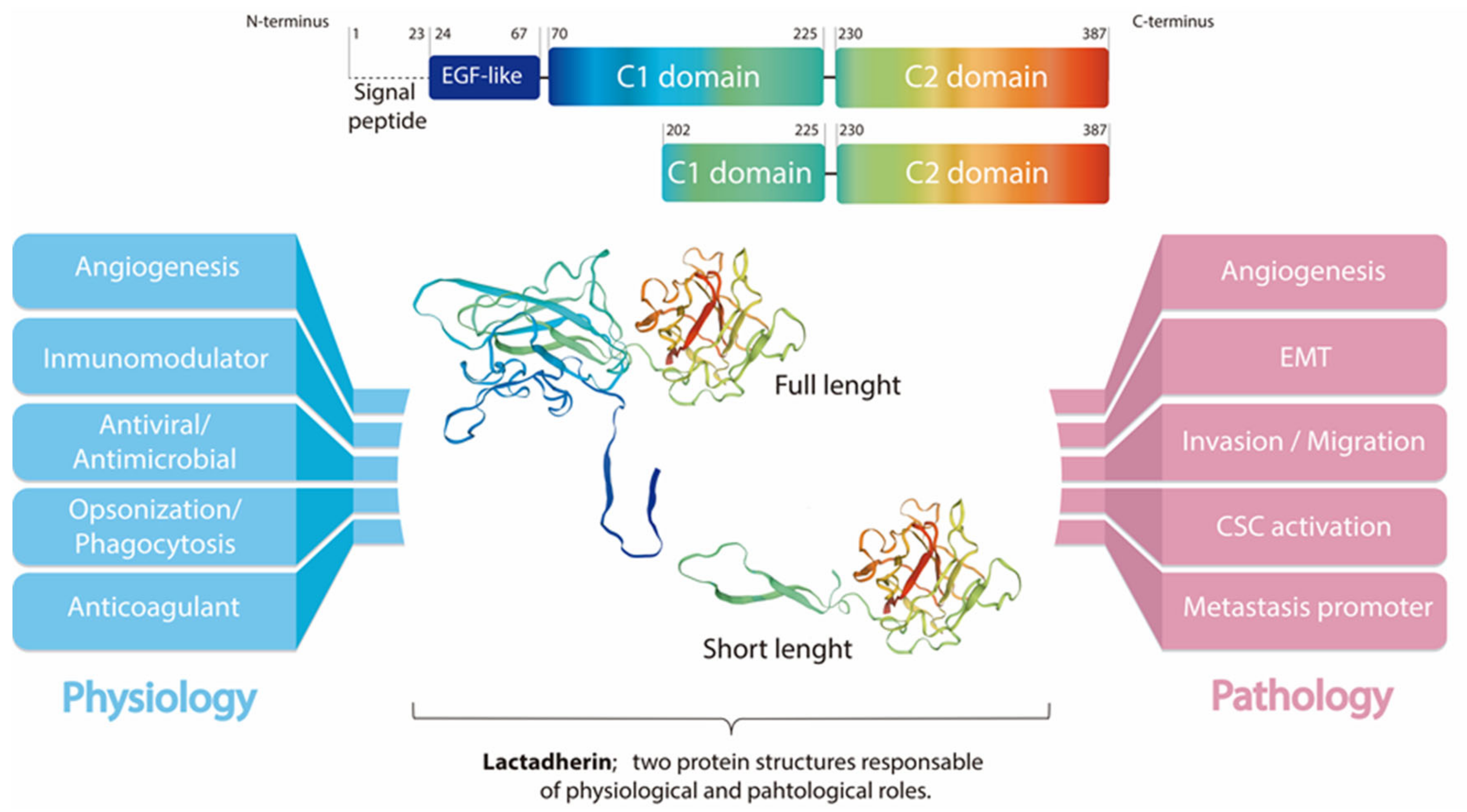
Lactadherin is a secreted glycoprotein associated with the milk fat globule membrane, which is highly present in the blood and in the mammary tissue of lactating women. Several biological functions have been associated with this protein, mainly attributable to its immunomodulatory role promoting phagocyte-mediated clearance of apoptotic cells. It has been shown that lactadherin also plays important roles in cell adhesion, promotion of angiogenesis, and tissue regeneration. On the other hand, this protein has been used as a marker of breast cancer and tumor progression. Recently, high levels of lactadherin has been associated with poor prognosis and decreased survival, not only in breast cancer, but also in melanoma, ovarian, colorectal, and other types of cancer. Although the mechanisms responsible for the tumor-promoting effects attributed to lactadherin have not been fully elucidated, a growing body of literature indicates that lactadherin could be a promising therapeutic target and/or biomarker for breast and other tumors. Moreover, recent studies have shown its presence in extracellular vesicles derived from cancer cell lines and cancer patients, which was associated with cancer aggressiveness and worse prognosis.
