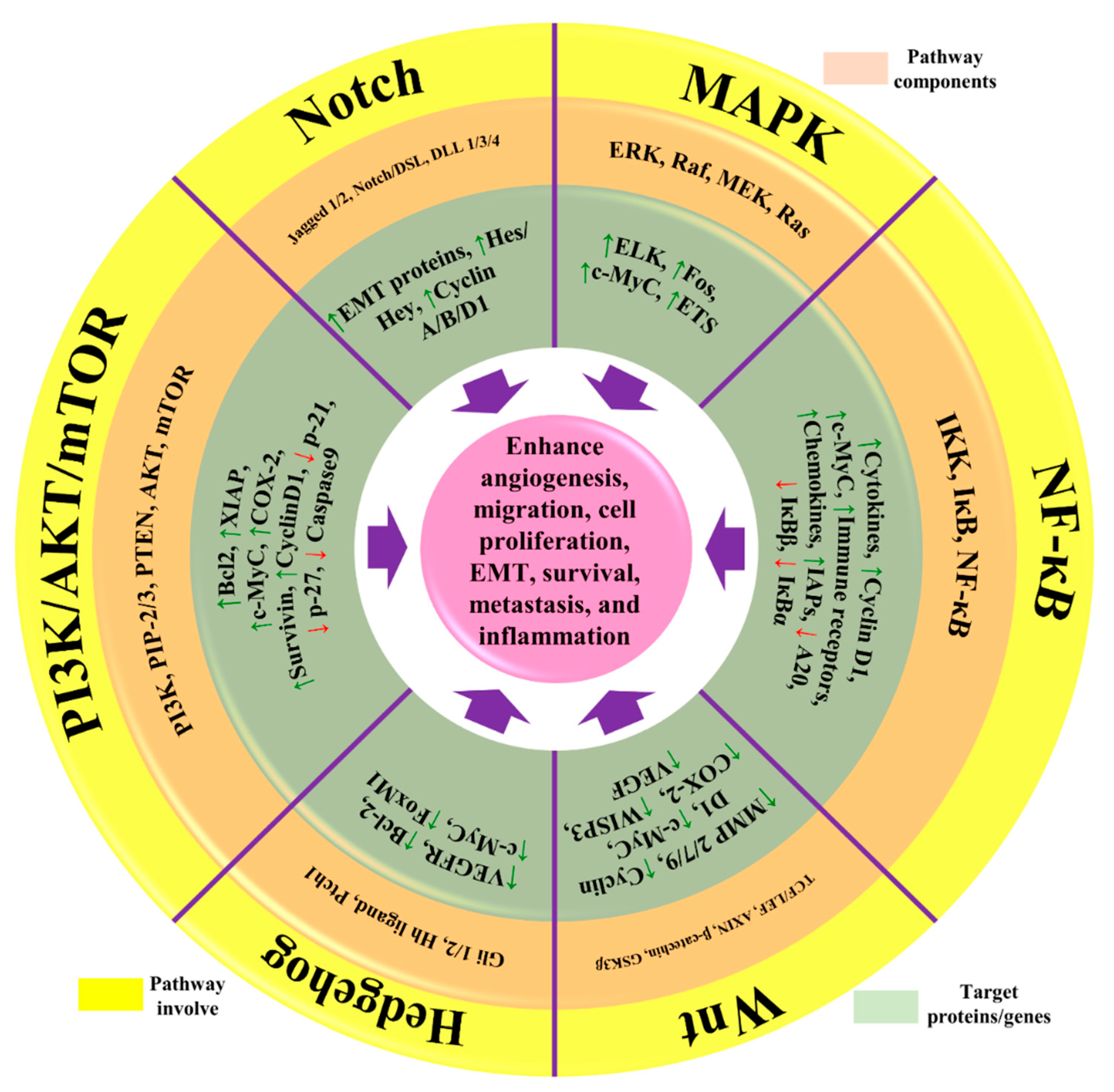
Breast cancer (BC) is the second leading cause of death among women, and it has become a global health issue due to the increasing number of cases. Different treatment options, including radiotherapy, surgery, chemotherapy and anti-estrogen therapy, aromatase inhibitors, anti-angiogenesis drugs, and anthracyclines, are available for BC treatment. However, due to its high occurrence and disease progression, effective therapeutic options for metastatic BC are still lacking. Considering this scenario, there is an urgent need for an effective therapeutic strategy to meet the current challenges of BC. Natural products have been screened as anticancer agents as they are cost-effective, possess low toxicity and fewer side effects, and are considered alternative therapeutic options for BC therapy. Natural products showed anticancer activities against BC through the inhibition of angiogenesis, cell migrations, proliferations, and tumor growth; cell cycle arrest by inducing apoptosis and cell death, the downstream regulation of signaling pathways (such as Notch, NF-κB, PI3K/Akt/mTOR, MAPK/ERK, and NFAT-MDM2), and the regulation of EMT processes. Natural products also acted synergistically to overcome the drug resistance issue, thus improving their efficacy as an emerging therapeutic option for BC therapy.
