Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Gabriel Santos and Version 3 by Rita Xu.
Diabetic peripheral neuropathies (DPNs) are conditions that impair the peripheral nervous system (PNS) component. These disorders may have numerous causes and are often presented in various forms. The monosialotetrahexosylganglioside (GM1) ganglioside, popularly known as Sygen, provides beneficial effects such as enhanced neuritic sprouting, neurotrophism, neuroprotection, anti-apoptosis, and anti-excitotoxic activity, being particularly useful in the treatment of neurological complications that arise from diabetes.
Diabetic peripheral neuropathies (DPNs) are conditions that impair the peripheral nervous system (PNS) component. These disorders may have numerous causes and are often presented in various forms.
- diabetic peripheral neuropathy
- gangliosides
- Sygen
- neurology
- regenerative medicine
- inflammation
- neurodegeneration
1. Introduction
According to previous studies [1][2][3][2,3,4], this condition has generalized a subset of varieties such as multiple mononeuropathy, lumbosacral, and thoracic and cervical radiculoplexus neuropathies. These varieties can be further separated into two major subgroups: diabetic sensorimotor polyneuropathy (DSPN) and atypical neuropathies. Tesfaye et al. [1][2] proposed four minimal criteria for typical DPN (Table 1). The incidence of DPN is estimated to lie between 6% and 51% in diabetic adults, according to variables such as age, glycemia, and the differences between diabetes types 1 and 2 [4][5]. Eventual complications can develop in about 50% of diabetic adults and cause significant morbidity such as pain, foot ulcers, and, ultimately, the amputation of lower limbs [5][6]. Clinical manifestations are often variable as patients can display extremely painful neuropathic symptoms or remain apparently asymptomatic.
Table 1. Minimal criteria for typical diabetic peripheral neuropathy.
| Classification | Definition |
|---|---|
| Possible DSPN | Symptoms: decreased sensation and numbness in lower limbs; signs: symmetric decrease of distal sensation or unequivocally decreased or absent ankle reflexes |
| Probable DSPNConfirmed DSPN | Detection of multiple signs and symptoms of neuropathy: neuropathic symptoms, decreased distal sensation, or unequivocally decreased or absent ankle reflexes Detection of nerve conduction test score abnormality + signs or symptoms of DSPN |
| Subclinical DSPN | No signs or symptoms of neuropathy are confirmed with neurophysiologic tests |
The impaired neuronal function in lower limbs is associated with poor outcomes (Figure 1) including minor accidents, the restriction of common daily routine activities, and a decreased quality of life [6][7]. It is extremely important for these individuals to receive continuous follow-up and extended examinations given the risk of foot ulcer development [4][5]. Although peripheral neuropathies can manifest in non-diabetic individuals, the management strategies of this disorder in diabetic individuals is generally more challenging as there are secondary approaches that must be implemented, especially glycemic control [7][8]. According to the American Diabetes Association, pain management and lifestyle adjustments (i.e., diet and exercise) are still regarded as an indispensable approach [7][8].
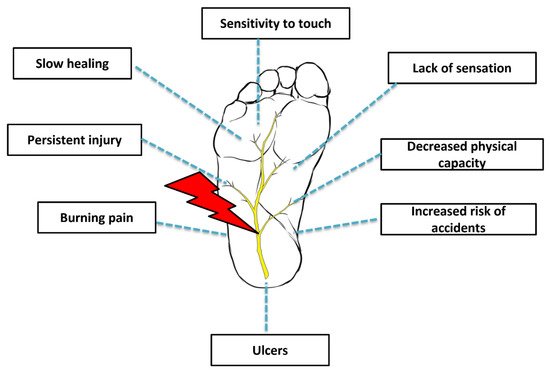
Figure 1. Diabetic peripheral neuropathy.
More recently, however, the potential application of gangliosides (glycosphingolipids) as a therapeutic tool has attracted some attention in the literature. These molecules are found in large quantities in mammalian tissues and exert vital functions in multiple physiological processes including cell signaling, differentiation, apoptosis, memory control, and neuroprotection and neuronal recovery [8][9][9,10]. Gangliosides are predominantly found in neurons of all animal species and participate in several biological events such as cell differentiation, cell signaling, memory control, apoptosis, and neuronal protection and recovery. These molecules also work as ‘biological anchors’ for several bacterial toxins, viruses, and autoantibodies.
