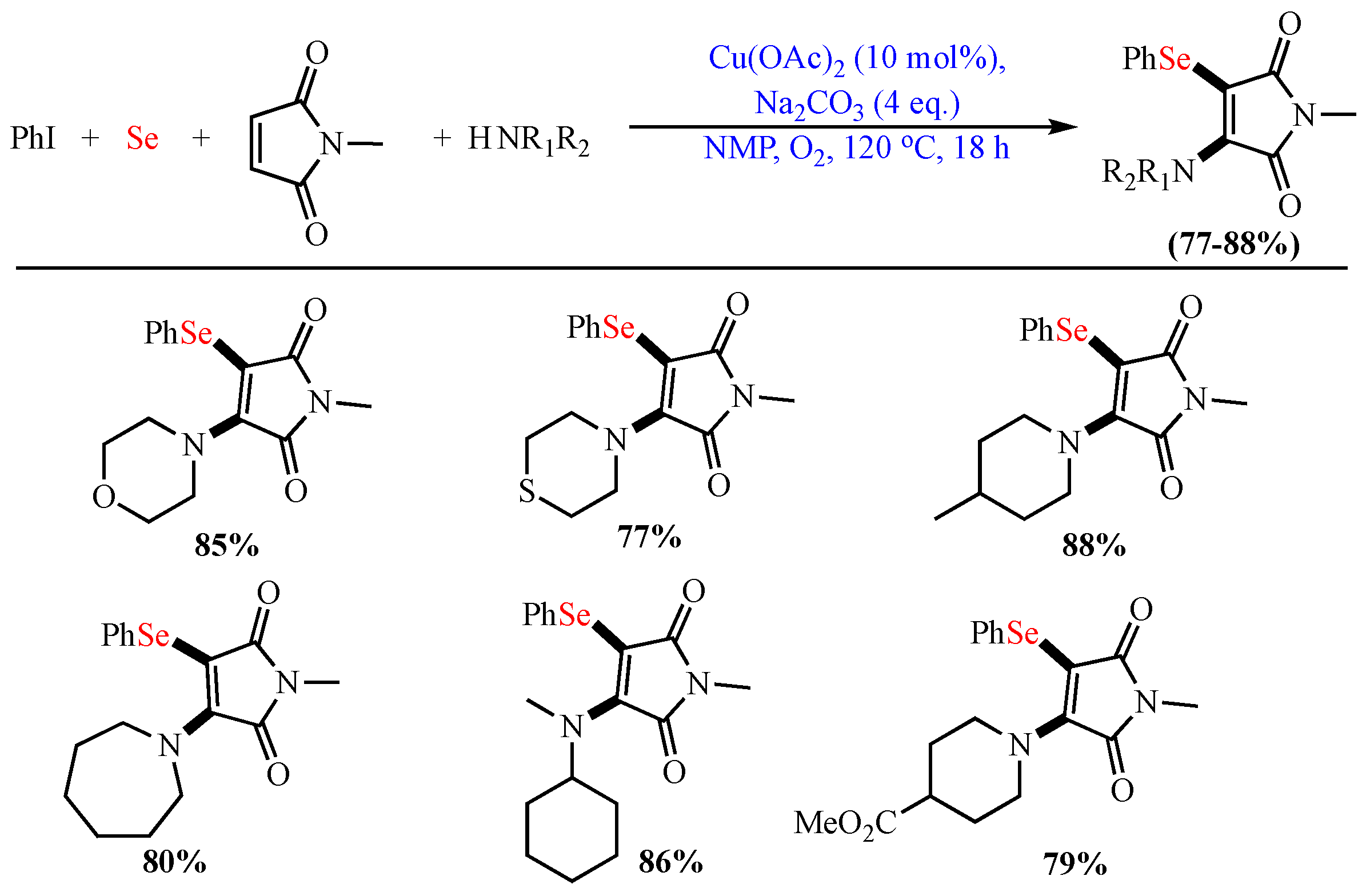
Scheme 5. Aminoarylselenation of maleimides.
Aminoarylselenation of maleimides.
Scheme 6.
Synthesis of 4-arylselanyl-1
H-1,2,3-triazoles.
-1,2,3-triazoles.
