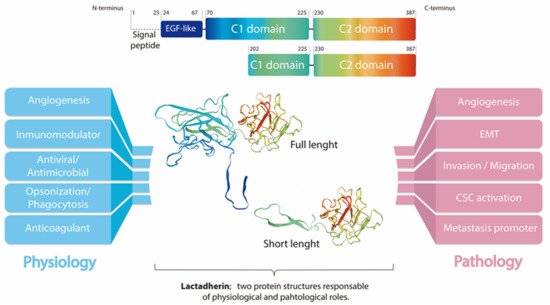
3. Lactadherin Role as Promotor of Tumor Progression
Lactadherin expression has been associated with the aggressiveness and progression of several types of cancer. In fact, most of the studies associated lactadherin overexpression in tumor samples with the promotion of pro-tumorigenic and pro-metastatic capacities, such as increased tumor cell proliferation, angiogenesis, migration, invasion, and epithelial-to-mesenchymal transition (EMT)
[11][27][34][38][39][40][41][42][43][11,19,21,22,39,40,41,42,48]; processes that are related to the physiological function of this protein. In this context, several studies in the cancer field, but also in other diseases, have demonstrated the activation of commonly pro-tumorigenic signaling pathways mediated by lactadherin overexpression, such as their interaction with β3-integrin, and the activation of the PI3K/AKT signaling
[39][42][44][45][46][39,42,49,50,51]. On the other hand, most of the studies have not deepened in the downstream intracellular signaling triggered by lactadherin, thus leaving the field open for more complete and comprehensive studies.
3.1. Tumor Cell Survival/Proliferation and EMT
Lactadherin is able to interact with αvβ3/β5-integrins through its RGD domain. This interaction allows the anchorage and rapprochement between cells (or extracellular matrix), thus favoring apoptotic cell clearance through phagocytosis
[2][5][7][2,5,7]. On the other hand, the activation of β3-integrin signaling can lead to the activation of pro-survival, anti-apoptotic, and pro-metastatic pathways through the activation of molecules such as Akt and Twist, as shown in murine melanoma studies
[39]. This lactadherin/β3-integrin interaction could promote the EMT process (through PI3K/Akt, Twist); an effect that has also been observed in CRC cancer models, where lactadherin promotes the migration and invasion of tumor cells
[42].
3.2. Lactadherin Immunomodulatory Role
Another important function of lactadherin is its immunomodulatory role, mainly as an immunosuppressive molecule
[1]. The first reports in this topic show that lactadherin is able to bind β3-integrin on the surface of phagocytes, mainly macrophages. In the same way, lactadherin can bind PS exposed in cells undergoing apoptosis, and promote their phagocytosis
[7] (
Figure 1). Subsequent studies have shown that other cells of the immune system also express lactadherin, such as dendritic cells, mast cells, and regulatory T lymphocytes, in addition to endothelial cells that are in direct contact with blood and blood cells
[1][11][1,11].
Lactadherin has also been involved in the modulation of the immune system in other types of cancer, such as oral cancer
[30][35], esophageal cancer
[31][36], and, recently, angiosarcoma
[32][37] and a murine glioma model
[47][55]. The immunomodulation in these types of cancer have been constantly associated with tumor development and bad prognosis.
4. Lactadherin as a Possible New Cancer Therapeutic Target
Although lactadherin has various regulatory physiological roles, and its expression and function have been targeted for the treatment of several other diseases (e.g., vascular and autoimmune pathologies)
[13][14][15][46][48][49][50][13,14,15,51,56,57,58], it has been established to be most relevant in the oncologic area. First studies by Ceriani et al. showed that an antibody cocktail, including MoAbs against HMFG components (one of them against lactadherin), worked as treatment and prevention against the engraftment of ER-positive and negative mammary tumors
[20][23][27,30]. Later, this group and others published several reports using this cocktail of antibodies, including an anti-lactadherin blocking antibody, demonstrating its beneficial effect against breast tumors
[24][25][51][31,32,59]. Subsequently, several studies reported lactadherin as a possible cancer therapeutic target
[28][34][38][41][43][20,21,22,41,48], either using gene therapy approaches, inhibiting its expression by siRNAs or shRNAs, or by using specific antibodies that block its function and interactions with αvβ3/β5 integrins
[35][45].
Recently, using TCGA data and machine learning, Kothari et al. have shown that lactadherin is one out of two proteins/genes that can differentiate TNBC from non-TNBC irrespective of their heterogeneity or subtype differences
[52][60]. Further affinity purification mass spectrometry and proximity biotinylation experiments identified a possible role for lactadherin in various tumor survival processes. Another study also using TCGA RNA-seq data showed that lactadherin, as well as KLK5/7 expression, are associated with COX-2 inhibitors treatment resistance in TNBC cells.
SThis study demonstrated that silencing of these genes markedly recovered COX-2 inhibitor sensitivity both in vitro and in vivo. Considering the difficulty in the treatment of TNBC, these results could support the possibility of using new combination therapies against TNBC involving COX-2 and lactadherin inhibition
[53][61].