The GM1 ganglioside (monosialotetrahexosylganglioside GM1; Sygen®, Abano Terme, Padua, Italy), in particular, has been studied for many years, and the comprehension of its biological properties seems to be well documented in regards to regenerative medicine. GM1 is the main component of mammalian cerebral tissue, and it is abundantly expressed in neurons. It has been one of the most widely investigated gangliosides; thus, theour comprehension of its properties is not limited. Scientists have been interested in the properties of GM1 since its discovery back in the early 1970s when it was proposed as a receptor for cholera toxin [8][9]. Gangliosides are glycosphingolipids highly abundant in the nervous system, carrying the majority of cerebral sialic acid residues. The lipid rafts on cell membranes are packed with gangliosides, where they are able to play key roles in the modulation of membrane proteins and ion channels, signaling cascades and cell communication.
Most of the focus is directed towards cerebral gangliosides because the loss of function mutations in ganglioside biosynthetic enzymes has been significantly linked to neurodegenerative disorders. Moreover, ganglioside profile alterations have been reported in regular aging and known neurological conditions such as amyotrophic lateral sclerosis, multiple sclerosis, Huntington’s disease (HD), Alzheimer’s disease (AD), Parkinson’s disease (PD), stroke, and epilepsy. At least in HD and some degrees of epilepsy, experimental evidence has indicated a potential therapeutic role for gangliosides in symptom alleviation. Previous clinical trials utilizing gangliosides for other neurological conditions including acute ischemic stroke [10][11] and PD [11][12] have sparked a fair share of curiosity and optimism. In these studies, there were no records of major adverse events except for minor cases of Guillain-Barré syndrome, which were reported in very few stroke patients receiving the treatment. The application of gangliosides, more specifically GM1, has since then been attracting considerable attention from the medical community.
2. Etiopathogenesis
DPN is a condition responsible for impaired neuronal function and death mainly by means of oxidative stress and inflammation [4][5]. Insulin resistance as well as the other key components of metabolic syndrome can significantly contribute to the dysregulation of metabolic pathways [12][13]. Consequently, metabolic stress destabilizes mitochondrial redox, causing an accumulation of reactive oxygen species in both the mitochondrion and cytosol [13][14]. Mitochondriopathies, in general, are responsible for damage and loss of energy in axonal structures, paving the way for neuropathy [14][15]. Additionally, the polyol pathway hyperactivity is also another major contributor as it increases the turnover of cofactors NADPH and NAD+. This ultimately leads to decreases in the redox potency and the regeneration of glutathione, elevated levels of advanced glycation end products (AGEs), and the activation of diacylglycerol and protein kinase C (PKC) isoforms [15][16]. Low levels of intracellular glutathione are recognized as one of the primary causes of oxidative stress and accumulation of toxic residues, being a major culprit in the development of many pathogenic processes [16][17].
Unmyelinated C fibers are the primary structures to be affected by these pathological changes, culminating in hyperesthesia, allodynia, and pain [17][18]. Further demyelination occurs and surpasses remyelination, resulting in neurodegeneration and a gradual loss of distal sensation in a distal-to-proximal course along nerves [18][19]. It is worthy to note that some researchers have also referred to DPN as “length dependent neuropathy”, which means that the longer the neuron, the greater the risk of developing neuropathy at that level [19][20]. Patients usually describe the pain as a burning or stabbing sensation, numbness, and increased sensitivity to touch or deep ache. In most cases, the pain worsens by night and is usually restricted to the lower extremities but can sometimes affect the hands as well [4][5].