- lactadherin
- breast cancer
- metastasis
- exosomes
- extracellular vesicles
1. Introduction
Lactadherin: A Milk Fat Globule Protein Associated with Cancer Development
2. Lactadherin as a Biomarker of Tumor Subtype, Progression and Metastasis
3. Lactadherin Role as Promotor of Tumor Progression
Lactadherin expression has been associated with the aggressiveness and progression of several types of cancer. In fact, most of the studies associated lactadherin overexpression in tumor samples with the promotion of pro-tumorigenic and pro-metastatic capacities, such as increased tumor cell proliferation, angiogenesis, migration, invasion, and epithelial-to-mesenchymal transition (EMT) [11,19,21,22,39,40,41,42,48][11][27][34][38][39][40][41][42][43]; processes that are related to the physiological function of this protein. In this context, several studies in the cancer field, but also in other diseases, have demonstrated the activation of commonly pro-tumorigenic signaling pathways mediated by lactadherin overexpression, such as their interaction with β3-integrin, and the activation of the PI3K/AKT signaling [39,42,49,50,51][39][42][44][45][46]. On the other hand, most of the studies have not deepened in the downstream intracellular signaling triggered by lactadherin, thus leaving the field open for more complete and comprehensive studies.3.1. Tumor Cell Survival/Proliferation and EMT
Lactadherin is able to interact with αvβ3/β5-integrins through its RGD domain. This interaction allows the anchorage and rapprochement between cells (or extracellular matrix), thus favoring apoptotic cell clearance through phagocytosis [2,5,7][2][5][7]. On the other hand, the activation of β3-integrin signaling can lead to the activation of pro-survival, anti-apoptotic, and pro-metastatic pathways through the activation of molecules such as Akt and Twist, as shown in murine melanoma studies [39]. This lactadherin/β3-integrin interaction could promote the EMT process (through PI3K/Akt, Twist); an effect that has also been observed in CRC cancer models, where lactadherin promotes the migration and invasion of tumor cells [42].3.2. Lactadherin Immunomodulatory Role
Another important function of lactadherin is its immunomodulatory role, mainly as an immunosuppressive molecule [1]. The first reports in this topic show that lactadherin is able to bind β3-integrin on the surface of phagocytes, mainly macrophages. In the same way, lactadherin can bind PS exposed in cells undergoing apoptosis, and promote their phagocytosis [7] (Figure 1). Subsequent studies have shown that other cells of the immune system also express lactadherin, such as dendritic cells, mast cells, and regulatory T lymphocytes, in addition to endothelial cells that are in direct contact with blood and blood cells [1,11][1][11]. Lactadherin has also been involved in the modulation of the immune system in other types of cancer, such as oral cancer [35][30], esophageal cancer [36][31], and, recently, angiosarcoma [37][32] and a murine glioma model [55][47]. The immunomodulation in these types of cancer have been constantly associated with tumor development and bad prognosis.4. Lactadherin as a Possible New Cancer Therapeutic Target
Although lactadherin has various regulatory physiological roles, and its expression and function have been targeted for the treatment of several other diseases (e.g., vascular and autoimmune pathologies) [13[13][14][15][46][48][49][50],14,15,51,56,57,58], it has been established to be most relevant in the oncologic area. First studies by Ceriani et al. showed that an antibody cocktail, including MoAbs against HMFG components (one of them against lactadherin), worked as treatment and prevention against the engraftment of ER-positive and negative mammary tumors [27,30][20][23]. Later, this group and others published several reports using this cocktail of antibodies, including an anti-lactadherin blocking antibody, demonstrating its beneficial effect against breast tumors [31,32,59][24][25][51]. Subsequently, several studies reported lactadherin as a possible cancer therapeutic target [20,21,22,41[28][34][38][41][43],48], either using gene therapy approaches, inhibiting its expression by siRNAs or shRNAs, or by using specific antibodies that block its function and interactions with αvβ3/β5 integrins [45][35]. Recently, using TCGA data and machine learning, Kothari et al. have shown that lactadherin is one out of two proteins/genes that can differentiate TNBC from non-TNBC irrespective of their heterogeneity or subtype differences [60][52]. Further affinity purification mass spectrometry and proximity biotinylation experiments identified a possible role for lactadherin in various tumor survival processes. Another study also using TCGA RNA-seq data showed that lactadherin, as well as KLK5/7 expression, are associated with COX-2 inhibitors treatment resistance in TNBC cells. ThSis study demonstrated that silencing of these genes markedly recovered COX-2 inhibitor sensitivity both in vitro and in vivo. Considering the difficulty in the treatment of TNBC, these results could support the possibility of using new combination therapies against TNBC involving COX-2 and lactadherin inhibition [61][53].5. Extracellular Vesicles and Exosomes as Promotors of Breast Cancer Metastasis
