You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Teja Senekovič Kojc and Version 2 by Dean Liu.
Novel biomarkers of heart failure are the subject of numerous studies. Biomarkers of heart failure can be determined in the blood and in the urine. The future of biomarker use is in multimarker panels that include a combination of biomarkers with different pathophysiological mechanisms in order to improve their diagnostic and prognostic predictive value.
- biomarkers
- heart failure
- myocardial stretch
- myocyte injury
- myocardial remodeling
- inflammation
- renal dysfunction
- oxidative stress
- child
1. Introduction
Despite advances in medicine, heart failure is still an important cause of morbidity and mortality in the modern world. Consequently, there is a considerable need to find new ways of predicting, screening, and prognosticating heart failure, especially in pediatrics [1]. Laboratory diagnostics is an important part of the decision-making process in everyday clinical practice in order to come to a diagnosis, and additionally for risk stratification and therapeutical choices [2].
Various novel biomarkers of heart failure have been studied in adults. However, reliable novel biomarkers of heart failure in pediatrics have not been sufficiently studied for everyday clinical practice yet, therefore, additional knowledge is very welcome. RIn this researchersview, we try to classify biomarkers according to the pathophysiological mechanisms that contribute to the development of heart failure. Several biomarkers of heart failure are still under evaluation and a detailed review of all of them is beyond the scope of this narrative review.
In pediatrics, biomarkers of heart failure are particularly important for the early identification and risk stratification of patients with systemic diseases and associated risk for early development of heart failure. Good biomarkers have the following characteristics: high sensitivity and specificity, the possibility of simultaneous processing of many samples, short analysis time, low cost, and good clinical applications, thus predicting the risk of heart failure and the associated prognosis as well as the adequacy of monitoring [3].
Two strategies are currently in place to detect newer biomarkers of heart failure, the first is based on proteomics and metabolomics, which means comparing blood and tissue samples from patients with heart failure with healthy individuals. It provides data on the expression of proteins and their breakdown products [4]. This first approach does not provide a lot of information about the pathophysiological processes that lead to the disease, which is typical for the second approach, based on the mechanisms underlying the development of cardiovascular disease [5]. Biomarkers of heart failure can be determined in blood samples and some also in urine samples. RIn this researchers view, we will present seven groups of newer biomarkers that are associated with heart failure based on pathophysiological mechanisms, as seen in Table 1. Normal values of some biomarkers of heart failure are presented in Table 2 [6][7][8][9][6,7,8,9]. In addition, rwesearchers will also highlight the possibilities of determining biomarkers in the urine, which allows less invasive sampling and better participation of patients and healthy individuals in potential clinical studies.
Table 1.
Biomarkers of heart failure based on pathophysiological mechanisms.
1 BNP, brain natriuretic peptide; 2 NT-proBNP, N-terminal-proBNP; 3 ANP, atrial natriuretic peptide; 4 MR-proANP, mid-regional proatrial natriuretic peptide; 5 cTn, cardiac troponins; 6 TnI, troponin I; 7 TnT, troponin T; 8 hs-cTn, high-sensitivity cardiac troponin; 9 H-FABPs, heart-type fatty acid-binding proteins; 10 GSTP1, glutathione transferase P1; 11 sST2, soluble isoform of suppression of tumorigenicity 2; 12 GDF-15, growth differentiation factor-15; 13 EMPs, endothelial microparticles; 14 EPCs, endothelial progenitor cells; 15 CRP, C-reactive protein; 16 hs-CRP, high-sensitivity C-reactive protein; 17 TNF-α, tumor necrosis factor alpha; 18 IL-6, interleukin-6; 19 NGAL, neutrophil gelatinase-associated lipocalin; 20 KIM-1, kidney injury molecule-1; 21 IL-18, interleukin-18; 22 L-FABP, liver-type fatty acid-binding protein; 23 NAG, N-acetyl-β-D-glucosaminidase; 24 MR-proADM, mid-regional pro-adrenomedullin; 25 MMPs, matrix metalloproteinases; 26 MPO, myeloperoxidase; 27 SUA, serum uric acid.