- breast cancer
- natural products
- transcription factors
- signaling pathways
- treatment
- therapeutic agents
- combination therapy
1. Pathogenesis of Breast Cancer
2. Recent Discoveries and Developments of Natural Products against BC
2.1. Novel Verminoside and Their Derivatives
| Extracted Compound | Biochemical Structure | Biochemical Nature | Source | Study Type | BC Type | Animal Model | Key Finding | Mechanism of Action | Reference |
|---|---|---|---|---|---|---|---|---|---|
| VOA | 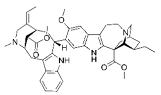 |
Alkaloid | V. africana | In vitro | ER-positive, TNBC, and HER2-positive BC | --- | Downregulating the PI3K/Akt/mTOR. VOA showed its usefulness against MCF-7 and 4T1 cells with IC50 values (0.99, 1.48 μM). | VOA significantly inhibits the phosphorylated AKT and mTOR in BC cells and also decreases the expression of CDK2, cyclin A, E. It also induces apoptosis and cell death in MCF-7 and 4T1 cells by arresting the S phase of the cell cycle. | [19] |
| Lin A | 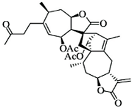 |
Sesquiterpenoid | I. lineariifolia | In vitro | TNBC, and HER2-positive BC | --- | Lin A induced apoptosis at a higher concentration of 50% in BC cells (MCF7 and MDA-MB-231 with IC50 (4.5 ± 0.3, 7.8 ± 0.6). | Lin A arrests the cell cycle at the G2/M phase, and inhibits cell invasion and cell proliferation in BC cells. | [20][21] |
| Fisetin | 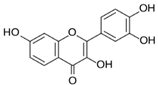 |
Flavonol | Cucumber, apple, strawberry | In vitro and in vivo | ER-positive, TNBC, and HER2-positive BC | BALB/c mice | Fisetin induced apoptosis in MCF-7, 4T1, and MDA-MB-231 at 40 and 80 μM. | Fisetin acts as an inhibitor of PI3K/Akt/mTOR signaling and inhibits the proliferation and dysregulation of this signaling pathway. | [22] |
| WG | 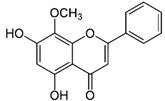 |
Flavone | S. baicalensis | In vitro and in vivo | ER-positive, TNBC, and HER2-positive BC | Chicken chorioallantoic membrane (CAM) | WG showed inhibitory effects on MCF-7 and MDA-MB-231 at 20 and 40 μM. | WG acts as an inhibitor of PI3K/Akt/mTOR signaling and shows inhibition in cell proliferation. | [23] |
| AP | 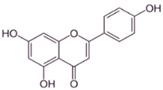 |
Flavone | A. cepa, C. sinensis | In vitro | ER-positive, HER2-positive BC | --- | It influenced the NF-κB pathway by suppressing the VEGF through deactivating progesterone receptors in BC cells. | It inhibits cell proliferation and migrations by arresting the cell cycle at the G2/M phase. It also suppresses the cyclin A, B, and CDK1 which controls the G2/M phase. | [24] |
| Oridonin | 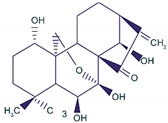 |
Diterpenoid | R. rubescens | In vivo | --- | BALB/C athymic nude mice | It induced apoptosis and cell death in BC cells. | Notch 1-4 protein expression is lowered by oridonin therapy, which hinders cancer cell migration and invasion. | [25] |
| Genistein | 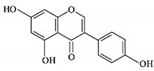 |
Isoflavones | Soy-based foods | In vitro | ER-positive, TNBC | --- | Activation of NF-κB showed potential against MCF-7 at an IC50 value of 20 µM. | It inhibits the phosphorylation of IκBα in MCF-7/T47D/MDA-MB-231 cell lines, thus playing a significant role in the regulation of IκBα to the p50. | [26] |
| GLA | 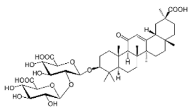 |