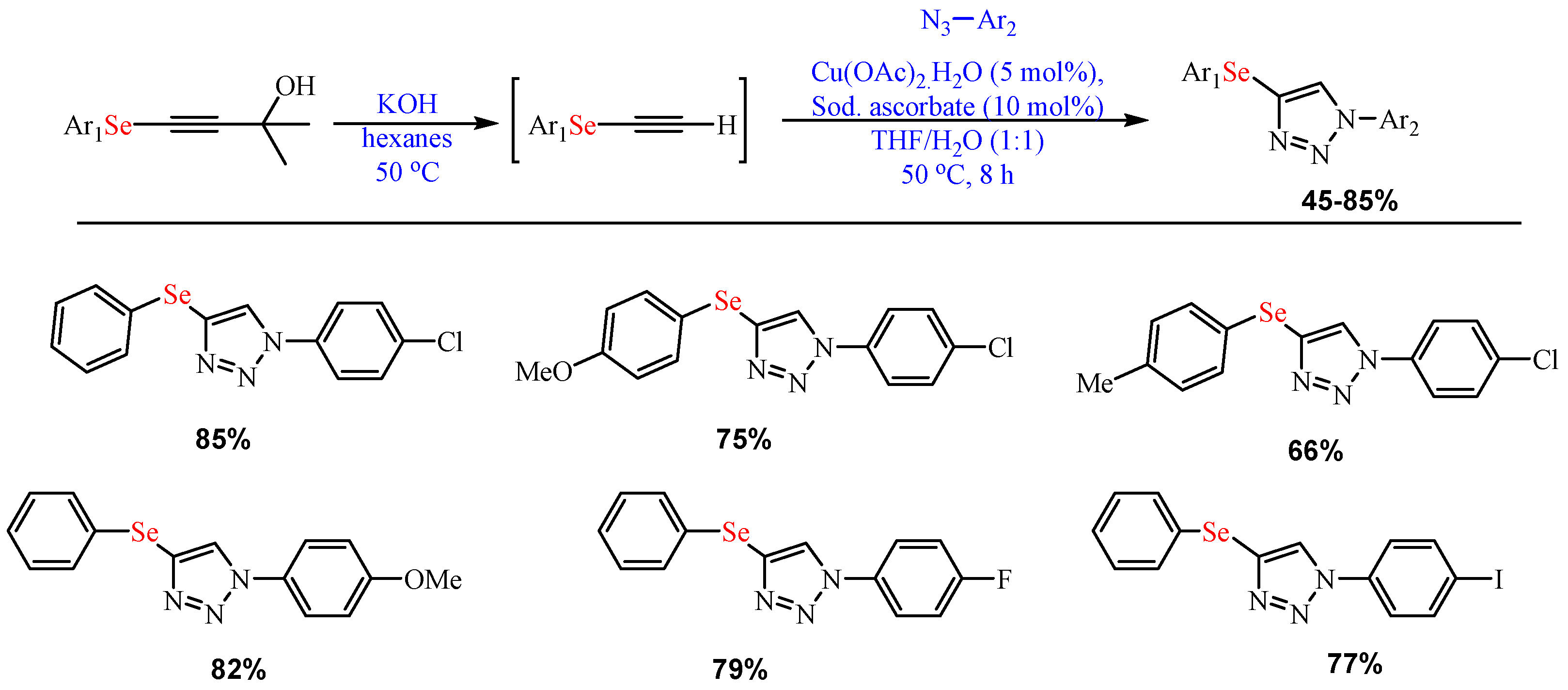
Scheme 7.
Synthesis of 3-((arylethynyl)selanyl)-1
H-indoles.
-indoles.
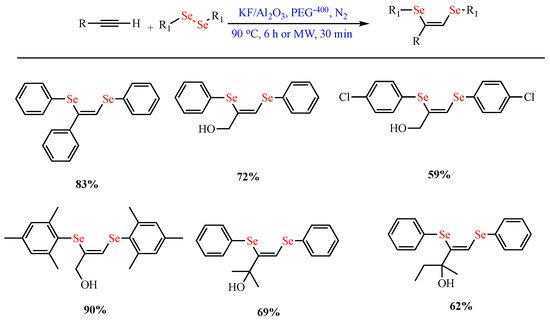
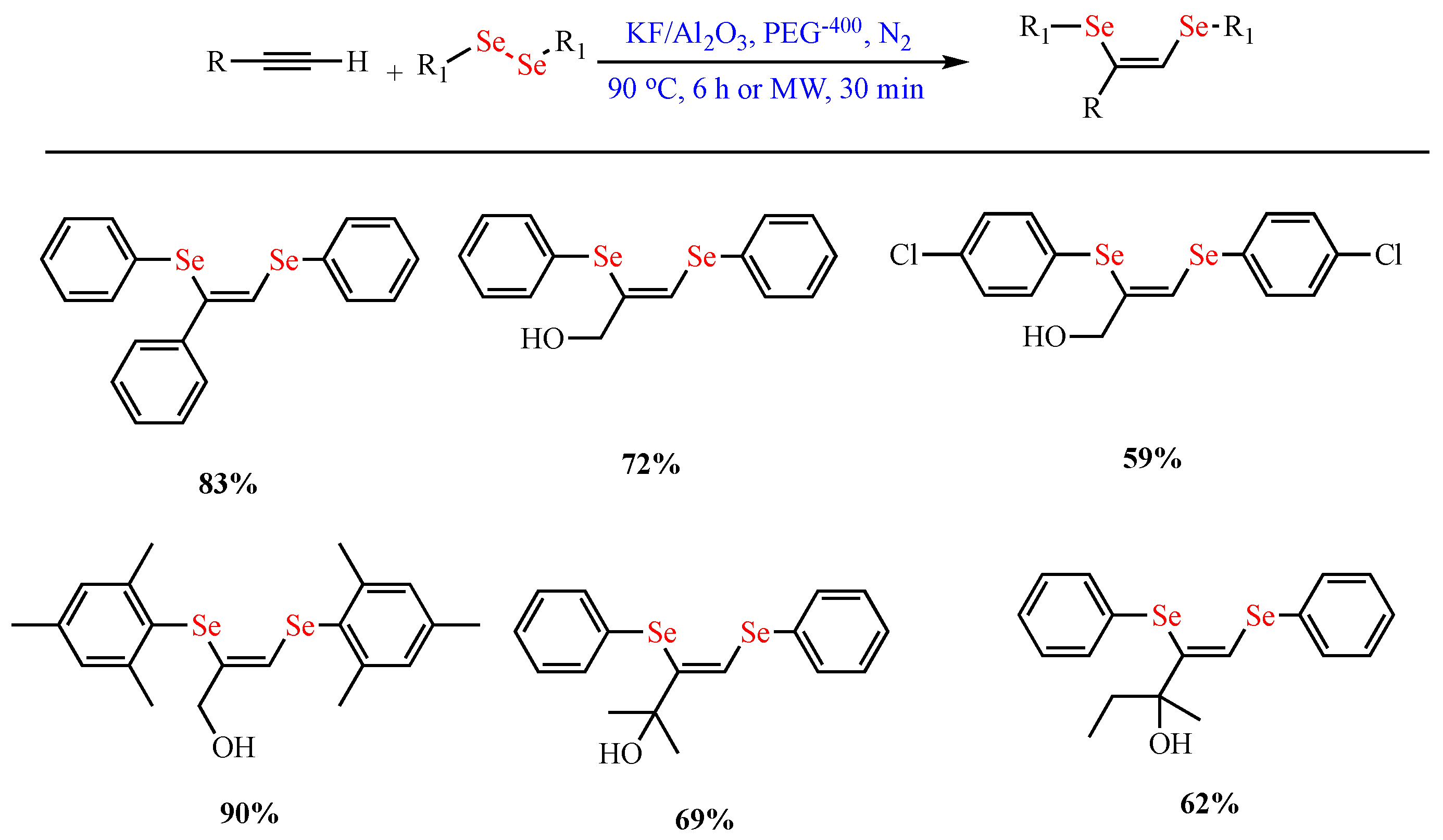
Scheme 8.
Synthesis of (
Z)-1,2-bis-arylselanyl alkenes.
)-1,2-bis-arylselanyl alkenes.
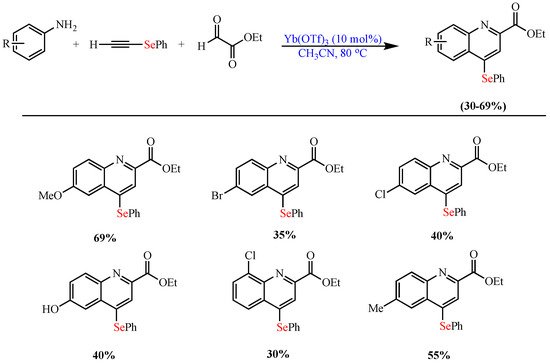
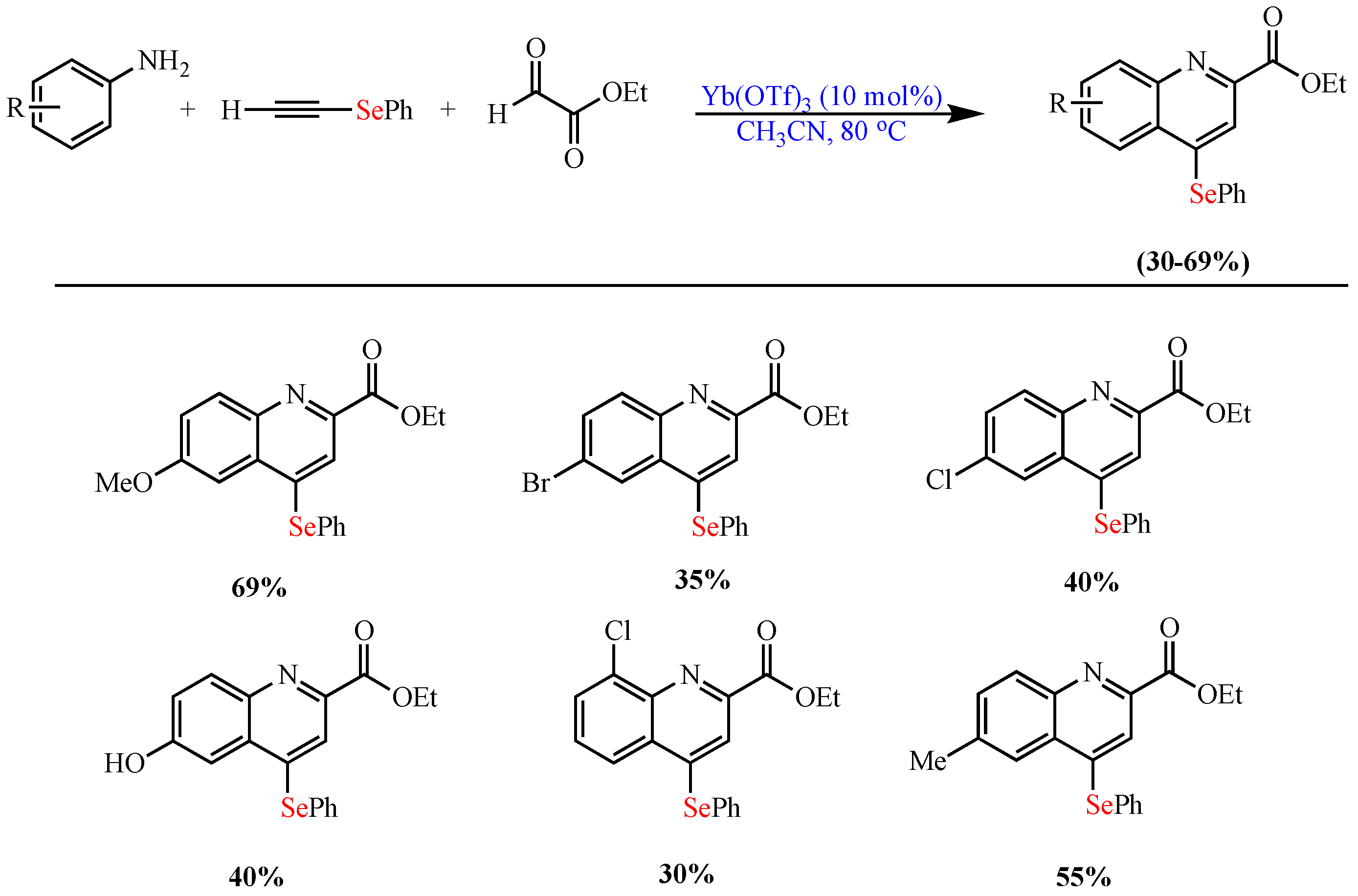
Scheme 9. Synthesis of 2,4- disubstituted Se-quinoline derivatives.
Synthesis of 2,4- disubstituted Se-quinoline derivatives.
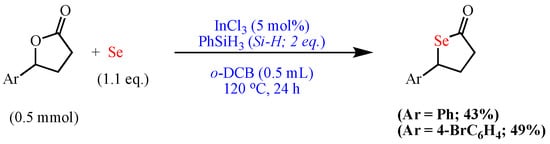
Scheme 10. Synthesis of selenolactones.
Synthesis of selenolactones.
