Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Luana Beatriz dos Santos Nascimento.
Plants evolved an impressive arsenal of specialized metabolites to cope with the novel environmental pressures imposed by the terrestrial habitat when moving from water. Flavonoids are maybe the most important specilized metabolites that show multifarious roles in the sucess of plant terrestrialization. These compounds modulated auxin transport and signaling and promoted the symbiosis between plants and fungi (e.g., arbuscular mycorrhizal, AM), a central event for the conquest of land by plants. AM improved the ability of early plants to take up nutrients and water from highly impoverished soils. Therefore, flavonoids were essential to plant development in the “new world” scarce of water and nutrients.
- arbuscular mycorrhizal
- auxin
- drought
- nutrient deficiency
- flavonoids
1. Introduction
Many evolutionary innovations, i.e., the adaptive traits of living organisms [1], occurred over a short time period when plants moved from water to populate land (approx. 460 mya) [2,3][2][3]. Together with other adaptive features, “molecular” innovations were driven by the novel environmental pressures that the early land plants faced in harsh terrestrial habitats. These included dramatic declines in water and nutrient availabilities, large fluctuations in air temperature, and substantial increases in visible light and UV radiation [4,5,6][4][5][6]. However, there is now conclusive evidence that most of the molecular toolkit enabling the conquest of the terrestrial habitat by plants was already present in their ancestors, i.e., members of the Charophyta algae clade (Zygnematophyceae) [4,7,8,9][4][7][8][9]. This molecular tool kit included a set of novel transcription factors, and genes involved in both phytohormone signaling and in the formation of the cell wall, all of them being closely linked to the emergence of land plants [8]. Therefore, in other words, plants were apparently “terrestrial from the beginning” [10].
For instance, the two- (2D) to three-dimensional (3D) growth transition was a key innovation increasing the plant complexity [11,12][11][12] and hence improving their ability to conquest more stressful habitats [13,14,15,16][13][14][15][16]. This is consistent with the notion that 3D plants bodies display higher resistance against desiccation and UV radiation, ultimately providing protection to both vegetative and reproductive tissues [6,17][6][17]. Interestingly, recent findings show that 2D to 3D growth transition mostly involves the phytohormone auxin and its asymmetrical distribution in different plant tissues, driven by the polar auxin transport (PAT) [12,18][12][18]. Indeed, PAT was already present in the ancestors of land plants, and it was regulated by a range of transporters, including the PIN-formed (PIN) proteins [19]. Nonetheless, PAT in Charophytes is not as tightly regulated as it is in bryophytes and angiosperms [20,21][20][21]. This only in part is due to the increased numbers of PIN proteins detected in more complex land plants. Instead, the evolution of regulatory mechanisms of both PIN activity and PIN-induced PAT likely represented a major developmental innovation responsible for the increased complexity observed from ancestors of land plants up to angiosperms [22,23][22][23]. Similar reasoning concerns core components of the ABA signaling pathway, such as SnRK and PP2C proteins, which were already present in land plants’ ancestors [24,25,26][24][25][26]. The complexity and robustness of the ABA signaling network observed in gymnosperms and angiosperms are due to not only the expansion of core components but also to the evolution of several downstream regulatory network components (MYB transcription factors and MAPKs proteins) [27,28,29,30][27][28][29][30]. The enhanced complexity in this pathway has expanded the function of the ABA signaling up to include the fine tuning of stomata movements, a trait that would allow land plants to later adapt to habitats characterized by severe soil water deficit and excessive sunlight irradiance [30,31][30][31].
The outstanding ability of terrestrial plants to adapt to this “ever-changing” new environment has depended not only on the evolution of a pre-existing molecular toolkit but largely on the impressive chemical diversity originated by the rise of a huge number of specialized metabolic pathways [32,33,34][32][33][34]. The pivotal role of specialized metabolism in the successful responses of plants to environmental stressors also depends on the huge numbers of metabolites synthesized by different taxa. It is long known that each specialized metabolite class serves a multiplicity of functions in plant-environment interaction [35], changing with the plant developmental stage and the type and intensity of environmental injuries [36,37,38][36][37][38]. Indeed, these metabolic networks enable plants’ diversification, biotic interactions, and, to a greater extent, the occupation of several niches [34]. Therefore, the multi-functionality represents an effective way to reduce the metabolic cost of specialized metabolite biosynthesis [39], being, indeed, a widespread property of most secondary metabolites [40].