Metastasis is defined as the dissemination of cancerous cells from the primary tumor, and the effective colonization of secondary target organs. It is widely accepted that intercellular communication is essential in all steps of the metastatic cascade. Exosomes are a particular subpopulation of EVs released by a variety of cell types. These exosomes are 40–200 nm in diameter, and are derived from the multivesicular endosome pathway, and can enter a recipient cell mainly through three different pathways: membrane fusion, endocytosis, or the interaction of proteins in exosomes with receptors in recipient cells [64,65,66,67][54][55][56][57]. Exosomes are thought to play important roles in intercellular communication, transferring a variety of molecules to target recipient cells. Exosomes contain several bioactive molecules, such as nucleic acids (mRNA, microRNA, DNA, and other non-coding RNAs), proteins (receptors, transcription factors, enzymes, extracellular matrix proteins), and lipids that can redirect the phenotype and function of a recipient cell [67,68,69,70][57][58][59][60]. Therefore, exosomes are emerging as local and systemic cell–cell mediators of oncogenic information that play an important role in cancer progression [65,66,67,68,69,70,71,72][55][56][57][58][59][60][61][62]. In breast cancer, there is a potential use of exosomes and other EVs as promising diagnostic and therapeutic biomarkers. On the other hand, exosomes secreted by metastatic cells potentiate tumorigenic capacities of less aggressive cells acting in a paracrine manner [68,69,70,71,72,73][58][59][60][61][62][63]. Moreover, recent reports indicate that exosomes are able to condition the microenvironment, where tissue recipient cells are waiting for the advent of a tumor cell, and which in turn, are capable of promoting angiogenic mechanisms in breast cancer [71][61]. At the same time, exosomes secreted by tumor stroma can also influence tumor progression. Breast-cancer-associated fibroblasts secrete exosomes that have been shown to promote tumor mobility, invasion, and dissemination of breast cancer cells through the Wnt-planar cell polarity pathway (Wnt-PCP pathway) [74][64].6. Role and Use of Lactadherin in EVs and Exosomes
Lactadherin expression has been reported in a variety of tissues and organs. However, its overexpression has been associated with bad prognosis and outcomes in different types of cancers. Moreover, lactadherin presence in EVs has recently been reported in several studies and databases [79[65][66][67],80,81], which has led to the consideration of it as a possible new marker of EVs. On the other hand, lactadherin C1C2 domains have been extensively used in EV designing and engineering. Due to its presence in EVs, and its interaction with PS (also present on EV surfaces), these domains are usually fused to a protein of interest, thus directing target protein to the EV surface [82,83,84,85][68][69][70][71]. This strategy has also been used to engineer EVs to expose anti-HER2 scFv on their EVs surface, and redirect EVs to HER2+ breast cancer cells to deliver a cargo of mRNA gene therapy [86][72]. More recently, a similar strategy was used by Kooijmans et al. to decorate EVs with EGFR-specific nanobodies fused to the C1C2 domains of lactadherin to further improve tumor cell targeting and incorporation [87][73]. Particularly in the case of breast tissue (normal or tumor), there are few studies describing the presence of lactadherin associated with exosomes or EVs. One of these articles indicates that lactadherin in EVs is required to transduce cellular signals from the basolateral side of adherent cells by accumulating exosomes in mammary epithelial cells [89]. However, this report did not describe the presence of lactadherin on the exosomes. Finally, recent studies by Lobos-Gonzalez et al., described the presence of lactadherin as part of the characterization of exosomes secreted by a metastatic breast cancer cell line (MDA-MB-231); however, functional experiments studying its specific and precise role were not performed [23,91]. As lactadherin has several functions, both as a cell adhesion molecule, and triggering intracellular signaling cascades, it is possible to think that it could have similar roles as part of EVs membranes of EVs cargo".7. Conclusions
Lactadherin is present widely in human tissues, and can easily be targeted by its special multi-domain structures. Lactadherin can promote tumor formation, and prompts cancer vascular angiogenesis, survival, and EMT, regulating multiple oncogenic pathways (p63/p73, PI3K/Akt, β-catenin, Akt/Twist), which can promote cancer cell resistance to chemotherapy and host immunity suppression. However, to date, the specific roles, molecular mechanisms, and signaling pathways by which lactadherin promotes tumorigenic and metastatic properties of tumor cells, especially breast cancer cells, are not completely elucidated. Despite the interesting fact that several patents have used lactadherin as a possible tumor marker or even therapeutic target, there are no reported clinical trials in which this protein is being tested. On the other hand, it is well known that EVs and exosomes can mediate cell–cell communication, and can promote the acquisition of oncogenic and pro-metastatic properties in recipient cells. Past and recent works have shown the presence of lactadherin in EVs secreted by different cell types. However, the role of lactadherin present in EVs, more specifically, in exosomes secreted by cancer cells (or other cells in the tumor microenvironment), is still unknown. Given the importance of this protein modulating tumor development and progression, deeper understanding on the role of lactadherin in EV- and exosome-mediated tumor progression and metastasis could be a promising focus of study in the future.References
- Sabha, B.H.; Alzahrani, F.; Almehdar, H.A.; Uversky, V.N.; Redwan, E.M. Disorder in milk proteins: Lactadherin multifunctionality and structure. Curr. Protein Pept. Sci. 2018, 19, 983–997.