Although strongly associated with glucose intolerance and metabolic syndrome components in general, the risk of DPN appears to be even greater in individuals with prevalent cardiovascular disease [20][21]. A plausible explanation for this tendency may be linked to the occurrence of subclinical atherosclerosis or vascular pathologies that contribute to the development of progressive cardiovascular and peripheral neuropathy morbidities [21][22]. In fact, DPN has been found to be strongly associated with microvascular damage. Clinical and preclinical studies revealed that in these specific conditions peripheral perfusion is reduced in both the nervous and epithelial tissue [22][23][24][23,24,25]. This promotes nerve ischemia due to arteriosclerosis, since this condition strongly affects the blood vessels that supply peripheral nerves [25][26]. Increased nerve swelling and interstitial pressure is also accompanied by higher capillary pressure, fibrin deposition, and thrombi development [15][16]. Moreover, under hyperglycemic conditions, sensory nerves suffer hypoxia and have their electrical stability disrupted; Schwann cells, in turn, lose their capacity to support myelin sheaths [26][27][27,28].
Another hypothesized mechanism responsible for DPN is damaged nerve endings. Improper action potentials are generated by the extremities of damaged nerves; therefore, they may be equivocally interpreted by the central nervous system (CNS) as pain or dysesthesia [15][16]. Altered ion channel expression in peripheral nerve fibers is directly related to nerve injury, hyperexcitability, and, inevitably, neuropathic pain [28][29]. An animal study [29][30] revealed that calcium ion channels are also dysregulated in diabetic conditions. This increases calcium flux in sensory neurons and triggers the rapid stimulation of substance P and glutamate release [29][30].
Microglial activation is also deeply involved in the pathogenic progression of nervous system disorders. Microglial cells are primarily associated with the maintenance of homeostasis, myelin sheath formation, and the protection and support for neurons from both the peripheral and central nervous systems [30][31]. Microglial activation occurs after peripheral nerve injury and can last up to 3 months. This event triggers the production and release of many inflammatory mediators such as chemokines, cytokines, and cytotoxic substances, including nitric oxide (NO) and free radicals. This leads to a shift towards a pro-inflammatory and catabolic microenvironment [31][32]. In cases of metabolic syndrome and subsequent development of DPN, the state of chronic inflammation does not only disrupt the standard healing process but also prolongs and even aggravates the inflammatory cascade [32][33][34][33,34,35].
3. Conventional Management of Peripheral Neuropathy
Peripheral neuropathies are often considered irreversible; however, in rare cases it may be managed effectively. Management strategies are employed as supportive approach and aim to prevent disease progression and the risk of complications [35][36]. Physicians target three main variables: glycemic control, pain, and foot care. Interestingly, glycemic control does not appear to reduce the symptoms in patients suffering from this condition, therefore remaining largely a preventative strategy along with foot care [36][37]. Regardless of circumstance, the primary objective is to confirm if the signs and symptoms displayed by the patient are actually related to peripheral nerve dysfunction, because neuropathies are often multifactorial in nature [37][1]. For instance, problems involving the spinal vertebrae, such as lumbosacral radiculopathy, can be responsible for peripheral neuropathy symptoms such as numbness of lower limbs [37][1]. Age-related vitamin B12 deficiency can also be responsible for nervous system dysfunction and the classic signs of peripheral neuropathy [37][1].
Lifestyle interventions such as dietary modifications and physical fitness can improve a patient’s metabolic health [12][13]. A recent prospective, double-blind, placebo-controlled study [38] evaluated the efficacy and safety of the combination of superoxide dismutase, alpha lipoic acid, vitamin b12, and carnitine for 12 months in patients with diabetic neuropathy. The combination of these nutrients was found to ameliorate DPN symptoms in these patients by improving sural nerve conduction velocity and amplitude, pain, and quality of life perception. However, larger studies are still required to further confirm the efficacy of these effects in longer follow-up periods.
Logically, such strategies really do seem to be helpful in the management of DPN; however, whilst supportive data continue to emerge, they are still largely preliminary [4][7][39][5,8,39].
Pharmacological alternatives are frequently recommended for the treatment of peripheral neuropathies and have demonstrated efficacy in randomized clinical trials and systematic reviews [7][40][8,40]. Medications such as duloxetine and pregabalin have been approved by the Food and Drug Administration (FDA) for the treatment of neuropathic pain [41]. Additional drugs such as tricyclic antidepressants may mitigate pain; however, they are not approved by regulatory bodies due to serious side effects [7][8]. Lastly, although opioids have also been shown to improve pain scores in some patients, these compounds are known to trigger addictive behavior and should only be considered as a last resource for neuropathic pain [42].
