Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Francesco Guerrini and Version 2 by Conner Chen.
Glioblastoma are the most common primary malignant brain tumors with a highly infiltrative behavior. The extent of resection of the enhancing component has been shown to be correlated to survival. Recently, it has been proposed to move the resection beyond the contrast-enhanced portion into the MR hyper intense tissue which typically surrounds the tumor, the so-called supra marginal resection (SMR).
- Glioblastoma
- high-grade glioma
- supramarginal resection
- Flairectomy
1. Definition of SMR, Its Impact on OS, and the Role of FLAIR
There is a discrete consensus about the definition of SMR since the majority of the studies state that SMR is the resection of any part of a T1w gadolinium-enhanced tumor exceeding FLAIR volume.
However, far less agreement can be found concerning the role of SMR and its impact on OS. In fact, in contrast to the well-established role of the EOR of the contrast-enhanced component, when considering the so-called “FLAIR-ectomy” there is much less agreement. Li et al. found that a resection larger than 53.2% of FLAIR volume confers an advantage on OS in previously untreated IDH mutated patients [1][23]. A quite similar value was found by Pessina et al. and Tripathi et al. who conducted a different analysis according to the radiological appearance of the tumor; while SMR seems to bring an OS advantage in patients with moderately and highly diffuse wtIDH glioblastomas; in case of nodular ones, a maximum of 29% of FLAIR volume would be advantageous [2][24]. Additionally, Yan et al. found the DTI sequence’s anisotropic component to bepositively associated with OS and PFS [3][27]. The review by Karschnia et al. tried to clarify the topic concluding that SMR is defined as any resection beyond contrast enhancement into T2w/FLAIR hyperintensity [4][10].
It is evident that many of the uncertainties come from the real significance of the hyperintense T2 signal. It is generally found that the GBM relapses just beyond the resected contrast-enhanced edges, as some works claimed it is plausible to think that stem-like cells can be found in this area [5][28]. Studies comparing MR and 18FET-PET demonstrated that the T2 hyper signal is likely to host tumor cells [6][29]. However, PET imaging is an advanced modality that is not available in every center, so the majority of the studies are based on standard MR imaging. From this perspective, the FLAIR hyper signal has been retained as a marker of tumoral infiltration, although it cannot actually distinguish infiltration from brain edema. To resolve that issue, a FLAIR hyper signal can be found around brain metastasis too, reflecting a vasogenic edema [7][30]. Some studies tried to develop methods to differentiate tumor infiltration areas from edema. Certo et al. described a manual segmentation method that distinguished Region of Interests (ROIs) with different hyperintensity values on FLAIR sequences; ROIs with higher values represented edema [8][17]. Other studies revealed that Apparent Diffusion Coefficient (ADC) mapping can have a prognostic value in patients with a Glioblastoma, as it reflects water sequestration and, as a consequence, hypercellularity. Elson et al. found that a <0.3 minimum ADC value was associated with a shorter OS and PFS [9][31]. Finally, a peritumoral FLAIR hyper signal could have a different significance, and hence, the benefit of removing apparently healthy tissue beyond the contrast-enhancing “meaty” tissue has conflicting evidence.
2. SMR, Tumor Volume, and Location
When talking about the resection of an infiltrating brain tumor, the first questions that arise in the surgeon’s mind are about the location (eloquent/critical area versus less “dangerous” areas) and volume, which are intimately bound. A large part of the studies included in theise content review showed a wide range of pre-operative tumor volumes and only a few differentiated between eloquent and non-eloquent locations. For example, Vivaz-Butraigo et al. reported a range between 1 and 124 cm3 for contrast-enhanced volumes that reached 182.74 cm3 in FLAIR sequences. They reported a positive influence in cases of 20% to 50% SMR, without a clear advantage for greater resection [10][32].