WResearchers examine here the multi-functionality of flavonoids, the ancient class of secondary metabolites synthesized through a branch-pathway of the general phenylpropanoid metabolism, which is unique to land plants [41,42][41][42], in the “plant terrestrialization”. The primary functions of flavonoids during the water-to-land transition are still uncertain [41,42][41][42]. This originates from (1) the inherent ability of flavonoids to serve multiple functions in the response of plants to environmental stimuli and (2) the contemporary action of novel environmental stressors plants faced in the terrestrial habitat [37,43–47][37][43][44][45][46][47]. WResearchers reason on the environmental drivers that were mostly responsible for the rise of the flavonoid metabolism, a pre-requisite to unveil their primary roles during the early steps of plant terrestrialization.
2. A “Revolutionary” Molecular Innovation: The Rise of Flavonoid Metabolism during Water-to-Land Transition of Plants
2. A “Revolutionary” Molecular Innovation: The Rise of Flavonoid Metabolism during Water-to-Land Transition of Plants
One of the most intriguing molecular innovations that accompanied the water-to-land transition of plants was the replacement of the mycosporine-like amino acids (MAAs) metabolism, typical of streptophytic algae, by the flavonoid biosynthetic pathway (Figure 1), a common feature of all extant land plant taxa [41,48]. Actually, there is evidence of the occurrence of early genes of the phenylpropanoid pathway, such as those involved in lignin and coumarin biosynthesis, in algal relatives closest to land plants [4,49][4][41]. This was of pivotal value for cell wall reinforcement and for the initial steps of both plant vascularization and defense, allowing their successful growth and development on land [50][48]. Indeed, the localization of phenolic compounds in different cell organelles in comparison to the predominantly cytoplasmatic position of MAAs [51,52][49][50] supports the importance of phenolics in the early steps of land conquest. WResearchers suggest that the presence of both MAA [53][51] and phenylpropanoid metabolism [49][41] in clades sisters of the early land plants was an expensive adaptation strategy, and it is likely due to reminiscence of the evolution of both basal and highly branched streptophytic algae in nutrient-rich habitats. Indeed, it is speculated that early land plants were firstly dependent on MAAs instead of flavonoids as UV protectants [54][52]. WResearchers observe that while flavonoids are merely carbon-based compounds, MAAs have nitrogen atoms in an amino-cyclohexanone or amino-cyclohexenimine core skeleton [48,52][50] (Figure 1). Therefore, it is conceivable that when plant ancestors moved to land, the MAA metabolism was unfavored and further definitively lost because of the large deprivation of nitrogen (as well as phosphorous) and water in land soils [2,48,55][2][53]. This “saving strategy” [56,57][54][55] allowed the nitrogen to be fully available for sustaining the growth of early plants in nutrient-poor terrestrial habitats. Indeed, several studies have shown a positive correlation between flavonoids’ biosynthesis and nitrogen depletion [58[56][57],59], and a high C/N ratio has been reported to promote the accumulation of flavonols [60][58]. It is suggested that, despite the biosynthesis of flavonoids requiring high-energy consumption, their preferential accumulation might represent a “power drain valve” in a scenario of higher solar irradiation [61[59][60],62], such as that faced by early plants. However, the matter is much more complex than wresearchers discuss here. As speculated by Harholt et al. [10], the Streptophyta algae were “terrestrial from the beginning”, which is consistent with the rise of phenylpropanoid metabolism in several of these lineages. Nonetheless, wresearchers note that Charophyceae ancestors of land plants first colonized moderately moist habitats near freshwater, then gradually moved into drier lands [56,63][54][61].