Terpenoid | G. glabra | In vitro | ER-positive, TNBC, and HER2-positive BC | --- | It showed anticancer activity against MDA-MB-231/BT549. | It inhibits invasion and cell proliferation, as well as promote the expression of E-cadherin. | [27] |
| ATG | 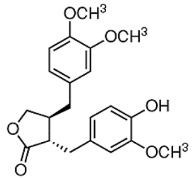 |
Isoflavones | S. heteromalla | In vitro and in vivo | ER-positive, TNBC | BALB/cA-nu | It showed anticancer potentials in MDA-MB-231 cells at 200 μM. | Inhibiting the phosphorylation of MAPK/ERK in MDA-MB-231 cells. | [28] |
| PPD | 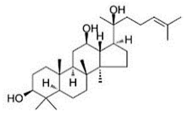 |
Glycoside | P. notoginseng | In vitro and in vivo | TNBC, and HER2-positive BC | BALB/C nude mice | It showed maximum activity below 20 μM against MDA-MB-231. | PPD targets BC cell lines by suppressing the MAPK pathway through the deactivation of ERK1/2, p38, and JNK. | [29] |
| Kaempferol | 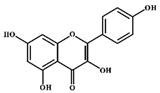 |
Flavonols | Onions, lettuce | In vitro | ER-positive, TNBC | --- | The number of cancerous cells decreased from 85.2% to 50.32% in the G1 phase of the cell cycle. Kaempferol significantly inhibited the BC cells (BT474 and MDA-MB-231) by blocking the critical phases of cell cycles. | Inhibitory actions against different breast cell lines can inhibit the expression of genes involved in MAPK/ERK. This shows that binding with estradiol causes degradation of Erα. | [30] |
| Cimigenoside | 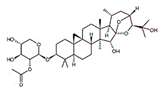 |
Glycoside | C. dahurica | In vitro and in vivo | ER-positive, TNBC | BALB/C nude Crlj mice | Cimigenoside showed maximum anticancer activity against BC cell lines (MDA-MB-231, MCF-7) with IC50 (12.6 ± 1.47, 15.6 ± 2.47 μM). | Cimigenoside induces apoptosis in BC cells by arresting the G2/M phase of the cell cycle. An in vitro study of cimigenoside also inhibits/attenuates BC cell proliferation and invasion. An in vivo study inhibited the growth of tumor growth in mice models. | [31][32] |
| Ginsenosides | 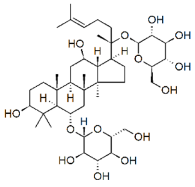 |
Glycosides | P. notoginseng | In vitro and in vivo | ER-positive, TNBC | Nu/nu mice | It showed maximum anticancer activity against BC cell lines (MDA-MB-231). | 25-OCH3-PPD is involved in arresting the G1 phase of the cell cycle and induces apoptosis in BC cells by downregulating MDM2. | [33] |
| BA | 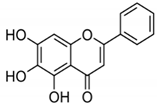 |
Flavonoid | Scutellaria baicalensis | In vitro and in vivo | ER-positive, HER2-positive BC | BALB/c mice | It showed suppression of the NF-κB pathway in the development of human breast epithelial cells (MCF10A). | Suppress the NF-κB signaling pathway, as well as IL-1β, Bcl-2, and VEGF. | [34][35] |
| VMS | 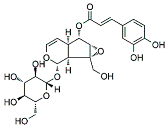 |
Monoterpenoids | P. rotundum | In vitro and in vivo | ER-positive, TNBC, and HER2-positive BC | PyMT/FP635 mouse | It showed maximum activity against MDA-MB-231 and MCF7 cells with IC50 value (10 µM). | VMS suppresses the growth of epithelial lining and the transition of mesenchymal breast cells. | [18] |
| Calcitrinone A | 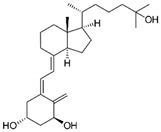 |