- Taylor, M.R.; Couto, J.R.; Scallan, C.D.; Ceriani, R.L.; Peterson, J.A. Lactadherin (Formerly BA46), a Membrane-Associated Glycoprotein Expressed in Human Milk and Breast Carcinomas, Promotes Arg-Gly-Asp (RGD)-Dependent Cell Adhesion. DNA Cell Biol. 1997, 16, 861–869.
- Larocca, D.; Peterson, J.A.; Urrea, R.; Kuniyoshi, J.; Bistrain, A.M.; Ceriani, R.L. A Mr 46,000 human milk fat globule protein that is highly expressed in human breast tumors contains Factor VHI-like domains. Cancer Res. 1991, 51, 4994.
- Couto, J.R.; Taylor, M.R.; Godwin, S.G.; Ceriani, R.L.; Peterson, J.A. Cloning and Sequence Analysis of Human Breast Epithelial Antigen BA46 Reveals an RGD Cell Adhesion Sequence Presented on an Epidermal Growth Factor-Like Domain. DNA Cell Biol. 1996, 15, 281–286.
- Andersen, M.H.; Graversen, H.; Fedosov, S.N.; Petersen, T.E.; Rasmussen, J.T. Functional analyses of two cellular binding domains of bovine lactadherin. Biochemistry 2000, 39, 6200–6206.
- Giuffrida, M.G.; Cavaletto, M.; Giunta, C.; Conti, A.; Godovac-Zimmermann, J. Isolation and characterization of full and truncated forms of human breast carcinoma protein BA46 from human milk fat globule membranes. J. Protein Chem. 1998, 17, 143–148.
- Hanayama, R.; Tanaka, M.; Miwa, K.; Shinohara, A.; Iwamatsu, A.; Nagata, S. Identification of a factor that links apoptotic cells to phagocytes. Nature 2002, 417, 182–187.
- Newburg, D.S.; Peterson, J.A.; Ruiz-Palacios, G.M.; Matson, D.O.; Morrow, A.L.; Shults, J.; Guerrero, M.L.; Chaturvedi, P.; Newburg, S.O.; Scallan, C.D.; et al. Role of human-milk lactadherin in protection against symptomatic rotavirus infection. Lancet 1998, 351, 1160–1164.
- Kvistgaard, A.; Pallesen, L.; Arias, C.; Lopez, S.; Petersen, T.; Heegaard, C.; Rasmussen, J.T. Inhibitory Effects of Human and Bovine Milk Constituents on Rotavirus Infections. J. Dairy Sci. 2004, 87, 4088–4096.
- Jinushi, M.; Chiba, S.; Yoshiyama, H.; Masutomi, K.; Kinoshita, I.; Dosaka-Akita, H.; Yagita, H.; Takaoka, A.; Tahara, H. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc. Natl. Acad. Sci. USA 2011, 108, 12425–12430.
- Silvestre, J.-S.; Théry, C.; Hamard, G.; Boddaert, J.; Aguilar, B.; Delcayre, A.; Houbron, C.; Tamarat, R.; Blanc-Brude, O.; Heeneman, S.; et al. Lactadherin promotes VEGF-dependent neovascularization. Nat. Med. 2005, 11, 499–506.