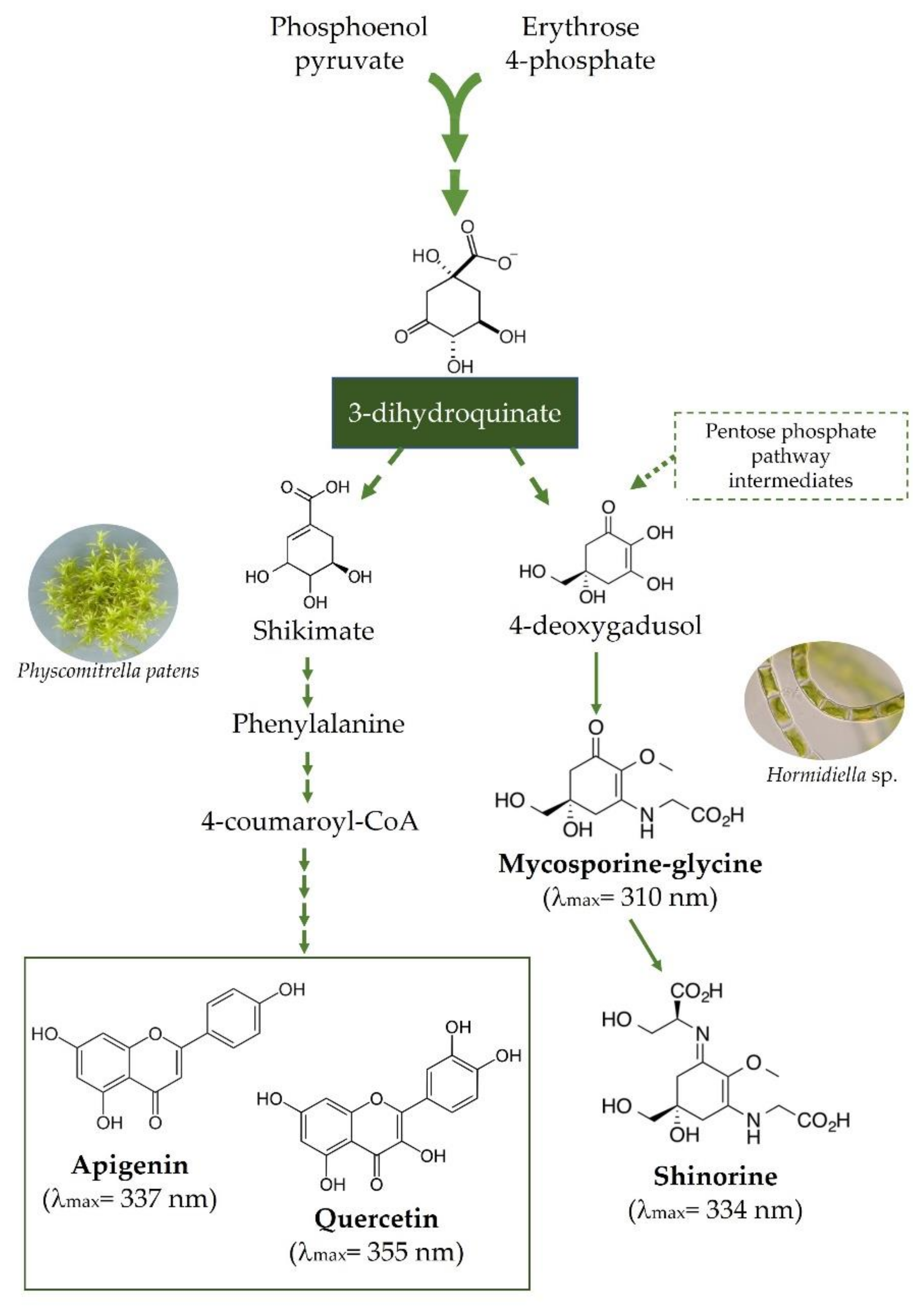 The replacement of nitrogen-containing MAAs with carbon-based products synthesized by the “late” flavonoid pathway, particularly the flavonol branch, was, therefore, a molecular event of outstanding significance during plant terrestrialization [30[30][62],48,64], although this matter has not received the deserved attention in the pertinent literature yet. The nutrient-centered hypothesis for the rise of flavonoid metabolism outlined above is reasonable but also raises a series of concerns that needs deep analysis. For instance: (i) it is necessary to determine which are the benefits associated with flavonoid metabolism in primitive terrestrial plants that were facing nutrient and water-deficient soils; (ii) it moves to the background of the UV-B centered hypothesis, i.e., the increase in UV-B irradiance was the evolutional environmental driver responsible for the rise of the flavonoid metabolism.