Phloroglucinol | C. citrinus | In vivo and in vitro | ER-positive | Chick chorioallantoic membrane (CAM) | Calcitrinone A induced apoptosis and cell death in MDA-MB-231 cells. | Calcitrinone A interferes with mitochondrial function by blocking succinate coenzyme Q reductase and ultimately inhibits the complex II that increases the production of ROS. | [36] |
| Vulpinic acid | 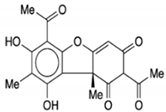 |
Butenolide | Lichens | In vitro | ER-positive, TNBC, and HER2-positive BC | --- | Vulpinic acid induced apoptosis in MCF-7. | Elevate the levels of FOXO-3 and Bax, and suppress the expression of Bcl-2 and procaspase-3/9 to enhance the activity of tumor suppressor miRNAs. | [37] |
| Genistein | 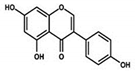 |
Isoflavone | Fabaceae family | In vitro | TNBC, and HER2-positive BC | --- | It induced apoptosis and cell death in MCF-7 and MDA-MB-231 cells. It also inhibited cell proliferation and progression in BC. | Arresting the cell cycle at G2/M phase, downregulating CDK-1, and inhibiting the expression of Bcl-2 and the function of DNA polymerase II. | [38][39] |
| CUR + BBR | 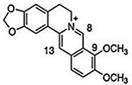 |
Diarylheptanoid, isoquinoline alkaloid | Curcuma longa, berberine from Rhizoma coptidis | In vitro | ER-positive, TNBC, and HER2-positive BC | --- | It showed effects against BC cell lines (MDA-MB-231 and MDA-MB-468) at p ≤ 0.010. | The EMT process in the case of BC is impaired. | [40][41] |
| BA + 5-FU | 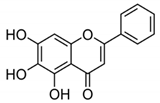 |
Flavonoid (BA) | Scutellaria baicalensis | In vivo | --- | Swiss albino mice | It showed inflammation by inhibiting the VEGF, IL-1β, and NF-κB. | Inflammation is inhibited by the VEGF, IL-1β, and NF-κB, which play significant roles in preventing BC. | [42] |
| MG + 5-FU | 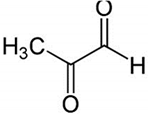 |
Polyphenolic (MG) | Coffee, wine | In vivo and in vitro | ER-positive, TNBC, and HER2-positive BC | BALB/c mice and Swiss albino mice | It showed anticancer activity against the BC cell line (MCF-7). | Arresting the cell cycle at MG G0/G1 phase induces apoptosis and cell death by increasing Bac-2 and caspase 9. | [43] |
| RSVL + SAL | 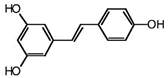 |
Polyphenol | Red grapes | In vivo and in vitro | ER-positive, TNBC, and HER2-positive BC | Swiss albino mice | It showed anticancer activity against MDA-MB-231. | Arresting the S1 phase of the cell cycle also induces apoptosis in BC cells. Other activities include inhibition and cell proliferation. It acts as an antioxidant by preventing the DNA dame and suppressing the tumor growth. SAL inhibits the epithelial mesenchymal transition, and suppresses p53, COX-2, and Beclin. | [44][45] |
2.2. Novel Phloroglucinol and Derivatives
2.3. Role of Viridiflorol against BC
2.4. Effect of Vulpinic Acid against BC
2.5. Action of Genistein against BC
References
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106.
- Reis-Filho, J.S.; Pusztai, L. Gene expression profiling in breast cancer: Classification, prognostication, and prediction. Lancet 2011, 378, 1812–1823.
- Hudis, C.A.; Gianni, L. Triple-negative breast cancer: An unmet medical need. Oncologist 2011, 16, 1–11.
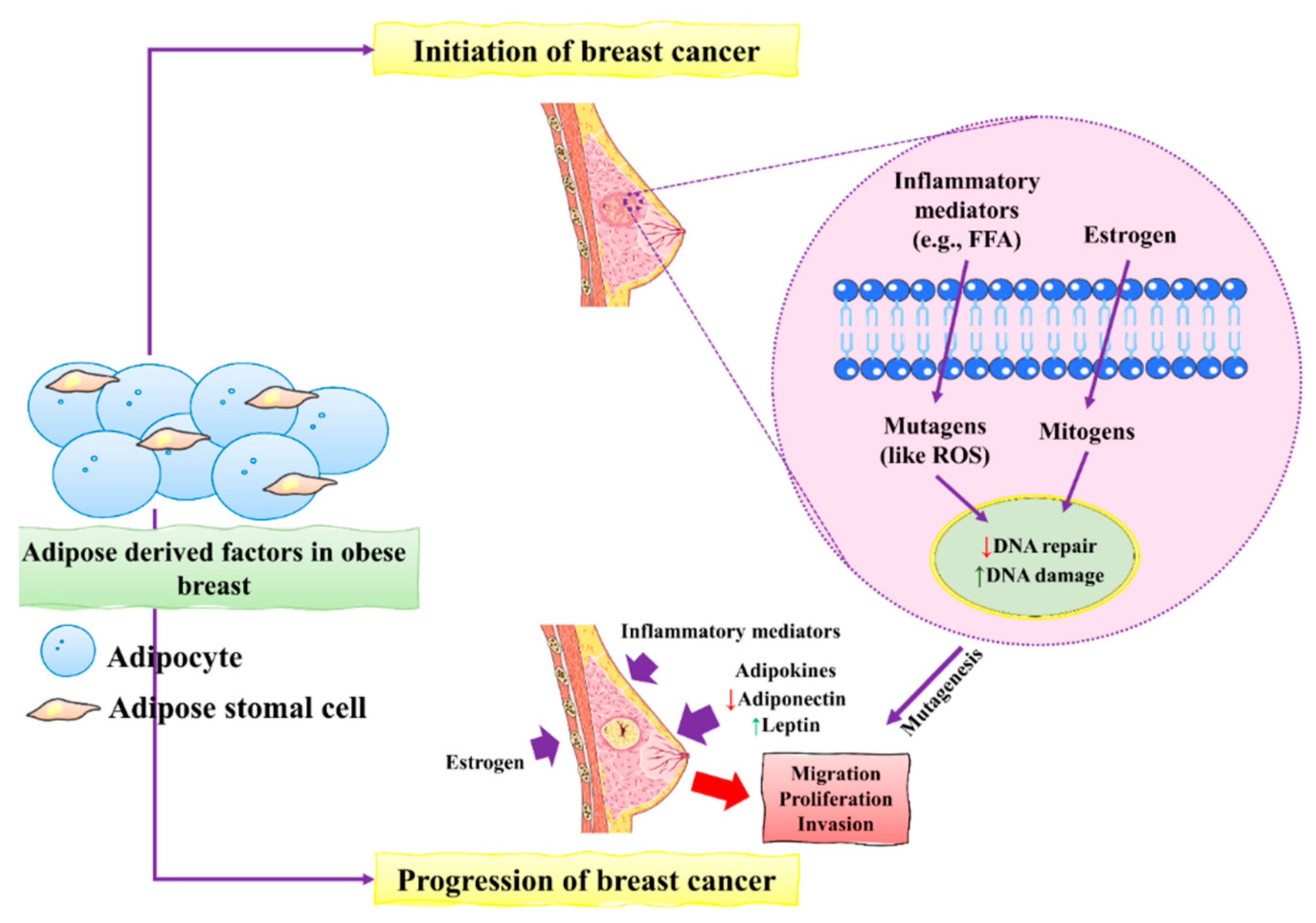
- Bhardwaj, P.; Brown, K.A. Obese adipose tissue as a driver of breast cancer growth and development: Update and emerging evidence. Front. Oncol. 2021, 11, 638918.
