1. Introduction
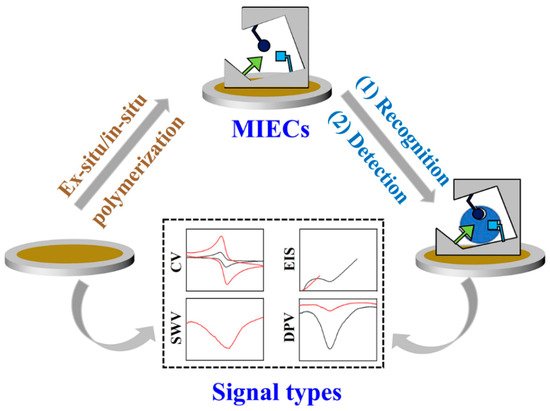
Molecular imprinting techniques (MIT)-based electrochemical sensors (MIECs) are integrated sensing techniques that use molecularly imprinted polymers (MIPs) as recognition elements and electrochemical platforms as signal transducers (
Figure 1). When the MIPs are well-prepared on the surface of the electrode, they can specifically recognize the analyte and indicate the molecular information (structure, concentration, conductivity, etc.) based on different electrochemical signals (CV for cyclic voltammetry, EIS for electrochemical impedance spectroscopy, SWV for Square-wave voltammetry, DPV for differential pulse voltammetry). As the analytes bind with MIPs, the direct detection can be achieved by the change in the state of MIPs or surface potential, which is due to a change in properties of the system without any electrodic reaction of analytes. As the specificity of MIPs towards analytes, the changes are only dependent on the amounts of analytes, and thus can be measured by the CV or EIS with variations of current or resistance. For example, Hedborg et al. reported the first MIPs-based capacitance sensor with capacitive or impedance detection, which is based on the principle of plate-capacitor with double layer phenomenon
[1][36]. When analytes rebind with MIPs, the capacitance will vary with the concentration of analytes. This impedometric binding detection can be achieved by MIECs without the electrodic reaction of analytes.
Figure 1.
Illustration of working principle of MIECs from the preparation of MIPs to electrochemical signal output.
2. Applications of MIECs in Food Safety and Drug Detection
As a new kind of polymer material, MIPs have long been used in the field of analytical science. Compared with natural antibodies, MIPs can be easily modified and controllable in the affinity of receptor sites, which makes it more accessible to recognize different kinds of molecules, no matter small ones or big macromolecules
[2][3][45,46]. In addition, MIPs show high stability, strong resistance to the environment and biocompatibility, which mean that they are of low cytotoxicity, and can be stored and used in harsh conditions, such as in low or high temperatures, extreme pH solutions, and strong ion strengths
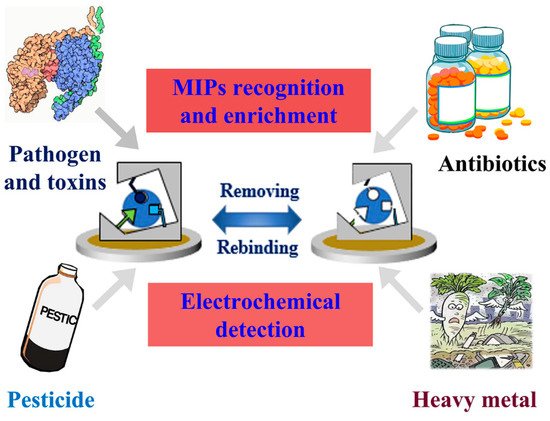
[4][5][47,48]. Because of their advantages, such as being lightweight, low-cost and easy to use, electrochemical sensors with a high diversity of electroanalytical techniques are expected to be the future generation of analytical systems. Taking the merits of MIPs and electrochemical sensors, MIECs behave promisingly in applications in food and drug safety detection (
Figure 2).
Figure 2.
Schematic presentation of MIECs in the applications of food and drug safety detection.