The replacement of nitrogen-containing MAAs with carbon-based products synthesized by the “late” flavonoid pathway, particularly the flavonol branch, was, therefore, a molecular event of outstanding significance during plant terrestrialization [30[30][62],48,64], although this matter has not received the deserved attention in the pertinent literature yet. The nutrient-centered hypothesis for the rise of flavonoid metabolism outlined above is reasonable but also raises a series of concerns that needs deep analysis. For instance: (i) it is necessary to determine which are the benefits associated with flavonoid metabolism in primitive terrestrial plants that were facing nutrient and water-deficient soils; (ii) it moves to the background of the UV-B centered hypothesis, i.e., the increase in UV-B irradiance was the evolutional environmental driver responsible for the rise of the flavonoid metabolism.
the subsequent steps toward drier habitats.

Figure 1. A synthetic diagram showing the metabolic pathways of mycosporine-like amino acids (MAAs, here exampled as the mycosporyne glycine and shinorine) and flavonoids (here represented by the flavone apigenin and the flavonol quercetin). The two pathways diverge early in the shikimate pathway. Mycosporyne glycine and shinorine have, on average, molar extinction coefficients 80% higher than those of apigenin and quercetin over the UV-B portion of the solar spectrum (280–315 nm, G. Agati personal communication).
3. Arbuscular Mycorrhizal Associations: A Central Event in Plant Terrestrialization Aided by Flavonoids
As outlined above, one of the most severe challenges faced by early land plants was the drastic reduction in the availability of water and nutrients [65,66][63][64]. Once again, land plant ancestors were already equipped with a toolkit potentially enabling their adaptations to land, which includes their ability to form symbiotic associations with fungi and bacteria [67][65]. This is intriguingly in line with the findings that nutrient-impoverished-ancient soils are indeed hotspots of plant biodiversity [68,69][66][67]. It is also consistent with the recent suggestion that ancestral and modern streptophyte algae could recognize fungi since they have homologous genes of LysM-RLK receptors, through which land plants recognize fungi [67,70,71][65][68][69]. However, even the most branched/complex algae miss other genes required for mycorrhizal association (MA), including those involved in the formation of arbuscules [72][70]. In fact, mycorrhizal symbiosis is a shared derived character, unique to land plants [67][65], playing a key role in the terrestrialization 450 million years ago [73,74,75][71][72][73]. The current paradigm is that the earliest rootless terrestrial plants coevolved with Glomeromycota arbuscular mycorrhiza fungi that, in exchange for plant photosynthates, enhanced their access to mineral nutrients and water [76,77,78,79][74][75][76][77]. There are few examples of AM or similar associations in streptophytic algae [71][69], whereas liverworts, hornworts, and lycophytes have recently been shown to form symbioses with members of two ancient fungal lineages: arbuscular mycorrhizal fungi of the Glomeromycotina and the symbiotic fungi of the Mucoromycotina [74,80][72][78]. Even if recent reports have revealed that fungi associations between liverworts and members of the Glomeromycotina are particularly few [81][79], a limited number of species have been studied so far [82][80]. The outstanding significance of arbuscular mycorrhiza fungi in assisting not only the water-to-land transition [83][81] but mainly in the expansive conquest of land by plants is also well exemplified by the observation that more than 80% of plant species form AM [84,85][82][83]. Notably, a study conducted with members of the different plant groups revealed that all the gymnosperms surveyed were mycorrhizal, most of them obligate [86][84], which was attributed to their evolution in nutrient-poor habitats. Moreover, there is compelling evidence that even symbioses between “modern” flowering plants and nitrogen-fixing bacteria (e.g., Rhizobium) must probably have evolved from the ancient AM symbiosis since several of the pathway signals are common between both biological interactions [77,87][75][85]. Auxin is a major regulator of plant growth and developmental processes, and it is a central modulator/regulator in AM symbiosis [88][86]. Increased auxin level in colonized cells has promoting effects on hyphal branching (possibly by weakening the cell wall) and, consequently, on arbuscule incidence [89,90][87][88]. There is also evidence of auxin involvement in the early stages of AM formation, e.g., during pre-symbiotic signal exchange [91][89], in part through the control of the strigolactone levels [92][90]. The feedback regulation of the host IAA biosynthesis offers strong support