- Zhou, Y.; Bond, A.M.; Shade, J.E.; Zhu, Y.; Davis, C.-H.O.; Wang, X.; Su, Y.; Yoon, K.-J.; Phan, A.T.; Chen, W.J.; et al. Autocrine Mfge8 Signaling Prevents Developmental Exhaustion of the Adult Neural Stem Cell Pool. Cell Stem Cell 2018, 23, 444–452.e4.
- Kamińska, A.; Enguita, F.J.; Stępień, E. Lactadherin: An unappreciated haemostasis regulator and potential therapeutic agent. Vasc. Pharmacol. 2018, 101, 21–28.
- Uchiyama, A.; Yamada, K.; Perera, B.; Ogino, S.; Yokoyama, Y.; Takeuchi, Y.; Ishikawa, O.; Motegi, S.-I. Protective Effect of MFG-E8 after Cutaneous Ischemia–Reperfusion Injury. J. Investig. Dermatol. 2015, 135, 1157–1165.
- Motegi, S.-I.; Leitner, W.; Lu, M.; Tada, Y.; Sárdy, M.; Wu, C.; Chavakis, T.; Udey, M.C. Pericyte-Derived MFG-E8 Regulates Pathologic Angiogenesis. Arter. Thromb. Vasc. Biol. 2011, 31, 2024–2034.
- Motegi, S.; Ishikawa, O. Mesenchymal stem cells: The roles and functions in cutaneous wound healing and tumor growth. J. Dermatol. Sci. 2017, 86, 83–89.
- Nakashima, Y.; Miyagi-Shiohira, C.; Noguchi, H.; Omasa, T. The Healing Effect of Human Milk Fat Globule-EGF Factor 8 Protein (MFG-E8) in A Rat Model of Parkinson’s Disease. Brain Sci. 2018, 8, 167.
- Bocca, S.; Anderson, S.; Amaker, B.; Swanson, R.; Franchi, A.; Lattanzio, F.; Oehninger, S. Milk fat globule epidermal growth factor 8 (MFG-E8): A novel protein in the mammalian endometrium with putative roles in implantation and placentation. Placenta 2012, 33, 795–802.
- Arklie, J.; Taytor-Papadimitrou, J.; Bodmer, W.; Egar, M.; Millis, R. Differentiation antigens expressed by epithelial cells in the lactating breast are also detectable in breast cancer. Int. J. Cancer 1981, 28, 23–29.
- Ceriani, R.L.; Peterson, J.A.; Blank, E.W. Breast Cancer Diagnosis with Human Mammary Epithelial Antigens and the Prospective Use of Antibodies against Them in Therapy. In Mechanisms of Cancer Metastasis. Developments in Oncology; Honn, K.V., Powers, W.E., Sloane, B.F., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 235–257.
- UCSC Xena Open Access. Available online: https://xena.ucsc.edu (accessed on 13 May 2020).
- Human Protein Atlas. (Shows Expression of Lactadherin mRNA and Protein in All Female Reproductive Organs). Available online: https://www.proteinatlas.org/ENSG00000140545-MFGE8/tissue (accessed on 13 May 2020).
- Taylor-Papadimitriou, J.; Peterson, J.A.; Arklie, J.; Burchell, J.; Ceriani, R.L.; Bodmer, W.F. Monoclonal antibodies to epithelial-specific components of the human milk fat globule membrane: Production and reaction with cells in culture. Int. J. Cancer 1981, 28, 17–28.
- Sasaki, M.; Peterson, J.A.; Ceriani, R.L. Monoclonal antibodies against breast epithelial cell surface antigens in breast cancer therapy. Hybridoma 1983, 2, 120.
- Peterson, J.A.; Zava, D.T.; Duwe, A.K.; Blank, E.W.; Battifora, H.; Ceriani, R.L. Biochemical and histological characterization of antigens preferentially expressed on the surface and cytoplasm of breast carcinoma cells identified by monoclonal antibodies against the human milk fat globule. Hybridoma 1990, 9, 221–235.
- Ceriani, R.L.; Sasaki, M.; Sussman, H.; Wara, W.M.; Blank, E.W. Circulating human mammary epithelial antigens in breast cancer. Proc. Natl. Acad. Sci. USA 1982, 79, 5420–5424.
- Neutzner, M.; Lopez, T.; Feng, X.; Bergmann-Leitner, E.S.; Leitner, W.W.; Udey, M.C. MFG-E8/lactadherin promotes tumor growth in an angiogenesis-dependent transgenic mouse model of multistage carcinogenesis. Cancer Res. 2007, 67, 6777–6785.