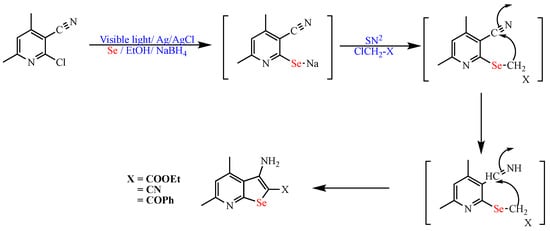
Scheme 11. Synthesis of seleno [2,3-b]pyridine derivatives.
Synthesis of seleno [2,3-b]pyridine derivatives.
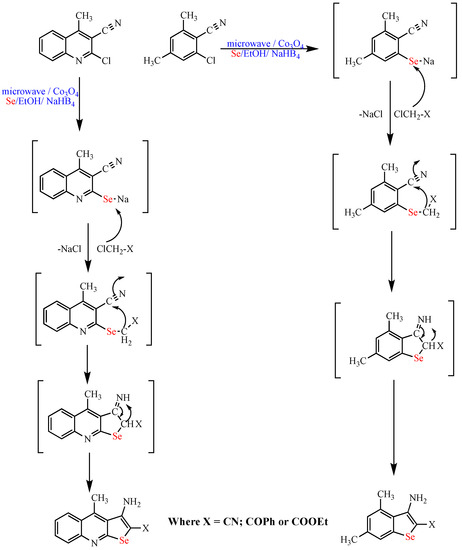
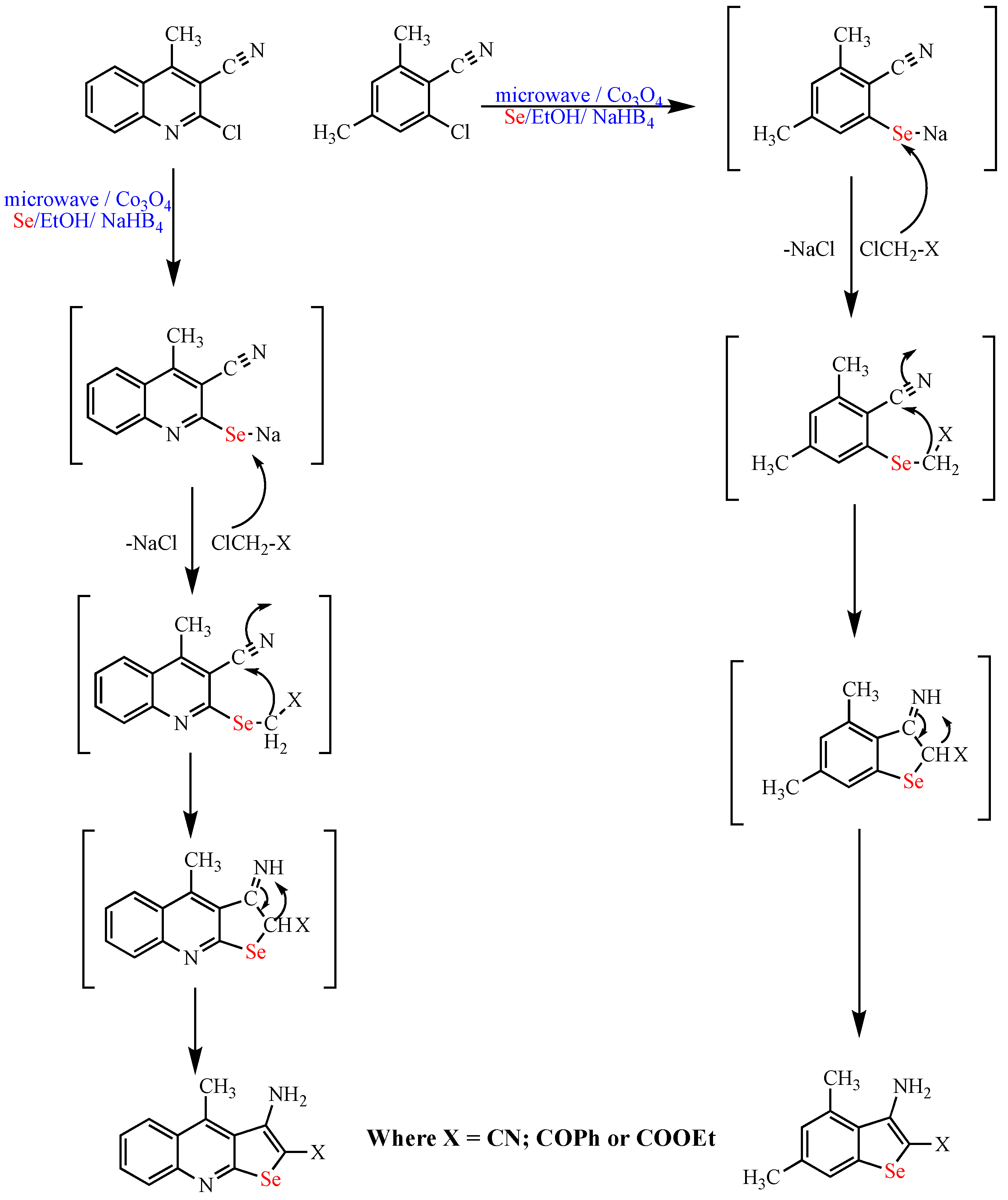
Scheme 12. Synthesis of selenopyridine and quinoline derivatives.
Synthesis of selenopyridine and quinoline derivatives.
References
- Kalimuthu, K.; Keerthana, C.K.; Mohan, M.; Arivalagan, J.; Christyraj, J.R.S.S.; Firer, M.A.; Choudry, M.H.A.; Anto, R.J.; Lee, Y.J. The emerging role of selenium metabolic pathways in cancer: New therapeutic targets for cancer. J. Cell. Biochem. 2022, 123, 532–542.