Although in a standardized predictive model for Glioblastoma pre-operative tumors volume is not usually considered, it is intuitive that larger tumors can hamper SMR, especially when coupled to an eloquent or near-eloquent location. Such a consideration found important feedback in the work by Roh et al. which showed that a frontal or temporal lobectomy for glioblastoma located in the non-dominant hemisphere was associated with longer OS and PFS [11][14]. Schneider et al. showed an anterior temporal lobectomy was able to prolong both OS and PFS, both on the dominant and non-dominant sides. On the contrary, Figueroa et al. did not find a survival advantage in using this technique. Regardless, according to the survey by Rakovec et al., the neurosurgical oncology community seems to agree with limiting the SMR to right anterior temporal and right frontal lobe GBM [12][11].
In some cases, such as a small GBM located in the eloquent area, SMR can be advocated (see Figure 12); however, this only concerns single cases and a very attentive selection. Other than these very specific cases, it seems hard to conceive that patients with larger lesions near or inside eloquent areas could benefit from SMR without putting them at higher risk of neurological outcomes. As discussed below, accumulated evidence demonstrated that a worsened post-operative neurological performance abrogates survival benefits from complete tumor resection or unilobed tumor location [13][14][15][33,34,35]. An analog consideration could be done for tumors infiltrating deep neural and vascular structures (i.e., insular glioblastoma) which typically represent the boundaries of the resection cavity. It appears that a deeper analysis is needed in order to clarify the role of tumor volume and location on SMR, with special attention regarding the eloquent area location.
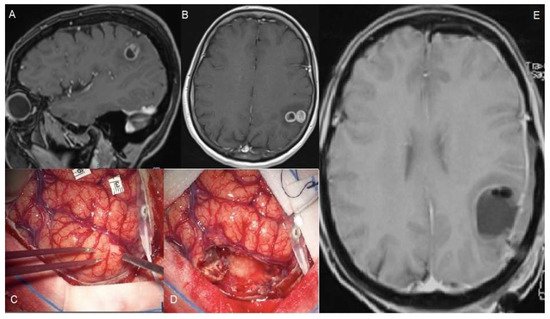
Figure 12. A 56-year-old female suffered a single seizure (speech articulation impairment lasting 10 min). The upper figures (A,B) show pre-operative T1 gadolinium-enhanced MRIs of a Glioblastoma infiltrating the left supramarginal gyrus. The patient was operated on through an awake craniotomy and direct language mapping (C,D). Since the mapping did not show activation areas on the supramarginal gyrus, a complete gyrus resection was performed. (E) The post-operative MRI confirmed the complete resection not only of the tumor but also of the gyrus. Post-operatively, the patient did not experience any speech disturbances.
3. SMR and Tumors Infiltrating Periventricular White Matter
Tumor-initiating brain cells are thought to be placed into the so-called Subventricular Zone (SVZ) and evidence supporting this assumption is still accumulating. The contact between cancerous cells and SVZ seems to confer higher resistance to traditional radio- and chemotherapy [16][36]. Additionally, a lateral ventricle wall involvement is considered the source of leptomeningeal dissemination and, finally, for obstructive hydrocephalus [17][37]. Hallaert et al. analyzed 214 patients and found that contact with SVZ was associated with unmethylated MGMT and a shorter OS [18][38]. Vivaz-Buitrago et al. and Tripathi et al. examined the involvement of the lateral ventricle, confirming its role as a poor prognostic factor in case the contrast-enhancement reaches the ependyma [10][32]. However, the role of the infiltration of the ependyma by the FLAIR hyper signal received less attention. Mistry et al. demonstrated that the distance between glioblastoma and SVZ did not influence OS, which, on the contrary, suffers from the contact between SVZ and contrast-enhancement edges [19][22]. This data seems conflicting since as long as the tumor grows toward the SVZ, it is difficult to explain why it appears true for the CE portion only. In other words, if FLAIR volume contains tumoral cells, it should have the same role in the SVZ involvement. As a consequence, does the resection of this volume confer increased OS? Undoubtedly, more studies are necessary and this factor should be well analyzed if the neurosurgical community wants to establish criteria for SMR in the case of high-grade gliomas.
4. SMR and Intraoperative Techniques
There are several technological tools used intraoperatively to guide tumor resection which help in better visualizing tumor tissue such as intraoperative MRI, ultrasound, fluorescent agents, and 5-ALA. These latter two are specifically addressed to detect tissue that corresponds to the contrast-enhancing MR images.