2.1. Pathogen and Toxins
The ingestion of pathogen and toxin-contaminated food can cause severe illnesses, which pose a huge threat to human health
[2][3][45,46]. A trace level of pathogen or toxin in the human body could inflict biological damage or even death
[4][47]. Foodborne pathogens such as Salmonella enterica, Listeria monocytogenes, Escherichia coli, and Staphylococcus aureus, are responsible for poisoning food and water. Some mycotoxins produced by fungi, such as aflatoxins, fumonisins, ochratoxin A, and patulin can also induce physiological abnormalities in humans and animals, and most of them are tumorigenic
[5][6][48,49]. Various analytical methods have been developed for the detection of pathogens and toxins in food samples, and MIECs have attracted much attention due to their high selectivity and sensitivity, as well as low cost and easy operation.
For instance, Golabi et al. reported an electrochemical biosensor based on a whole-cell imprinting approach, which can deliver the rapid detection of
S. epidermidis [7][50]. The cell-imprinted polymer with a boronic acid group endows a high affinity for bacteria, which was further used for the label-free detection of
S. epidermidis via EIS with a linear response in the range of 10
3–10
7 CFU/mL. However, the presence of boronic acid groups will lead to non-specific absorption, making it less sensitive to the target. Thus, further studies can be carried out to eliminate the undesirable effects. Furthermore, Li et al. developed MIECs for Listeria monocytogenes (LM) based on 3-thiopheneacetic acid (TPA) as the functional monomer
[8][51]. MIPs were prepared via the in-situ electropolymerization of TPA on the GCE surface in the presence of LM, which was denoted as LIP/GCE. In this case, [Fe(CN)
6]
4−/3− is used as a probe to indicate the amount of LM. When LM cells are captured by LIP/GCE, the imprinted cavity will be filled up with LM and the access of [Fe(CN)
6]
4−/3− to the electrode surface is blocked. As a result, the peak current of [Fe(CN)
6]
4−/3− decreases with the increasing LM concentration. Finally, the MIECs behaved at a low limit of detection (6 CFU/mL) and a wide linear range (10 to 10
6 CFU/mL).
In addition, Guo et al. constructed MIECs for the determination of patulin based on electropolymerization technology with modifications of carbon dots, chitosan, and Au NP
[9][52]. Wherein, 2–oxindole was adopted as a template to replace patulin and form the molecularly imprinted cavity at a lower cost, and the modifiers were used to increase electroactive areas and acquire distinct signals. The MIECs showed a linear range from 1 pM to 1 nM with the limit of detection (LOD) of 0.757 pM. Munawar et al. fabricated an ex- situ MIPs for the electrochemical detection of fumonisin B
1 (FB
1)
[10][53]. The MIPs were firstly prepared and then covalently attached to the working electrode. With the probing redox couple of [Fe(CN)
6]
4−/3−, this sensor was allowed to detect FB
1 via the impedimetric or voltammetric technique. The EIS and DPV techniques behaved in a linear detection range from 1 fM to 10 pM with LODs of 0.03 and 0.7 fM, respectively.
With the aid of MIPs, MIECs can easily achieve specific and sensitive detection of pathogens and toxins in food samples. However, the cell imprinting strategy still suffers from partial non-specific recognition, which requires further improvement in the blotting templates and preparation methods. On the other hand, though the electropolymerization strategy is convenient to prepare MIPs, functionalized nanomaterials are still needed to enhance the conductive properties of the electrodes.
2.2. Pesticide Residue
Pesticides are favored in agriculture for crops and seed protection, as they help raise the output of agricultural products. Though the use of pesticides can produce significant market prospects and huge social benefits, the pesticide residues in food materials also have deleterious effects on human health
[11][12][54,55]. Most of the used pesticides and their residues have long-term stability and biological effects due to their high persistence in the environment
[13][56]. For months or years, the toxicity and potential carcinogenicity of the pesticide residues still exist
[14][15][57,58]. So, the pesticide residues are easy to accumulate in the human body through the food chain. To ensure food safety for consumers, it is vital to develop sensitive and effective methods for pesticide residue detection. MIECs could be a potential candidate for pesticide residue monitoring.