to the tight auxin-AM relationship. This mechanism avoids the excessive auxin accumulation in colonized cells and modulates the subsequent auxin-induced gene expression, both contributing to the phenotypical changes during mycorrhizal colonization [90,93][88][91]. The strong dependence of AM on the auxin perception and signaling highlights the importance of endogenous regulators of this hormone, such as the flavonoids [94,95][92][93]. Actually, several secondary metabolic pathways are interconnected with phytohormone networks, making most specialized metabolites active in plant growth and development regulation, besides their general metabolic function [34]. Flavonoids have long been reported to be involved in AM formation [96[94][95][96][97],97,98,99], being active in root colonization, spore germination, hyphal growth, and branching [47,100,101][98][99][47]. Nonetheless, the molecular mechanisms that drive the effects of flavonoids in AM are largely unexplored. It is conceivable that the flavonoid-induction of AM partially involves the regulation of local auxin levels (by acting on its transport and catabolism) [102][100] and of the level of downstream components of the auxin signaling pathway, as well known to occur in flavonoid-induced nodulation [103,104][101][102]. As outlined above, some features of root nodule endosymbiosis have been likely recruited from the more ancient AM symbiosis [105[103][104],106], and there is compelling evidence that flavonoids act as both essential signals for the establishment of legume nodulation and prime candidates in AM symbiosis [107][105]. The observation that flavonoid aglycones are much more active than flavonoid glycosides in the promotion of AM, likely through the inhibition of auxin transport, similarly to what is observed during nodulation [108][106], further corroborates the idea of a strong relationship between flavonoids, auxins and AM [47][98]. Overall, this is consistent with the relatively old suggestions that flavonoids play a crucial role as developmental regulators [109,110][107][108], which is closely linked to their ability to modulate phytohormone signaling pathways, as also recently shown for the ABA signaling network [111,112][109][110]. As already reported, this signaling ability is deeply dependent on the capacity of flavonoids to regulate “key downstream” components of phytohormone signaling, such as H2O2 and a range of protein kinases, including PID and mitogen-activated protein kinases (MAPKs) [30,110][30][108]. In general, this supports the idea of a primary function of flavonoids as developmental regulators in both the process of plant terrestrialization andthe subsequent steps toward drier habitats.
4. Conclusions
Plants were challenged against a wide range of novel environmental pressures when moving to land. The excellent capacity of plants to adapt to the harsh terrestrial habitat has depended on both the evolution of a pre-existing molecular toolkit and the rise of a huge number of specialized metabolites. Among the latter, the replacement of MAAs with flavonoids was an extraordinary molecular innovation, producing more than 9000 different flavonoid structures known to date. Modern land plants are capable of finely modulating flavonoid biosynthesis depending on the type and intensity of environmental stressors. In addition, the occurrence of flavonoids in different tissues and subcellular compartments well explains their ability to play multiple functions in response to environmental stimuli. The multiple roles served by a nascent flavonoid metabolism during the “early steps” of plants in a land environment were crucial. Although these molecules undoubtedly allowed primitive plants to effectively deal with the most damaging solar wavelengths by acting as both UV filters and ROS scavengers, it is unlikely that these were their most prominent function in the process of terrestrialization. Indeed, the benefits associated with the replacement of MAAs (excellent UV filters) with flavonoids mostly have involved the ability of these last to modulate phytohormone signaling and to assist the plant-fungus symbiosis, which is essential to plant development in the “new world” scarce of water and nutrients.References
- Wagner, A. The molecular origins of evolutionary innovations. Trends Genet. 2011, 27, 397–410.
- Delaux, P.M.; Nanda, A.K.; Mathé, C.; Sejalon-Delmas, N.; Dunand, C. Molecular and biochemical aspects of plant terrestrialization. Perspec. Plant Ecol. Evol. Syst. 2012, 14, 49–59.
- Bowles, A.M.; Bechtold, U.; Paps, J. The origin of land plants is rooted in two bursts of genomic novelty. Curr. Biol. 2020, 30, 530–536.
- De Vries, J.; de Vries, S.; Slamovits, C.H.; Rose, L.E.; Archibald, J.M. How embryophytic is the biosynthesis of phenylpropanoids and their derivatives in streptophyte algae? Plant Cell Physiol. 2017, 58, 934–945.