- Phadnis, P.P. Synthesis Strategies for Organoselenium Compounds and Their Potential Applications in Human Life. In Handbook on Synthesis Strategies for Advanced Materials; Springer: Berlin/Heidelberg, Germany, 2021; pp. 537–641.
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawska, A.; Bielawski, K. Selenium as a Bioactive Micronutrient in the Human Diet and Its Cancer Chemopreventive Activity. Nutrients 2021, 13, 1649.
- Handy, D.E.; Joseph, J.; Loscalzo, J. Selenium, a Micronutrient that Modulates Cardiovascular Health via Redox Enzymology. Nutrients 2021, 13, 3238.
- Schomburg, L. The other view: The trace element selenium as a micronutrient in thyroid disease, diabetes, and beyond. Hormones 2020, 19, 15–24.
- Xu, J.; Gong, Y.; Sun, Y.; Cai, J.; Liu, Q.; Bao, J.; Yang, J.; Zhang, Z. Impact of Selenium Deficiency on Inflammation, Oxidative Stress, and Phagocytosis in Mouse Macrophages. Biol. Trace Elem. Res. 2020, 194, 237–243.
- Li, B.; Li, W.; Tian, Y.; Guo, S.; Qian, L.; Xu, D.; Cao, N. Selenium-Alleviated Hepatocyte Necrosis and DNA Damage in Cyclophosphamide-Treated Geese by Mitigating Oxidative Stress. Biol. Trace Elem. Res. 2020, 193, 508–516.