As an instance, Dai et al. developed novel MIECs for selective and sensitive detection of imidacloprid residues
[16][59]. With the strategy of dual-template molecularly imprinted polymers (DMIPs), two different templates and thionine (TH) were electropolymerized, and TH with redox peak acts as an internal signal. For the two templates, one is a non-electroactive template for a single signal, and another is an electroactive template for dual signals. Thus, non-electroactive bensulfuron-methyl (BSM) and electroactive imidacloprid (IMI) can be detected with different modes: the occupation of the imprinted cavities with BSM indicates a single-signal output of TH, and that of IMI shows an on-off ratiometric signal. By this means, the sensor behaved in wide linear ranges of 10 nM–10 μM and 0.1 Μm–0.1 mM, with LODs of 7.8 × 10
−9 M and 6.5 × 10
−8 M for BSM and IMI, respectively. This study demonstrated the feasibility of DMIPs coupled with electrochemical techniques in the analysis of pesticide residues, which provided a new idea to construct selective MIECs for electroactive and non-electroactive template detection.
Based on Co
3O
4 nanowire and core-shell Co
3O
4@MOF-74 nanocomposite, Karimi-Maleh et al. developed a MIECs method for fenamiphos (FEN) analysis
[17][60]. After the modification of nanocomposites on pretreated carbon electrodes, the preparation of MIPs was carried out by the in-situ electropolymerization with pyrrole as monomer and FEN as a template. The MIECs behaved in a linear detection range from 0.01 nM to 1.0 nM with a limit of quantification (LOQ) and LOD of 1.0 × 10
−11 M and 3.0 × 10
−12 M, respectively. Co
3O
4@MOF-74 nanocomposites provided a high surface area and fast electron transfer rate in the detection. However, the synthesis of nanocomposites with a high temperature may increase the cost, so it would be better to develop greener and more energy-efficient nanomaterials.
As discussed above, MIECs can achieve specific and sensitive detection of pesticides. Still, there are some limitations with MIECs. For example, with the popularization of pesticides, different pesticides often exist in one food sample. It is difficult for MIECs to detect multiple pesticides at the same time. Therefore, it can be an alternative to exploring the MIPs with diverse functional monomers.
2.3. Heavy Metal Ions
Heavy metal ions are commonly found in wastewater and classified as water pollutants. However, even in the soil, heavy metal ions are dangerous because they can be adsorbed by crops, fruits, and vegetables
[18][61]. For instance, heavy metals such as mercury, lead, cadmium, chromium and arsenic are non-degradable and ubiquitously distributed, and they are considered hazardous compounds even at a low concentration
[19][62]. With the intake of those heavy metal ions, people could suffer from enzyme inhibition, oxidative stress and impaired antioxidant metabolism
[20][63]. Hence, it is critical to develop suitable techniques for the fast and accurate detection of metal ions. Most heavy metal ions are electrically active and highly susceptible to exchange electrons and produce characteristic electrochemical signals. MIECs can be the appropriate tool for the detection and quantification of heavy metal ions.
To impart selectivity of electrochemical sensors, modifiers with a strong affinity are commonly used to recognize target ions. For example, Motlagh et al. prepared a novel nanostructured cadmium(II) ion-imprinted polymers (IIPs) by a sol-gel process
[21][64]. For the preparation of Cd-IIP materials, an ex-situ surface imprinting strategy combined with a sol–gel process was adopted to fabricate the carbon paste electrode. The electrochemical sensor was employed for voltammetry detection of Cd(II) with a linear range of 0.5–40 μg L
−1 and LOD of 0.15 μg L
−1. Similarly, Sebastian et al. adopted Pb(II) ions as a template to prepare IIPs by modifying multiwalled carbon nanotubes (MWCNT) on Pt electrodes
[22][65]. The sites imprinted in MWCNT/IIPs are highly selective to Pb(II) ions and the Pt electrode showed a sensitive response with the modified nanostructure. CV and DPV tests were conducted to discuss the features of the IIP electrochemical sensor. The sensing system behaved with an LOD of 2 × 10
−2 μM for Pb(II) ions, revealing promising applications in the detection of environmental and food samples.
Similar to MIPs, the IIPs can impart electrochemical techniques with selectivity and simplicity in the detection of real samples. However, IIPs may suffer some limitations including low binding capacity, irregular shape, poor target site accessibility, and heterogeneous binding site distributions. To avoid the limitations indicated, new technologies should be developed to prepare IIPs with good accessibility, high affinity, and selectivity to the target ions. Due to their high porosity, adjustable structures, and good stability, metal–organic frameworks (MOFs) can be used as efficient substrates to prepare IIPs and construct electrochemical sensors
[23][66].