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017, 50, 33.
- Evans, D.G.; van Veen, E.M.; Byers, H.J.; Evans, S.J.; Burghel, G.J.; Woodward, E.R.; Harkness, E.F.; Eccles, D.M.; Greville-Haygate, S.L.; Ellingford, J.M. High likelihood of actionable pathogenic variant detection in breast cancer genes in women with very early onset breast cancer. J. Med. Genet. 2022, 59, 115–121.
- Ahmed, S.; Thomas, G.; Ghoussaini, M.; Healey, C.S.; Humphreys, M.K.; Platte, R.; Morrison, J.; Maranian, M.; Pooley, K.A.; Luben, R. Newly discovered breast cancer susceptibility loci on 3p24 and 17q23. 2. Nat. Genet. 2009, 41, 585–590.
- Singh, V.; Kumar, K.; Purohit, D.; Verma, R.; Pandey, P.; Bhatia, S.; Malik, V.; Mittal, V.; Rahman, M.H.; Albadrani, G.M. Exploration of therapeutic applicability and different signaling mechanism of various phytopharmacological agents for treatment of breast cancer. Biomed. Pharmacother. 2021, 139, 111584.
- Varghese, E.; Samuel, S.M.; Abotaleb, M.; Cheema, S.; Mamtani, R.; Büsselberg, D. The “yin and yang” of natural compounds in anticancer therapy of triple-negative breast cancers. Cancers 2018, 10, 346.
- Marino, N.; German, R.; Rao, X.; Simpson, E.; Liu, S.; Wan, J.; Liu, Y.; Sandusky, G.; Jacobsen, M.; Stoval, M. Upregulation of lipid metabolism genes in the breast prior to cancer diagnosis. NPJ Breast Cancer 2020, 6, 50.
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081.
- Suman, S.; Sharma, P.K.; Rai, G.; Mishra, S.; Arora, D.; Gupta, P.; Shukla, Y. Current perspectives of molecular pathways involved in chronic inflammation-mediated breast cancer. Biochem. Biophys. Res. Commun. 2016, 472, 401–409.
- Huang, A.; Cao, S.; Tang, L. The tumor microenvironment and inflammatory breast cancer. J. Cancer 2017, 8, 1884.
- Puar, Y.R.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; Sethi, G.; Tergaonkar, V. Evidence for the involvement of the master transcription factor NF-κB in cancer initiation and progression. Biomedicines 2018, 6, 82.
- Oshi, M.; Newman, S.; Tokumaru, Y.; Yan, L.; Matsuyama, R.; Endo, I.; Nagahashi, M.; Takabe, K. Intra-tumoral angiogenesis is associated with inflammation, immune reaction and metastatic recurrence in breast cancer. Int. J. Mol. Sci. 2020, 21, 6708.
- Michels, N.; van Aart, C.; Morisse, J.; Mullee, A.; Huybrechts, I. Chronic inflammation towards cancer incidence: A systematic review and meta-analysis of epidemiological studies. Crit. Rev. Oncol. Hematol. 2021, 157, 103177.
- Aung, T.N.; Qu, Z.; Kortschak, R.D.; Adelson, D.L. Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int. J. Mol. Sci. 2017, 18, 656.
- Lee, C.; Ryu, H.W.; Kim, S.; Kim, M.; Oh, S.-R.; Ahn, K.-S.; Park, J. Verminoside from Pseudolysimachion rotundum var. subintegrum sensitizes cisplatin-resistant cancer cells and suppresses metastatic growth of human breast cancer. Sci. Rep. 2020, 10, 20337.