- Singh, F.V.; Wirth, T. Selenium reagents as catalysts. Catal. Sci. Technol. 2019, 9, 1073–1091.
- Sanmartín, C.; Ruberte, A.C.; Ibáñez, E.; Moreno, E.; Espuelas, S.; Plano, D. Selenium Entities: Promising Scaffolds for the Treatment of Cancer and Leishmania. Curr. Org. Synth. 2017, 14, 1075–1081.
- Bevinakoppamath, S.; Ahmed, A.M.S.; Ramachandra, S.C.; Vishwanath, P.; Prashant, A. Chemopreventive and Anticancer Property of Selenoproteins in Obese Breast Cancer. Front. Pharmacol. 2021, 12, 618172.
- Nogueira, C.W.; Barbosa, N.V.; Rocha, J.B.T. Toxicology and pharmacology of synthetic organoselenium compounds: An update. Arch. Toxicol. 2021, 95, 1179–1226.
- Shaaban, S.; Zarrouk, A.; Vervandier-Fasseur, D.; Al-Faiyz, Y.S.; El-Sawy, H.; Althagafi, I.; Andreoletti, P.; Cherkaoui-Malki, M. Cytoprotective organoselenium compounds for oligodendrocytes. Arab. J. Chem. 2021, 14, 103051.
- Chen, Z.; Lai, H.; Hou, L.; Chen, T. Rational design and action mechanisms of chemically innovative organoselenium in cancer therapy. Chem. Commun. 2020, 56, 179–196.
- Bartolini, D.; Sancineto, L.; de Bem, A.F.; Tew, K.D.; Santi, C.; Radi, R.; Toquato, P.; Galli, F. Selenocompounds in Cancer Therapy: An Overview. Adv. Cancer Res. 2017, 136, 259–302.
- Zhu, E.; Fu, L.; Lu, Y.; Jiang, W.; Jee, M.H.; Liu, R.; Li, Z.; Che, G.; Woo, H.Y.; Liu, C. NIR-Absorbing Electron Acceptor Based on a Selenium-Heterocyclic Core Attaching to Phenylalkyl Side Chains for Polymer Solar Cells with 17.3% Efficiency. ACS Appl. Mater. Interfaces 2022, 14, 7082–7092.
- Fu, Y.; Sun, Y.; Tang, H.; Wang, L.; Yu, H.; Cao, D. Selenium-containing D-A-D-type dopant-free hole transport materials for perovskite solar cells. Dye. Pigment. 2021, 191, 109339.
- Thomas, J.; Dong, Z.; Dehaen, W.; Smet, M. Selenium/Tellurium-Containing Hyperbranched Polymers: Effect of Molecular Weight and Degree of Branching on Glutathione Peroxidase-Like Activity. Macromol. Rapid Commun. 2012, 33, 2127–2132.
- Pons, D.G.; Moran, C.; Alorda-Clara, M.; Oliver, J.; Roca, P.; Sastre-Serra, J. Micronutrients Selenomethionine and Selenocysteine Modulate the Redox Status of MCF-7 Breast Cancer Cells. Nutrients 2020, 12, 865.
- Cardoso, B.R.; Roberts, B.R.; Bush, A.I.; Hare, D.J. Selenium, selenoproteins and neurodegenerative diseases. Metallomics 2015, 7, 1213–1228.
- Metanis, N.; Hilvert, D. Natural and synthetic selenoproteins. Curr. Opin. Chem. Biol. 2014, 22, 27–34.
- Benelli, J.L.; Poester, V.R.; Munhoz, L.S.; Melo, A.M.; Trápaga, M.R.; Stevens, D.A.; Xavier, M.O. Ebselen and diphenyl diselenide against fungal pathogens: A systematic review. Med. Mycol. 2021, 59, 409–421.
- Weglarz-Tomczak, E.; Tomczak, J.M.; Talma, M.; Brul, S. Ebselen as a highly active inhibitor of PLProCoV2. bioRxiv 2020.
- Wang, L.; Yang, Z.; Fu, J.; Yin, H.; Xiong, K.; Tan, Q.; Jin, H.; Li, J.; Wang, T.; Tang, W.; et al. Ethaselen: A potent mammalian thioredoxin reductase 1 inhibitor and novel organoselenium anticancer agent. Free Radic. Biol. Med. 2012, 52, 898–908.
- Shaaban, S.; Ashmawy, A.M.; Negm, A.; Wessjohann, L.A. Synthesis and biochemical studies of novel organic selenides with increased selectivity for hepatocellular carcinoma and breast adenocarcinoma. Eur. J. Med. Chem. 2019, 179, 515–526.
- Shaaban, S.; Vervandier-Fasseur, D.; Andreoletti, P.; Zarrouk, A.; Richard, P.; Negm, A.; Manolikakes, G.; Jacob, C.; Cherkaoui-Malki, M. Cytoprotective and antioxidant properties of organic selenides for the myelin-forming cells, oligodendrocytes. Bioorganic Chem. 2018, 80, 43–56.