Table 2.
Normal values of some biomarkers of heart failure with pediatric specificities according to available data.
| Galectin-3 | ||
| <22.1 ng/mL | ||
| <33 ng/mL | ||
| sST2 7 | <49.3 ng/mL (male) <33.5 ng/mL (female) |
<50 ng/mL |
| GDF-15 8 | <584 pg/mL | |
| NGAL 9 | <50 ng/mL | |
| MR-proADM 10 | <0.55 nmol/L | |
| Copeptin | <11.25 pmol/L | <13.1 pmol/L |
1 BNP, brain natriuretic peptide; 2 NT-proBNP, N-terminal-proBNP; 3 MR-proANP, mid-regional proatrial natriuretic peptide; 4 hsTnT, high-sensitivity troponin T; 5 hsTnI, high-sensitivity troponin I; 6 H-FABPs, heart-type fatty acid-binding proteins; 7 sST2, soluble isoform of suppression of tumorigenicity 2; 8 GDF-15, growth differentiation factor-15; 9 NGAL, neutrophil gelatinase-associated lipocalin; 10 MR-proADM, mid-regional pro-adrenomedullin; 11 Y, year; 12 M, month.
Heart failure is a condition in which the heart is not able to pump enough blood to meet the needs of all tissues [5]. This causes an increase in blood volume by regulating sodium and retaining water in the body. Natriuretic peptides are produced in atrial and ventricular cells due to pressure or volume overload, as seen in Figure 1. ANP (atrial natriuretic peptide) and BNP (brain natriuretic peptide) are used in the diagnosis of heart failure and lead to natriuresis, diuresis, and vasodilatory mechanisms, which are compensatory mechanisms in heart failure [10]. In clinical practice, the precursor of BNP, i.e., NT-proBNP (N-terminal-proBNP), is used primarily in suspected heart failure and in the monitoring of patients with known heart failure. BNP and NT-proBNP values are influenced by age, sex, obesity, renal function, and lung disease [11]. In pediatric patients, NT-proBNP correlates well with the stage of disease and is a better predictive factor of heart failure than BNP [12].
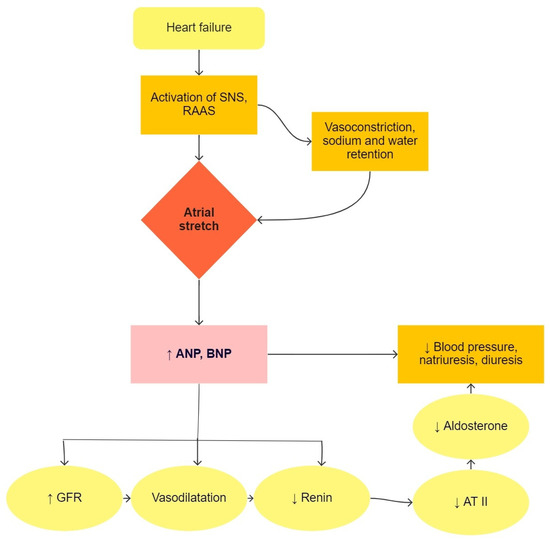
2. Biomarkers of Myocardial Stretch

Figure 1. The physiological function of natriuretic peptides in heart failure. SNS, sympathetic nervous system; RAAS, renin-angiotensin-aldosterone system; ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; GFR, glomerular filtration rate; AT II, angiotensin II.
3. Biomarkers of Myocyte Injury
Numerous studies have already been conducted in the field of biomarkers of myocardial damage, as the process of cell death of cardiomyocytes due to apoptosis or necrosis is at the forefront of heart failure. Various mechanisms lead to cell death, such as poorer tissue perfusion, poorer oxygen supply, increased heart muscle load, circulating neurohormones, adrenergic system activation, inflammation, and oxidative stress [13][18].