- Zuo, Y.; Zhang, C.-Z.; Ren, Q.; Chen, Y.; Li, X.; Yang, J.-R.; Li, H.-X.; Tang, W.-T.; Hing-Man, H.; Sun, C. Activation of mitochondrial-associated apoptosis signaling pathway and inhibition of PI3K/Akt/mTOR signaling pathway by voacamine suppress breast cancer progression. Phytomedicine 2022, 99, 154015.
- Wang, W.; Zafar, A.; Rajaei, M.; Zhang, R. Two birds with one stone: NFAT1-MDM2 dual inhibitors for cancer therapy. Cells 2020, 9, 1176.
- Qin, J.-J.; Sarkar, S.; Voruganti, S.; Agarwal, R.; Wang, W.; Zhang, R. Identification of lineariifolianoid A as a novel dual NFAT1 and MDM2 inhibitor for human cancer therapy. J. Biomed. Res. 2016, 30, 322–333.
- Sun, X.; Ma, X.; Li, Q.; Yang, Y.; Xu, X.; Sun, J.; Yu, M.; Cao, K.; Yang, L.; Yang, G. Anti-cancer effects of fisetin on mammary carcinoma cells via regulation of the PI3K/Akt/mTOR pathway: In vitro and in vivo studies. Int. J. Mol. Med. 2018, 42, 811–820.
- Zhao, K.; Song, X.; Huang, Y.; Yao, J.; Zhou, M.; Li, Z.; You, Q.; Guo, Q.; Lu, N. Wogonin inhibits LPS-induced tumor angiogenesis via suppressing PI3K/Akt/NF-κB signaling. Eur. J. Pharmacol. 2014, 737, 57–69.
- Bauer, D.; Mazzio, E.; Soliman, K.F. Whole transcriptomic analysis of apigenin on TNFα immuno-activated MDA-MB-231 breast cancer cells. Cancer Genom. Proteom. 2019, 16, 421–431.
- Xia, S.; Zhang, X.; Li, C.; Guan, H. Oridonin inhibits breast cancer growth and metastasis through blocking the Notch signaling. Saudi Pharm. J. 2017, 25, 638–643.
- Pons, D.G.; Vilanova-Llompart, J.; Gaya-Bover, A.; Alorda-Clara, M.; Oliver, J.; Roca, P.; Sastre-Serra, J. The phytoestrogen genistein affects inflammatory-related genes expression depending on the ERα/ERβ ratio in breast cancer cells. Int. J. Food Sci. Nutr. 2019, 70, 941–949.
- He, L.; Wang, X.; Ma, Q.; Zhao, W.; Jia, Y.; Dong, G.; Zhu, Y.; Jia, X.; Tong, Z. Glycyrrhizin inhibits the invasion and metastasis of breast cancer cells via upregulation of expressions of miR-200c and e-cadherin. Trop. J. Pharm. Res. 2020, 19, 1807–1813.
- Hsieh, C.-J.; Kuo, P.-L.; Hsu, Y.-C.; Huang, Y.-F.; Tsai, E.-M.; Hsu, Y.-L. Arctigenin, a dietary phytoestrogen, induces apoptosis of estrogen receptor-negative breast cancer cells through the ROS/p38 MAPK pathway and epigenetic regulation. Free Radic. Biol. Med. 2014, 67, 159–170.
- Peng, B.; He, R.; Xu, Q.; Yang, Y.; Hu, Q.; Hou, H.; Liu, X.; Li, J. Ginsenoside 20 (S)-protopanaxadiol inhibits triple-negative breast cancer metastasis in vivo by targeting EGFR-mediated MAPK pathway. Pharmacol. Res. 2019, 142, 1–13.
- Zhu, L.; Xue, L. Kaempferol suppresses proliferation and induces cell cycle arrest, apoptosis, and DNA damage in breast cancer cells. Oncol. Res. 2019, 27, 629.
- Jia, H.; Liu, M.; Wang, X.; Jiang, Q.; Wang, S.; Santhanam, R.K.; Lv, C.; Zhao, Q.; Lu, J. Cimigenoside functions as a novel γ-secretase inhibitor and inhibits the proliferation or metastasis of human breast cancer cells by γ-secretase/Notch axis. Pharmacol. Res. 2021, 169, 105686.
- Huyen, C.T.T.; Luyen, B.T.T.; Khan, G.J.; Oanh, H.V.; Hung, T.M.; Li, H.-J.; Li, P. Chemical constituents from Cimicifuga dahurica and their anti-proliferative effects on MCF-7 breast cancer cells. Molecules 2018, 23, 1083.
- Wang, W.; Zhang, X.; Qin, J.-J.; Voruganti, S.; Nag, S.A.; Wang, M.-H.; Wang, H.; Zhang, R. Natural product ginsenoside 25-OCH3-PPD inhibits breast cancer growth and metastasis through down-regulating MDM2. PLoS ONE 2012, 7, e41586.