- Shaaban, S.; Negm, A.; Sobh, M.A.; Wessjohann, L.A. Organoselenocyanates and symmetrical diselenides redox modulators: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2015, 97, 190–201.
- Yao, Q.; Kinney, A.E.P.; Zheng, C. Selenium-Ligated Palladium (II) Complexes as Highly Active Catalysts for Carbon−Carbon Coupling Reactions: The Heck Reaction. Org. Lett. 2004, 6, 2997–2999.
- Shaaban, S.; Diestel, R.; Hinkelmann, B.; Muthukumar, Y.; Verma, R.P.; Sasse, F.; Jacob, C. Novel peptidomimetic compounds containing redox active chalcogens and quinones as potential anticancer agents. Eur. J. Med. Chem. 2012, 58, 192–205.
- Shaaban, S.; Arafat, M.A.; Gaffer, H.E.; Hamama, W.S. Synthesis and anti-tumor evaluation of novel organoselenocyanates and symmetrical diselenides dyestuffs. Pharma Chem. 2014, 6, 186–193.
- El-Lateef, H.M.A.; Shaaban, S.; Khalaf, M.M.; Toghan, A.; Shalabi, K. Synthesis, experimental, and computational studies of water soluble anthranilic organoselenium compounds as safe corrosion inhibitors for J55 pipeline steel in acidic oilfield formation water. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 625, 126894.
- Ma, Y.T.; Liu, M.C.; Zhou, Y.B.; Wu, H.Y. Synthesis of Organoselenium Compounds with Elemental Selenium. Adv. Synth. Catal. 2021, 363, 5386–5406.
- Zhao, X.; Liao, L. Modern Organoselenium Catalysis: Opportunities and Challenges. Synlett 2021, 32, 1262–1268.
- Lenardão, E.J.; Santi, C.; Sancineto, L. New Frontiers in Organoselenium Compounds; Springer: Berlin/Heidelberg, Germany, 2018.
- Jain, V.K.; Priyadarsini, K.I. Organoselenium Compounds in Biology and Medicine; The Royalty Society of Chemistry: London, UK, 2017.
- Shaaban, S.; Arafat, M.A.; Hamama, W.S. Vistas in the domain of organo selenocyanates. Arkivoc 2014, i, 470–505.
- Khan, T.; Ahmad, R.; Azad, I.; Raza, S.; Joshi, S.; Khan, A.R. Computer-aided drug design and virtual screening of targeted combinatorial libraries of mixed-ligand transition metal complexes of 2-butanone thiosemicarbazone. Comput. Biol. Chem. 2018, 75, 178–195.
- Shaaban, S.; Abdel-Wahab, B.F. Groebke-Blackburn-Bienaymé multicomponent reaction: Emerging chemistry for drug discovery. Mol. Divers. 2016, 20, 233–254.
- Wang, S.-L.; Zhang, G.; Jie, D.; Jiang, B.; Wang, X.-H.; Tu, S.-J. Microwave-Assisted Multicomponent Reactions: Rapid and Regioselective Formation of New Extended Angular Fused Aza-Heterocycles. Comb. Chem. High Throughput Screen. 2012, 15, 400–410.
- Dömling, A.; Beck, B.; Fuchs, T.; Yazbak, A. Parallel Synthesis of Arrays of Amino-Acid-Derived Isocyanoamides Useful as Starting Materials in IMCR. J. Comb. Chem. 2006, 8, 872–880.
- Ulaczyk-Lesanko, A.; Hall, D.G. Wanted: New multicomponent reactions for generating libraries of polycyclic natural products. Curr. Opin. Chem. Biol. 2005, 9, 266–276.
- Pirrung, M.C.; Das Sarma, K. Multicomponent Reactions Are Accelerated in Water. J. Am. Chem. Soc. 2004, 126, 444–445.
- Kolb, J.; Beck, B.; Almstetter, M.; Heck, S.; Herdtweck, E.; Domling, A. New MCRs: The first 4-component reaction leading to 2,4-disubstituted thiazoles. Mol. Divers. 2003, 6, 297–313.
- Ugi, I.; Heck, S. The multicomponent reactions and their libraries for natural and preparative chemistry. Comb. Chem. High Throughput Screen. 2001, 4, 1–34.
- Burchak, O.N.; Mugherli, L.; Ostuni, M.; Lacapère, J.J.; Balakirev, M.Y. Combinatorial Discovery of Fluorescent Pharmacophores by Multicomponent Reactions in Droplet Arrays. J. Am. Chem. Soc. 2011, 133, 10058–10061.
- Domling, A. The discovery of new isocyanide-based multi-component reactions. Curr. Opin. Chem. Biol. 2000, 4, 318–323.
- Suay-García, B.; Bueso-Bordils, J.I.; Falcó, A.; Antón-Fos, G.M.; Alemán-López, P.A. Virtual Combinatorial Chemistry and Pharmacological Screening: A Short Guide to Drug Design. Int. J. Mol. Sci. 2022, 23, 1620.