5. Extracellular Vesicles and Exosomes as Promotors of Breast Cancer Metastasis
Metastasis is defined as the dissemination of cancerous cells from the primary tumor, and the effective colonization of secondary target organs. It is widely accepted that intercellular communication is essential in all steps of the metastatic cascade. Exosomes are a particular subpopulation of EVs released by a variety of cell types. These exosomes are 40–200 nm in diameter, and are derived from the multivesicular endosome pathway, and can enter a recipient cell mainly through three different pathways: membrane fusion, endocytosis, or the interaction of proteins in exosomes with receptors in recipient cells
[54][55][56][57][64,65,66,67]. Exosomes are thought to play important roles in intercellular communication, transferring a variety of molecules to target recipient cells. Exosomes contain several bioactive molecules, such as nucleic acids (mRNA, microRNA, DNA, and other non-coding RNAs), proteins (receptors, transcription factors, enzymes, extracellular matrix proteins), and lipids that can redirect the phenotype and function of a recipient cell
[57][58][59][60][67,68,69,70]. Therefore, exosomes are emerging as local and systemic cell–cell mediators of oncogenic information that play an important role in cancer progression
[55][56][57][58][59][60][61][62][65,66,67,68,69,70,71,72].
In breast cancer, there is a potential use of exosomes and other EVs as promising diagnostic and therapeutic biomarkers. On the other hand, exosomes secreted by metastatic cells potentiate tumorigenic capacities of less aggressive cells acting in a paracrine manner
[58][59][60][61][62][63][68,69,70,71,72,73]. Moreover, recent reports indicate that exosomes are able to condition the microenvironment, where tissue recipient cells are waiting for the advent of a tumor cell, and which in turn, are capable of promoting angiogenic mechanisms in breast cancer
[61][71]. At the same time, exosomes secreted by tumor stroma can also influence tumor progression. Breast-cancer-associated fibroblasts secrete exosomes that have been shown to promote tumor mobility, invasion, and dissemination of breast cancer cells through the Wnt-planar cell polarity pathway (Wnt-PCP pathway)
[64][74].
6. Role and Use of Lactadherin in EVs and Exosomes
Lactadherin expression has been reported in a variety of tissues and organs. However, its overexpression has been associated with bad prognosis and outcomes in different types of cancers. Moreover, lactadherin presence in EVs has recently been reported in several studies and databases
[65][66][67][79,80,81], which has led to the consideration of it as a possible new marker of EVs. On the other hand, lactadherin C1C2 domains have been extensively used in EV designing and engineering. Due to its presence in EVs, and its interaction with PS (also present on EV surfaces), these domains are usually fused to a protein of interest, thus directing target protein to the EV surface
[68][69][70][71][82,83,84,85]. This strategy has also been used to engineer EVs to expose anti-HER2 scFv on their EVs surface, and redirect EVs to HER2+ breast cancer cells to deliver a cargo of mRNA gene therapy
[72][86]. More recently, a similar strategy was used by Kooijmans et al. to decorate EVs with EGFR-specific nanobodies fused to the C1C2 domains of lactadherin to further improve tumor cell targeting and incorporation
[73][87]. Particularly in the case of breast tissue (normal or tumor), there are few studies describing the presence of lactadherin associated with exosomes or EVs. One of these articles indicates that lactadherin in EVs is required to transduce cellular signals from the basolateral side of adherent cells by accumulating exosomes in mammary epithelial cells [
89]. However, this report did not describe the presence of lactadherin on the exosomes. Finally, recent studies by Lobos-Gonzalez et al., described the presence of lactadherin as part of the characterization of exosomes secreted by a metastatic breast cancer cell line (MDA-MB-231); however, functional experiments studying its specific and precise role were not performed [
23,
91]. As lactadherin has several functions, both as a cell adhesion molecule, and triggering intracellular signaling cascades, it is possible to think that it could have similar roles as part of EVs membranes of EVs cargo".
7. Conclusions
Lactadherin is present widely in human tissues, and can easily be targeted by its special multi-domain structures. Lactadherin can promote tumor formation, and prompts cancer vascular angiogenesis, survival, and EMT, regulating multiple oncogenic pathways (p63/p73, PI3K/Akt, β-catenin, Akt/Twist), which can promote cancer cell resistance to chemotherapy and host immunity suppression. However, to date, the specific roles, molecular mechanisms, and signaling pathways by which lactadherin promotes tumorigenic and metastatic properties of tumor cells, especially breast cancer cells, are not completely elucidated. Despite the interesting fact that several patents have used lactadherin as a possible tumor marker or even therapeutic target, there are no reported clinical trials in which this protein is being tested. On the other hand, it is well known that EVs and exosomes can mediate cell–cell communication, and can promote the acquisition of oncogenic and pro-metastatic properties in recipient cells. Past and recent works have shown the presence of lactadherin in EVs secreted by different cell types. However, the role of lactadherin present in EVs, more specifically, in exosomes secreted by cancer cells (or other cells in the tumor microenvironment), is still unknown. Given the importance of this protein modulating tumor development and progression, deeper understanding on the role of lactadherin in EV- and exosome-mediated tumor progression and metastasis could be a promising focus of study in the future.