- Chung, H.; Choi, H.S.; Seo, E.-K.; Kang, D.-H.; Oh, E.-S. Baicalin and baicalein inhibit transforming growth factor-β1-mediated epithelial-mesenchymal transition in human breast epithelial cells. Biochem. Biophys. Res. Commun. 2015, 458, 707–713.
- Zeng, A.; Liang, X.; Zhu, S.; Liu, C.; Luo, X.; Zhang, Q.; Song, L. Baicalin, a potent inhibitor of NF-κB signaling pathway, enhances chemosensitivity of breast cancer cells to docetaxel and inhibits tumor growth and metastasis both in vitro and in vivo. Front. Pharmacol. 2020, 11, 879.
- El Gaafary, M.; Saber, F.R.; Mahrous, E.A.; Ashour, R.M.; Okba, M.M.; Jin, L.; Lang, S.J.; Schmiech, M.; Simmet, T.; Syrovets, T. The phloroglucinol calcitrinone A, a novel mitochondria-targeting agent, induces cell death in breast cancer cells. Food Chem. Toxicol. 2022, 162, 112896.
- Cansaran-Duman, D.; Yangin, S.; Çolak, B. The role of vulpinic acid as a natural compound in the regulation of breast cancer-associated miRNAs. Biol. Res. 2021, 54, 37.
- Liu, Y.; Zou, T.; Wang, S.; Chen, H.; Su, D.; Fu, X.; Zhang, Q.; Kang, X. Genistein-induced differentiation of breast cancer stem/progenitor cells through a paracrine mechanism. Int. J. Oncol. 2016, 48, 1063–1072.
- Tarkowski, M.; Kokocińska, M.; Latocha, M. Genistein in chemoprevention and treatment. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2013, 34, 54–57.
- Wang, K.; Zhang, C.; Bao, J.; Jia, X.; Liang, Y.; Wang, X.; Chen, M.; Su, H.; Li, P.; Wan, J.-B. Synergistic chemopreventive effects of curcumin and berberine on human breast cancer cells through induction of apoptosis and autophagic cell death. Sci. Rep. 2016, 6, 26064.
- Kashyap, A.; Umar, S.M.; Dev, A., Jr.; Mendiratta, M.; Prasad, C.P. In vitro anticancer efficacy of a polyphenolic combination of Quercetin, Curcumin, and Berberine in triple negative breast cancer (TNBC) cells. Phytomed. Plus 2022, 2, 100265.
- Shehatta, N.H.; Okda, T.M.; Omran, G.A.; Abd-Alhaseeb, M.M. Baicalin; a promising chemopreventive agent, enhances the antitumor effect of 5-FU against breast cancer and inhibits tumor growth and angiogenesis in Ehrlich solid tumor. Biomed. Pharmacother. 2022, 146, 112599.
- Ghosh, S.; Pal, A.; Ray, M. Methylglyoxal in combination with 5-Fluorouracil elicits improved chemosensitivity in breast cancer through apoptosis and cell cycle inhibition. Biomed. Pharmacother. 2019, 114, 108855.
- Rai, G.; Suman, S.; Mishra, S.; Shukla, Y. Evaluation of growth inhibitory response of Resveratrol and Salinomycin combinations against triple negative breast cancer cells. Biomed. Pharmacother. 2017, 89, 1142–1151.
- Chimento, A.; Santarsiero, A.; Iacopetta, D.; Ceramella, J.; De Luca, A.; Infantino, V.; Parisi, O.I.; Avena, P.; Bonomo, M.G.; Saturnino, C. A phenylacetamide resveratrol derivative exerts inhibitory effects on breast cancer cell growth. Int. J. Mol. Sci. 2021, 22, 5255.
- Saini, M.; Sangwan, R.; Khan, M.F.; Kumar, A.; Verma, R.; Jain, S. Specioside (SS) & verminoside (VS)(Iridoid glycosides): Isolation, characterization and comparable quantum chemical studies using density functional theory (DFT). Heliyon 2019, 5, e01118.
- Huang, J.; Li, H.; Ren, G. Epithelial-mesenchymal transition and drug resistance in breast cancer. Int. J. Oncol. 2015, 47, 840–848.
- Singh, I.P.; Sidana, J.; Bharate, S.B.; Foley, W.J. Phloroglucinol compounds of natural origin: Synthetic aspects. Nat. Prod. Rep. 2010, 27, 393–416.
- Larayetan, R.; Ololade, Z.S.; Ogunmola, O.O.; Ladokun, A. Phytochemical constituents, antioxidant, cytotoxicity, antimicrobial, antitrypanosomal, and antimalarial potentials of the crude extracts of Callistemon citrinus. Evid. Based Complement. Altern. Med. 2019, 2019, 5410923.