- Sugano, G.; Bernard-Pierrot, I.; Laé, M.; Battail, C.; Allory, Y.; Stransky, N.; Krumeich, S.; Lepage, M.-L.; Maille, P.; Donnadieu, M.-H.; et al. Milk fat globule–epidermal growth factor–factor VIII (MFGE8)/lactadherin promotes bladder tumor development. Oncogene 2010, 30, 642–653.
- Na, L.; Dai, C.; Yang, Y.; Wu, X.; Wang, L.; Wang, P. The expression levels and clinical significance of MFG-E8 and CD133 in epithelial ovarian cancer. Gynecol. Endocrinol. 2020, 36, 803–807.
- Okamoto, A.; Sakakura, K.; Takahashi, H.; Motegi, S.I.; Kaira, K.; Yokobori-Kuwabara, Y.; Ishikawa, O.; Chikamatsu, K. Immunological and clinicopathological significance of MFG-E8 expression in patients with oral squamous cell carcinoma. Pathol. Oncol. Res. 2019, 26, 1263–1268.
- Kanemura, T.; Miyata, H.; Makino, T.; Tanaka, K.; Sugimura, K.; Hamada-Uematsu, M.; Mizote, Y.; Uchida, H.; Miyazaki, Y.; Takahashi, T.; et al. Immunoregulatory influence of abundant MFG -E8 expression by esophageal cancer treated with chemotherapy. Cancer Sci. 2018, 109, 3393–3402.
- Fujiwara, C.; Motegi, S.-I.; Ohira, A.; Yamaguchi, S.; Sekiguchi, A.; Yasuda, M.; Nakamura, H.; Makiguchi, T.; Yokoo, S.; Hoshina, D.; et al. The significance of tumor cells-derived MFG-E8 in tumor growth of angiosarcoma. J. Dermatol. Sci. 2019, 96, 18–25.
- Yang, W.; Lai, Z.; Li, Y.; Mu, J.; Yang, M.; Xie, J.; Xu, J. Immune signature profiling identified prognostic factors for gastric cancer. Chin. J. Cancer Res. 2019, 31, 463–470.
- Carrascosa, C.; Obula, R.G.; Missiaglia, E.; Lehr, H.A.; Delorenzi, M.; Frattini, M.; Rüegg, C.; Mariotti, A. MFG-E8/lactadherin regulates cyclins D1/D3 expression and enhances the tumorigenic potential of mammary epithelial cells. Oncogene 2012, 31, 1521–1532.
- Yang, C.; Hayashida, T.; Forster, N.; Li, C.; Shen, D.; Maheswaran, S.; Chen, L.; Anderson, K.S.; Ellisen, L.W.; Sgroi, D.; et al. The integrin alpha(v)beta(3–5) ligand MFG-E8 is a p63/p73 target gene in triple-negative breast cancers but exhibits suppressive functions in ER(+) and erbB2(+) breast cancers. Cancer Res. 2011, 71, 937–945.
- Yu, L.; Zhao, L.; Jia, Z.; Bi, J.; Wei, Q.; Song, X.; Jiang, L.; Lin, S.; Wei, M. MFG-E8 overexpression is associated with poor prognosis in breast cancer patients. Pathol. Res. Pr. 2018, 215, 490–498.
- Wang, M.-Y.; Huang, M.; Wang, C.-Y.; Tang, X.-Y.; Wang, J.-G.; Yang, Y.-D.; Xiong, X.; Gao, C.-W. Transcriptome Analysis Reveals MFGE8-HAPLN3 Fusion as a Novel Biomarker in Triple-Negative Breast Cancer. Front. Oncol. 2021, 11, 682021.
- Tibaldi, L.; Leyman, S.; Nicolas, A.; Notebaert, S.; Dewulf, M.; Ngo, T.H.; Zuany-Amorim, C.; Amzallag, N.; Bernard-Pierrot, I.; Sastre-Garau, X.; et al. New Blocking Antibodies Impede Adhesion, Migration and Survival of Ovarian Cancer Cells, Highlighting MFGE8 as a Potential Therapeutic Target of Human Ovarian Carcinoma. PLoS ONE 2013, 8, e72708.
- Jinushi, M.; Nakazaki, Y.; Carrasco, D.R.; Draganov, D.; Souders, N.; Johnson, M.; Mihm, M.C.; Dranoff, G. Milk Fat Globule EGF-8 Promotes Melanoma Progression through Coordinated Akt and Twist Signaling in the Tumor Microenvironment. Cancer Res. 2008, 68, 8889–8898.
- Oba, J.; Moroi, Y.; Nakahara, T.; Abe, T.; Hagihara, A.; Furue, M. Expression of milk fat globule epidermal growth factor-VIII may be an indicator of poor prognosis in malignant melanoma. Br. J. Dermatol. 2011, 165, 506–512.