Remodeling of the matrix leading to cardiac fibrosis is a crucial factor in the progression of heart failure, as evidenced by impaired systolic and diastolic ventricular function [14][37].
Chronic inflammation is one of the pivotal mechanisms in developing heart failure and is related to the progression and prognosis of heart failure. Inflammatory mediators have a direct impact on the heart muscle as well as on the adrenergic system, which leads to hypertrophy, fibrosis, and impaired cardiac function [1]. In the group of inflammatory biomarkers of heart failure are some traditional biomarkers, such as C-reactive protein (CRP) and high-sensitivity C-reactive protein (hs-CRP), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), as well as some new biomarkers [5]. Anti-inflammatory therapies are under investigation in patients with heart failure [15][52].
Cardiovascular and renal diseases are strongly related, as impaired function of one organ often leads to deterioration of function of the other. In patients with cardiorenal syndrome, i.e., with the involvement of both organ systems, morbidity and mortality are greatly increased. Many biomarkers are already used in the field of renal impairment, such as cystatin C, NGAL (neutrophil gelatinase-associated lipocalin), KIM-1 (kidney injury molecule-1), interleukin-18, L-FABP (liver-type fatty acid-binding protein), NAG (N-acetyl-β-D-glucosaminidase), β-2 microglobulin, and glutathione-S-transferase [16][58]. In the case of heart failure, panels of renal dysfunction biomarkers are often used [17][47].
Cardiac failure is characterized by the activation of the neurohumoral system, namely the sympathetic nervous system. At the initial stage of heart failure, the body tries to provide adequate tissue perfusion through compensatory mechanisms, including activation of the sympathetic nervous system, renin-angiotensin-aldosterone system, decreased activity of the parasympathetic system, and dysregulation of the signaling pathway with nitric oxide (NO), and synthesis of inflammatory cytokines [18][70]. In addition to the classic biomarkers of neurohumoral activation, such as norepinephrine, plasma renin activity, angiotensin II and aldosterone, newer biomarkers are also the subject of research.
Heart failure relates to oxidative stress due to circulating neurohormones, hemodynamic changes, inflammation, and poor oxygen supply. Then, disorders of redox balance even further impair vital structures and affect signaling pathways of cell renewal and cell death, additionally deteriorating heart failure [19][78]. There are many biomarkers in the group of oxidative stress, such as serum uric acid, myeloperoxidase (MPO), vitamin D3, ceruloplasmin, and 8-hydroxy-2-0-deoxyguanosine. An elevated level of serum uric acid is common in patients with heart failure, hypertension, atherosclerosis, obesity, diabetes mellitus, and chronic renal disease [20][79]. Serum levels of myeloperoxidase, vitamin D3, ceruloplasmin, and 8-hydroxy-2-0-deoxyguanosine correlate well with the stage of heart failure [17][47].
With the development of extended research on biomarkers in patients with heart failure and cardiovascular risk, the possibility to determine biomarkers in urine has emerged, which further simplifies investigation and improves the compliance of patients and healthy individuals in clinical trials, even more so in pediatrics. Biomarkers of renal dysfunction associated with heart failure, such as neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1), may be determined in urine. NGAL is a good independent prognostic factor in patients with heart failure [21][62]. KIM-1 is used as a biomarker of renal tubular impairment in patients with acute or chronic heart failure, furthermore, it correlates well with the stage of disease and may also be used as a predictor of cardiorenal syndrome [22][68]. Galectin-3, which is involved in myocardial remodeling and fibrosis, can also be detected in urine and has been shown as a good prognostic factor in patients with heart failure with preserved ejection fraction [23][86]. In addition, natriuretic peptides, which are the main biomarkers of myocardial stretch may also be determined in the urine, as well as sodium concentration, β-2 microglobulin, and albumin/creatinine ratio in order to achieve risk stratification of patients with heart failure [24][87].