- Kim, R.K.; Suh, Y.; Yoo, K.C.; Cui, Y.H.; Hwang, E.; Kim, H.J.; Kang, J.S.; Kim, M.J.; Lee, Y.Y.; Lee, S.J. Phloroglucinol suppresses metastatic ability of breast cancer cells by inhibition of epithelial-mesenchymal cell transition. Cancer Sci. 2015, 106, 94–101.
- Vidic, D.; Maksimovic, M.; Cavar, S.; Solic, M.E. Comparison of essential oil prof iles of Satureja montana l. and Endemic Satureja visianii Šilic. J. Essent. Oil Bear. Plants 2009, 12, 273–281.
- Esmaeili, A.; Rustaiyan, A.; Masoudi, S.; Nadji, K. Composition of the essential oils of Mentha aquatica L. and Nepeta meyeri Benth. from Iran. J. Essent. Oil Res. 2006, 18, 263–265.
- Medjahed, F.; Merouane, A.; Saadi, A.; Bader, A.; Cioni, P.L.; Flamini, G. Chemical profile and antifungal potential of essential oils from leaves and flowers of Salvia algeriensis (Desf.): A comparative study. Chil. J. Agric. Res. 2016, 76, 195–200.
- Loizzo, M.R.; Menichini, F.; Tundis, R.; Bonesi, M.; Nadjafi, F.; Saab, A.M.; Frega, N.G.; Menichini, F. Comparative chemical composition and antiproliferative activity of aerial parts of Salvia leriifolia Benth. and Salvia acetabulosa L. essential oils against human tumor cell in vitro models. J. Med. Food 2010, 13, 62–69.
- Ghantous, A.; Gali-Muhtasib, H.; Vuorela, H.; Saliba, N.A.; Darwiche, N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today 2010, 15, 668–678.
- Memariani, T.; Hosseini, T.; Kamali, H.; Mohammadi, A.; Ghorbani, M.; Shakeri, A.; Spandidos, D.A.; Tsatsakis, A.M.; Shahsavand, S. Evaluation of the cytotoxic effects of Cyperus longus extract, fractions and its essential oil on the PC3 and MCF7 cancer cell lines. Oncol. Lett. 2016, 11, 1353–1360.
- Furtado, F.B.; Borges, B.C.; Teixeira, T.L.; Garces, H.G.; de Almeida Junior, L.D.; Alves, F.C.B.; da Silva, C.V.; Junior, A.F. Chemical composition and bioactivity of essential oil from Blepharocalyx salicifolius. Int. J. Mol. Sci. 2018, 19, 33.
- Akiel, M.A.; Alshehri, O.Y.; Aljihani, S.A.; Almuaysib, A.; Bader, A.; Al-Asmari, A.I.; Alamri, H.S.; Alrfaei, B.M.; Halwani, M.A. Viridiflorol induces anti-neoplastic effects on breast, lung, and brain cancer cells through apoptosis. Saudi J. Biol. Sci. 2022, 29, 816–821.
- Solárová, Z.; Liskova, A.; Samec, M.; Kubatka, P.; Büsselberg, D.; Solár, P. Anticancer potential of lichens’ secondary metabolites. Biomolecules 2020, 10, 87.
- Sahin, E.; Dabagoglu Psav, S.; Avan, I.; Candan, M.; Sahinturk, V.; Koparal, A.T. Vulpinic acid, a lichen metabolite, emerges as a potential drug candidate in the therapy of oxidative stress-related diseases, such as atherosclerosis. Hum. Exp. Toxicol. 2019, 38, 675–684.
- Kılıç, N.; Derici, M.K.; Buyuk, I.; Aydın, S.; Aras, S.; Duman, D. Evaluation of in vitro anticancer activity of vulpinic acid and its apoptotic potential using gene expression and protein analysis. Indian J. Pharm. Educ. Res. 2018, 52, 46–54.
- Kılıç, N.; Aras, S.; Cansaran-Duman, D. Determination of vulpinic acid effect on apoptosis and mRNA expression levels in breast cancer cell lines. Anti-Cancer Agents Med. Chem. 2018, 18, 2032–2041.
- Zhang, L.; Yang, B.; Zhou, K.; Li, H.; Li, D.; Gao, H.; Zhang, T.; Wei, D.; Li, Z.; Diao, Y. Potential therapeutic mechanism of genistein in breast cancer involves inhibition of cell cycle regulation. Mol. Med. Rep. 2015, 11, 1820–1826.
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83.
- Xie, Q.; Bai, Q.; Zou, L.Y.; Zhang, Q.Y.; Zhou, Y.; Chang, H.; Yi, L.; Zhu, J.D.; Mi, M.T. Genistein inhibits DNA methylation and increases expression of tumor suppressor genes in human breast cancer cells. Genes Chromosom. Cancer 2014, 53, 422–431.