- Yamada, K.; Uchiyama, A.; Uehara, A.; Perera, B.; Ogino, S.; Yokoyama, Y.; Takeuchi, Y.; Udey, M.C.; Ishikawa, O.; Motegi, S.-I. MFG-E8 Drives Melanoma Growth by Stimulating Mesenchymal Stromal Cell–Induced Angiogenesis and M2 Polarization of Tumor-Associated Macrophages. Cancer Res. 2016, 76, 4283–4292.
- Zhao, Q.; Xu, L.; Sun, X.; Zhang, K.; Shen, H.; Tian, Y.; Sun, F.; Li, Y. MFG-E8 overexpression promotes colorectal cancer progression via AKT/MMPs signalling. Tumor Biol. 2017, 39, 1010428317707881.
- Yang, Y.; Li, J.; Song, Q.; Zhu, K.; Yu, X.; Tian, Y.; Zhang, J. Reduction in milk fat globule-EGF factor 8 inhibits triple-negative breast cancer cell viability and migration. Oncol. Lett. 2019, 17, 3457–3465.
- Xu, X.; Zhang, A.; Zhu, Y.; He, W.; Di, W.; Fang, Y.; Shi, X. MFG-E8 reverses microglial-induced neurotoxic astrocyte (A1) via NF-κB and PI3K-Akt pathways. J. Cell. Physiol. 2018, 234, 904–914.
- Li, H.; Xu, W.; Ma, Y.; Zhou, S.; Xiao, R. Milk fat globule membrane protein promotes C2C12 cell proliferation through the PI3K/Akt signaling pathway. Int. J. Biol. Macromol. 2018, 114, 1305–1314.
- Gao, Y.-Y.; Zhang, Z.-H.; Zhuang, Z.; Lu, Y.; Wu, L.-Y.; Ye, Z.N.; Zhang, X.-S.; Chen, C.-L.; Li, W.; Hang, C.-H. Recombinant milk fat globule-EGF factor-8 reduces apoptosis via integrin β3/FAK/PI3K/AKT signaling pathway in rats after traumatic brain injury. Cell Death Dis. 2018, 9, 845.
- Wu, J.; Yang, H.; Cheng, J.; Zhang, L.; Ke, Y.; Zhu, Y.; Wang, C.; Zhang, X.; Zhen, X.; Zheng, L.T. Knockdown of milk-fat globule EGF factor-8 suppresses glioma progression in GL261 glioma cells by repressing microglial M2 polarization. J. Cell. Physiol. 2019, 235, 8679–8690.
- Zhuowang, G.; Chen, Y.; Wang, B.; Zhang, X.; Yan, Y.; Zhou, L.; Zhang, Y.; Xie, Y. MFGE8 attenuates Ang-II-induced atrial fibrosis and vulnerability to atrial fibrillation through inhibition of TGF-β1/Smad2/3 pathway. J. Mol. Cell. Cardiol. 2020, 139, 164–175.
- Matsuda, A.; Jacob, A.; Wu, R.; Zhou, M.; Aziz, M.; Wang, P. Milk fat globule–EGF factor VIII ameliorates liver injury after hepatic ischemia-reperfusion. J. Surg. Res. 2012, 180, e37–e46.
- Deng, K.-Q.; Li, J.; She, Z.-G.; Gong, J.; Cheng, W.-L.; Gong, F.-H.; Zhu, X.-Y.; Zhang, Y.; Wang, Z.; Li, H. Restoration of Circulating MFGE8 (Milk Fat Globule-EGF Factor 8) Attenuates Cardiac Hypertrophy Through Inhibition of Akt Pathway. Hypertension 2017, 70, 770–779.
- Ceriani, R.L.; Blank, E.W.; Peterson, J.A. Experimental immunotherapy of human breast carcinomas implanted in nude mice with a mixture of monoclonal antibodies against human milk fat globule components. Cancer Res. 1987, 47, 532–540.
- Kotari, C.; Osseni, M.A.; Agbo, L.; Ouellette, G.; Déraspe, M.; Laviolette, F.; Corbeil, J.; Lambert, J.-P.; Diorio, C.; Durocher, F. Machine learning analysis identifies genes differentiating triple negative breast cancers. Sci. Rep. 2020, 10, 10464.
- Tian, J.; Wang, V.; Wang, N.; Khadang, B.; Boudreault, J.; Bakdounes, K.; Ali, S.; Lebrun, J.-J. Identification of MFGE8 and KLK5/7 as mediators of breast tumorigenesis and resistance to COX-2 inhibition. Breast Cancer Res. 2021, 23, 23.
