Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Roger Clotet and Version 4 by Jamir Pitton Rissardo.
Parkinson Disease (PD) primarily affects older adults. It is the second-most common neurodegenerative disease after Alzheimer’s disease. Freezing of Gait (FoG) is a symptom present in approximately 80% of advanced-stage PD’s patients. FoG episodes alter the continuity of gait, and may be the cause of falls that can lead to injuries and even death. The recent advances in the development of hardware and software systems for the monitoring, stimulus, or rehabilitation of patients with FoG has been of great interest to researchers because detection and minimization of the duration of FoG events is an important factor in improving the quality of life.
- Parkinson Disease
- freezing of gait
1. Introduction
Parkinson Disease (PD) is the second-most common neurodegenerative disease, after Alzheimer’s disease, among the elderly. At present, there are 10 million people patients with PD worldwide [1][2][1,2]. Statistics show that the prevalence of PD is higher in Europe, North America, and South America in comparison with Africa and Asia, with an incidence of 13.4 per 100,000 people per year [2][3][4][2,3,4]. The causes of PD remain unknown, but some studies have attributed it to environmental exposure factors and genetic factors [5]. Moreover, sex and ethnicity have been shown to be influencing factors, with the male:female ratio of patients with PD being approximately 3:2 [3]. Age remains the main risk factor for the development of PD, and the prevalence and incidence of the disease increases exponentially after 60 years of age [3][6][3,6]. This trend has important implications for public health, since greater longevity, which is the trend in most countries, is expected to increase the number of people with PD by 50% in 2030 [3][7][3,7].
Parkinson disease is associated with non-motor and motor symptoms. Non-motor symptoms include dementia, depression, psychotic characteristics, autonomic dysfunction, oculomotor abnormalities, and olfactory and visual impairments. Though not as visible as these motor symptoms, non-motor symptoms are also experienced by many individuals with PD as a part of their disease. Patients with PD may experience nonmotor symptoms related to the disease itself or to the medications used to treat it.[8] The moto include tr symptoms include tremor, stiffness, bradykinesia, postural instability, festination, decreased blink frequency, blepharospasm, and Freezing of Gait (FoG) [9][8]. Medical evaluation of non-motor symptoms is usually performed by a neuropsychologist, while motor symptoms are diagnosed by a neurologist on the basis of medical history, a review of signs and symptoms, and physical and neurological examinations.
The FoG symptom is related on bradykinesia, rigidity, tremor, and postural instability, together with perceptive malfunction and frontal executive dysfunction [9][10][8,9]. It presents as a reduction in the forward progression of the feet, despite the person’s intention to walk [11][12][13][10,11,12]. This symptom occurs in 21–27% of patients in the early stages of PD [14][15][13,14], and this percentage continues to increase during PD evolution, with the symptom appearing in 80% of patients more than 17 years from the initial diagnosis. FoG may be the cause of falls that can lead to injuries and even death [16][15].
FoG has its origin in the brain, specifically in the mesencephalic locomotive region (MLR), where the performance of the pedunculopontinal nucleus (PPN) diminishes their connections with basal ganglia. Similarly, the region of the brain stem is related to the condition of freezing, which has been validated with Functional Magnetic Resonance Imaging (FMRI) and ElectroEncephaloGraphy (EEG) data. FoG is no longer considered a strictly motor symptom but is a part of cognitive impairments that originate from areas of the brain that allow the body to be able to walk without hindrance [17][18][16,17].
FoG does not respond to existing drugs, and neurorehabilitation exercises tend to be repetitive and tiring. Several invasive methods, such as deep brain stimulation (DBS) [19][18] or vagus nerve invasive stimulation (VNS) [20][19], have been developed for the treatment of FoG, but they are expensive, do not guarantee elimination of freezing, and may increase other symptoms. Patients with an episode of FoG can resume walking after receiving external stimulation, and non-invasive methods such as visual, vibratory, and tactile stimulation devices can provide such stimulation at a relatively low cost and without health risks.
2. Brain Activity during a FoG Episode
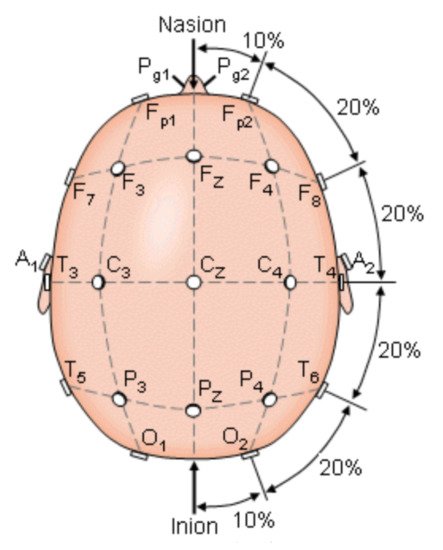
To understand the parameters to be measured by those systems, it was necessary to analyze the brain motor activity involved in PD during episodes FoG. Brain activity occurs when the brain generates electrical impulses known as action potentials, which travel through neurons. Electrical impulses contain information that travels from neuron to neuron making use of hundreds of thousands of them to get transported and perform a specific function, any alteration provokes a change in their contiguous connections [21][20]. When the brain generates an impulse to move a muscle, the impulse passes through the basal ganglia that help to smooth muscle movements and coordinate changes in posture, such as the gait. A statistical parametric mapping analysis applied to healthy subjects during the gait revealed that the following areas were activated in their brain activity: supplementary motor, medial primary sensorimotor, striatum, cerebellar vermis, and visual cortex. These results indicate that the cerebral cortexes that control: motor functions, visual cortex, basal ganglia, and cerebellum, may be involved in the bipedal locomotor activities in humans [22][21]. When a person has PD, there is a degeneration in the cells of the basal ganglia that causes a decrease production of dopamine and reduces connectivity between nerve cells and muscles [23][22]. Encephalography was used to understand the bioelectrical connections of those who suffer from PD and present FoG. The Figure 1 shows the electrode arranged system in a 10–20 scheme. This scheme was used to analyze brain activity utilizing electrodes on the hair scalp in FOG patients. The presence of FOG episodes generates different levels of energy in the brain waves of the parietal zone (P4), suggesting that this zone has been deeply affected by the disease. Measurements of P4 and the central zone (Cz) are the features that most contributed to the analysis for detecting FoG transition in PD patients [24][25][26][23,24,25].