There is no clarity on how intraoperative technologies make SMR feasible in glioma surgery. Certainly, the use of iMR (intraoperative magnetic resonance) and fluorophores helps in the best extension of tumor resection [20][21][39,40].
When coming to the eloquent location of tumors, intraoperative mapping is useful to guide resection according to functional boundaries rather than only anatomical. This strategy has shown robust results both for LGG and HGG. However, despite the use of functional monitoring and mapping, the idea to pursue aggressive resection in GBM has to take into account the fact that rapidly growing tumors bring a more destructive behavior compared to their slow-growing counterparts. This biological difference implies that brain plasticity has much less time to intervene, not allowing the brain to reshape and potentially increasing the risk for post-operative definitive impairments [22][41].
Pessina et al. performed a resection guided by neuronavigation and ultrasounds, extending until cortical and subcortical stimulation enhanced the risk of neurological deficits [23][15].
Some authors assert that sodium fluorescein, which accumulates in the extracellular space when the barrier is damaged, may be the intraoperative equivalent of the radiological signal given by gadolinium. The same authors state that this marker extends beyond tumor regions with contrast pinch and therefore can predict the pathological tissue facilitating resection [24][42]. Other authors recommend associating fluorescein with Raman spectroscopy which has been shown to be able to identify tumor versus healthy tissue at the margins of resection [25][43]. Furthermore, laser endomicroscopy, associated with fluorescein, can also have the same effect [26][27][28][44,45,46]. However, the use of 5-ALA appears questionable. In fact, despite Eyopoglu et al. finding an OS advantage in the DiVA group, Roh et al. did not obtain a better survival in their subgroup of patients in which 5-ALA was employed [10][29][21,32]. Nevertheless, experiences are limited and they cannot be elevated as a standard methodology.
5. SMR and Functional Outcome
It has been established that the total or near-total resection of the contrast-enhancing component is a strong predictor of prolonged OS [30][31][32][33][34][47,48,49,50,51]. In more recent years, it has also been demonstrated how radical resection must be balanced with the preservation of adequate functional outcomes, since this latter can negatively affect the deployment of adjuvant treatments and the OS. A first retrospective study published by McGirt et al. on Glioblastoma patients who received tumor resection introduced the prominent role of surgically acquired language and motor deficit on survival impairment (9.0-months and 9.6-months median survival, respectively, compared to 12.8 months without a new deficit, p < 0.05) [13][33]. Furthermore, in 2015, Verlut et al. showed that post-operative motor deterioration was associated with poor outcomes in patients receiving surgery followed chemo-radiotherapy [28][46]. Specifically, it has more recently been demonstrated how severe post-operative neurological deficits significantly reduce survival rates and become a predominant negative prognosticator over EOR, tumor location, KPS, age at the date of surgery, MGMT promoter methylation status, and adjuvant treatment regimen. Rahman and colleagues published a comprehensive work demonstrating that post-surgical acquired neurological deficits abrogate the survival benefit gained by EOR of 95% and more [14][34].
So, if these considerations hold true for radical resection, it is still more of a concern when dealing with SMR, especially when tumors are located near or inside eloquent locations. Aabaedi et al. claimed that as far as the subgroup of wild-type IDH Glioblastoma is concerned, no difference between CE and non-CE resection emerged in terms of OS; on the contrary, they reinforced the concept that a neurological impairment represents the real key factor for survival of these patients [35][52].
The greatest part of these contentpapers included in hereour review compared pre- and post-operative KPS and they did not find any difference, concluding that SMR was not associated with a worse clinical outcome. Nevertheless, even if KPS has a great value in the oncological field, it is necessary to remember that it does not explore every clinical aspect. In fact, less attention has been paid to post-operative cognitive status evaluation; only a few studies mentioned abovein this review conducted a deep analysis of specific symptoms, above all neurocognitive ones. As emerged from the review by Gately et al., a longer OS should be balanced with a certain quality of life that permits them to maintain real functional independence [36][53]. Therefore, as Roh et al. stated, a study that analyzes the effects of SMR on neuropsychological functions is desirable [11][14].