- Kennedy, J.P.; Williams, L.; Bridges, T.M.; Daniels, R.N.; Weaver, D.; Lindsley, C.W. Application of Combinatorial Chemistry Science on Modern Drug Discovery. J. Comb. Chem. 2008, 10, 345–354.
- Semreen, M.H.; El-Awady, R.; Abu-Odeh, R.A.; Saber-Ayad, M.; Al-Qawasmeh, R.; Chouaib, S.; Voelter, W.; Al-Tel, T.H. Tandem Multicomponent Reactions Toward the Design and Synthesis of Novel Antibacterial and Cytotoxic Motifs. Curr. Med. Chem. 2013, 20, 1445–1459.
- Chéron, N.; Ramozzi, R.; El Kaïm, L.; Grimaud, L.; Fleurat-Lessard, P. Challenging 50 Years of Established Views on Ugi Reaction: A Theoretical Approach. J. Org. Chem. 2012, 77, 1361–1366.
- Gulevich, A.V.; Zhdanko, A.G.; Orru, R.V.A.; Nenajdenko, V.G. Synthetic Application of Isocyanoacetic Acid Derivatives. In Isocyanide Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 109–158.
- de Oliveira, I.M.; Pimenta, D.C.; Zukerman-Schpector, J.; Stefani, H.A.; Manarin, F. Copper(i)/succinic acid cooperatively catalyzed one-pot synthesis of organoselenium-propargylamines via A3-coupling. New J. Chem. 2018, 42, 10118–10123.
- Liu, H.; Fang, Y.; Yin, L.; Wang, S.-Y.; Ji, S.-J. Copper(I)-Catalyzed Ligand-Promoted Multicomponent Reactions of Isocyanides, Selenium, Amines, and Iodoarenes: Access to Highly Functionalized Carbamimidoselenoates. J. Org. Chem. 2017, 82, 10866–10874.
- Aquino, T.B.; do Nascimento, J.E.R.; Dias, Í.F.C.; de Oliveira, D.H.; Barcellos, T.; Lenardão, E.J.; Perin, G.; Alves, D.; Jacob, R.G. Synthesis of (arylselanyl)- and (arylsulfenyl)-alkyl-1,2,3-triazolo-1,3,6-triazonines via a copper-catalyzed multicomponent reaction. Tetrahedron Lett. 2018, 59, 1080–1083.
- Zhang, L.-L.; Li, Y.-T.; Gao, T.; Guo, S.-S.; Yang, B.; Meng, Z.-H.; Dai, Q.-P.; Xu, Z.-B.; Wu, Q.-P. Efficient Synthesis of Diverse 5-Thio- or 5-Selenotriazoles: One-Pot Multicomponent Reaction from Elemental Sulfur or Selenium. Synthesis 2019, 51, 4170–4182.
- Gao, X.; Tang, L.; Huang, L.; Huang, Z.-S.; Ma, Y.; Wu, G. Oxidative Aminoarylselenation of Maleimides via Copper-Catalyzed Four-Component Cross-Coupling. Org. Lett. 2019, 21, 745–748.
- Begini, F.; Balaguez, R.A.; Larroza, A.; Lopes, E.F.; Lenardão, E.J.; Santi, C.; Alves, D. Synthesis of 4-Arylselanyl-1H-1,2,3-triazoles from Selenium-Containing Carbinols. Molecules 2021, 26, 2224.
- Rather, R.A.; Ara, T. Copper catalyzed synthesis of 3-((arylethynyl)selanyl)-1H-indoles via selenium insertion reaction by using elemental selenium. Tetrahedron 2021, 96, 132386.
- Lara, R.G.; Rosa, P.C.; Soares, L.K.; Silva, M.S.; Jacob, R.G.; Perin, G. A simple and stereoselective synthesis of (Z)-1,2-bis-arylselanyl alkenes from alkynes using KF/Al2O3. Tetrahedron 2012, 68, 10414–10418.
- de Oliveira, I.M.; Vasconcelos, S.S.N.; Barbeiro, C.S.; Correra, T.C.; Shamim, A.; Pimenta, D.C.; Caracelli, I.; Zukerman-Schpector, J.; Stefani, H.A.; Manarin, F. Ytterbium(iii)-catalyzed three-component reactions: Synthesis of 4-organoselenium-quinolines. New J. Chem. 2017, 41, 9884–9888.
- Sakai, N.; Horikawa, S.; Ogiwara, Y. Indium-Catalyzed Direct Conversion of Lactones into Thiolactones and Selenolactones in the Presence of Elemental Sulfur and Selenium. Synthesis 2017, 50, 565–574.
- Attia, Y.A.; Abdel-Hafez, S.H. Reusable photoresponsive Ag/AgCl nanocube-catalyzed one-pot synthesis of selenopyridine derivatives. Res. Chem. Intermed. 2020, 46, 3165–3177.
- Attia, Y.A.; Abdel-Hafez, S.H. Nano-Co3O4-catalyzed microwave-assisted one-pot synthesis of some seleno pyridine/quinoline derivatives. Res. Chem. Intermed. 2021, 47, 3719–3732.
More