2.4. Antibiotics Monitoring
Antibiotics are a class of antimicrobial compounds that are widely used in human or veterinary medicine to treat diseases, especially in the livestock industry and aquaculture
[24][67]. However, the abuse of antibiotics could result in sustainable adverse effects on human health and the environment. The constant intake of antibiotics could cause immunopathological effects, hepatotoxicity, carcinogenicity, bone marrow toxicity, reproductive disorders, or even anaphylactic shock
[25][68]. When antibiotics enter the water and land environment, the cycle of water will make it a significant local point of contamination. The overdosage of antibiotics in animals can lead to antibiotic residues in foodstuffs such as meat, chicken, egg, milk, and fish
[26][69]. Through the enrichment of the food chain or the transfer of water, the antibiotics will finally accumulate in the human body and pose potential risks to human health. Therefore, it is imperative to develop effective methods of monitoring the antibiotic residues.
Paracetamol (PR), a kind of analgesic, antipyretic and anti-inflammatory drug, and is one of the most commonly consumed pharmaceuticals. In a normal dose, PR does not produce any harmful side effects, however, overdose intake of the drug could cause pancreas inflammation or even kidney damage and hepatotoxicity
[27][70]. For instance, Dai et al. reported MIECs for the detection of PR based on Prussian blue (PB) embedded MIPs as a reference signal
[28][71]. Herein, the inner layer of PB acts as an internal electrochemical signal and the target PR as another signal. When PR molecules were captured and incorporated with the cavity on the outer layer of MIPs, the redox current of PR increased while that of PB decreased due to the occupied sites’ blocked electron transfer, which finally manifested as an “on and off” signal output mode. As a result, the sensor displayed a concentration range from 1.0 nM to 0.1 mM with a LOD of 0.53 nM, as well as recoveries in the range between 94.6 and 104.9%, revealing it is acceptable in the practical applications.
Detection resolution has been identified as an important factor in newly developed analytical techniques, which reflect their ability to distinguish the details of analytes with similar structures. For instance, propranolol (prop), an important and widely used β-adrenaline antagonist for the treatment of cardiovascular diseases, has a similar chemical structure to salbutamol
[29][72]. Moreover, prop has two enantiomers of S-prop and r-prop, and only S-prop has pharmacological performance. Based on the reduced graphene oxide (rGO) and chitosan-based MIPs, Liu et al. developed a differential potential ratio sensing platform for binary molecular recognition of prop
[30][73]. In the platform, MIPs specifically recognize and capture prop enantiomers, rGO acts as a conductive substrate to produce an amplified signal, and the potential difference between the R-/S-prop offers the ratiometric signal. As a result, the method gained a distinct potential difference of 135 mV with a detection range from 50 μM to 1000 μM in the racemic mixture, which reveals great potential in the fields of pharmacological detection and clinical analysis.
Chlorpromazine (CPZ) is an antipsychotic drug used to treat psychiatric and personality disorders, and clinical monitoring of CPZ is necessary. Liu et al. presented Pt/Co
3O
4 nanoparticles and methylene blue (MB) monomer-based MIPs for selective detection of CPZ
[31][74]. Wherein, MB molecule in MIPs provides a fixed internal signal, and the signal of CPZ changes with concentrations, which is a typical on/off ratiometric signal output mode. Under optimal conditions, the method showed a linear range of 0.005–9 μM with a LOD of 2.6 nM and recoveries of 95.3–108.0% in pharmaceutical samples. The dual-signal output mode provides built-in signal calibration to eliminate interference and adjusts the signal fluctuation, thus can effectively improve detection stability and accuracy.
Besides food safety and drug detection, MIECs combined with nanomaterials have also shown good performance for the screening of biomarkers. As biomarkers are closely related to some diseases, it is important to achieve stable and sensitive detection of biomarkers for the early diagnosis. To fulfill the rapid screening, comprehensive procedures must be taken to treat the complex media, which usually involves preconcentration and separation. MIECs can take the role to realize the separation and detection at the same time. For example, Anirudhan et al. reported MIECs for the detection of 2-aminoadipic acid (2-AAA), a diabetes biomarker, based on the surface modification of electrode with a drop-casting method
[32][75]. The modified MIP electrode showed good DPV results for 2-AAA with an LOD of 0.40 × 10
−11 M, demonstrating the high selectivity and sensitivity of the MIECs for real sample analysis.