- Thery, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579.
- Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357.
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977.
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17.
- Lee, K.W.; Cho, J.A.; Park, H.; Lim, E.H. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int. J. Oncol. 2011, 40, 130–138.
- Gorczynski, R.M.; Erin, N.; Zhu, F. Serum-derived exosomes from mice with highly metastatic breast cancer transfer increased metastatic capacity to a poorly metastatic tumor. Cancer Med. 2016, 5, 325–336.
- Harris, D.A.; Patel, S.H.; Gucek, M.; Hendrix, A.; Westbroek, W.; Taraska, J.W. Exosomes Released from Breast Cancer Carcinomas Stimulate Cell Movement. PLoS ONE 2015, 10, e0117495.
- Feng, Q.; Zhang, C.; Lum, D.; Druso, J.E.; Blank, B.; Wilson, K.F.; Welm, A.; Antonyak, M.A.; Cerione, R.A. A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis. Nat. Commun. 2017, 8, 14450.
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Mark, M.T.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335.
- Galindo-Hernandez, O.; Serna-Marquez, N.; Castillo-Sanchez, R.; Salazar, E.P. Extracellular vesicles from MDA-MB-231 breast cancer cells stimulated with linoleic acid promote an EMT-like process in MCF10A cells. Prostaglandins Leukot. Essent. Fat. Acids 2014, 91, 299–310.
- Luga, V.; Zhang, L.; Viloria-Petit, A.M.; Ogunjimi, A.A.; Inanlou, M.R.; Chiu, E.; Buchanan, M.; Hosein, A.N.; Basik, M.; Wrana, J.L. Exosomes Mediate Stromal Mobilization of Autocrine Wnt-PCP Signaling in Breast Cancer Cell Migration. Cell 2012, 151, 1542–1556.
- Novikova, S.; Shushkova, N.; Farafonova, T.; Tikhonova, O.; Kamyshinsky, R.; Zgoda, V. Proteomic Approach for Searching for Universal, Tissue-Specific, and Line-Specific Markers of Extracellular Vesicles in Lung and Colorectal Adenocarcinoma Cell Lines. Int. J. Mol. Sci. 2020, 21, 6601.
- Pathan, M.; Fonseka, P.; Chitti, S.V.; Kang, T.; Sanwlani, R.; Van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019, 47, D516–D519.
- Véron, P.; Segura, E.; Sugano, G.; Amigorena, S.; Théry, C. Accumulation of MFG-E8/lactadherin on exosomes from immature dendritic cells. Blood Cells Mol. Dis. 2005, 35, 81–88.
- Kenari, A.N.; Cheng, L.; Hill, A.F. Methods for loading therapeutics into extracellular vesicles and generating extracellular vesicles mimetic-nanovesicles. Methods 2020, 177, 103–113.
- Miyasaka, K.; Hanayama, R.; Tanaka, M.; Nagata, S. Expression of milk fat globule epidermal growth factor?8 in immature dendritic cells for engulfment of apoptotic cells. Eur. J. Immunol. 2004, 34, 1414–1422.
- Zeelenberg, I.S.; Ostrowski, M.; Krumeich, S.; Bobrie, A.; Jancic, C.; Boissonnas, A.; Delcayre, A.; Le Pecq, J.-B.; Combadière, B.; Amigorena, S.; et al. Targeting Tumor Antigens to Secreted Membrane Vesicles In vivo Induces Efficient Antitumor Immune Responses. Cancer Res. 2008, 68, 1228–1235.
- Komuro, H.; Kawai-Harada, Y.; Aminova, S.; Pascual, N.; Malik, A.; Contag, C.H.; Harada, M. Engineering Extracellular Vesicles to Target Pancreatic Tissue In Vivo. Nanotheranostics 2021, 5, 378–390.
- Wang, J.H.; Forterre, A.V.; Zhao, J.; Frimannsson, D.O.; Delcayre, A.; Antes, T.J.; Efron, B.; Jeffrey, S.S.; Pegram, M.D.; Matin, A.C. Anti-HER2 scFv-Directed Extracellular Vesicle-Mediated mRNA-Based Gene Delivery Inhibits Growth of HER2-Positive Human Breast Tumor Xenografts by Prodrug Activation. Mol. Cancer Ther. 2018, 17, 1133–1142.
- Kooijmans, S.A.A.; Gitz-Francois, J.J.J.M.; Schiffelers, R.M.; Vader, P. Recombinant phosphatidylserine-binding nanobodies for targeting of extracellular vesicles to tumor cells: A plug-and-play approach. Nanoscale 2018, 10, 2413–2426.