- Han, X.; Chang, X.; Zhang, Z.; Chen, H.; He, H.; Zhong, B.; Deng, X.W. Origin and evolution of core components responsible for monitoring light environment changes during plant terrestrialization. Mol. Plant 2019, 12, 847–862.
- Buschmann, H.; Holzinger, A. Understanding the algae to land plant transition. J. Exp. Bot. 2020, 71, 3241–3246.
- Cheng, S.; Xian, W.; Fu, Y.; Marin, B.; Keller, J.; Wu, T.; Sun, W.; Li, X.; Xu, Y.; Zhang, Y.; et al. Genomes of subaerial Zygnematophyceae provide insights into land plant evolution. Cell 2019, 179, 1057–1067.
- Donoghue, P.; Paps, J. Plant evolution: Assembling land plants. Curr. Biol. 2020, 30, R81–R83.
- Fürst-Jansen, J.M.; de Vries, S.; de Vries, J. Evo-physio: On stress responses and the earliest land plants. J. Exp. Bot. 2020, 71, 3254–3269.
- Harholt, J.; Moestrup, Ø.; Ulvskov, P. Why plants were terrestrial from the beginning. Trends Plant Sci. 2016, 21, 96–101.
- Moody, L.A. Three-dimensional growth: A developmental innovation that facilitated plant terrestrialization. J. Plant Res. 2020, 133, 283–290.
- Blázquez, M.A.; Nelson, D.C.; Weijers, D. Evolution of plant hormone response pathways. Ann. Rev. Plant Biol. 2020, 71, 327–353.
- Pires, N.D.; Dolan, L. Morphological evolution in land plants: New designs with old genes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 508–518.
- Weng, J.K. The evolutionary paths towards complexity: A metabolic perspective. New Phytol. 2014, 201, 1141–1149.
- Nishiyama, T.; Sakayama, H.; De Vries, J.; Buschmann, H.; Saint-Marcoux, D.; Ullrich, K.K.; Rensing, S.A. The Chara genome: Secondary complexity and implications for plant terrestrialization. Cell 2018, 174, 448–464.
- Leslie, A.B.; Simpson, C.; Mander, L. Reproductive innovations and pulsed rise in plant complexity. Science 2021, 373, 1368–1372.
- Moody, L.A. The 2D to 3D growth transition in the moss Physcomitrella patens. Curr. Opin. Plant Biol. 2019, 47, 88–95.
- Hata, Y.; Kyozuka, J. Fundamental mechanisms of the stem cell regulation in land plants: Lesson from shoot apical cells in bryophytes. Plant Mol. Biol. 2021, 107, 213–225.
- Michniewicz, M.; Zago, M.K.; Abas, L.; Weijers, D.; Schweighofer, A.; Meskiene, I.; Heisler, M.G.; Ohno, C.; Zhang, J.; Huang, F.; et al. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 2007, 130, 1044–1056.
- Viaene, T.; Landberg, K.; Thelander, M.; Medvecka, E.; Pederson, E.; Feraru, E.; Friml, J. Directional auxin transport mechanisms in early diverging land plants. Curr. Biol. 2014, 24, 2786–2791.
- Vosolsobě, S.; Skokan, R.; Petrášek, J. The evolutionary origins of auxin transport: What we know and what we need to know. J. Exp. Bot. 2020, 71, 3287–3295.
- Bennett, T. PIN proteins and the evolution of plant development. Trends Plant Sci. 2015, 20, 498–507.
- Morffy, N.; Strader, L.C. Old Town Roads: Routes of auxin biosynthesis across kingdoms. Curr. Opin. Plant Biol. 2020, 55, 21–27.
- Komatsu, K.; Suzuki, N.; Kuwamura, M.; Nishikawa, Y.; Nakatani, M.; Ohtawa, H.; Sakata, Y. Group A PP2Cs evolved in land plants as key regulators of intrinsic desiccation tolerance. Nat. Commun. 2013, 4, 2219.
- Stevenson, S.R.; Kamisugi, Y.; Trinh, C.H.; Schmutz, J.; Jenkins, J.W.; Grimwood, J.; Cuming, A.C. Genetic analysis of Physcomitrella patens identifies ABSCISIC ACID NON-RESPONSIVE, a regulator of ABA responses unique to basal land plants and required for desiccation tolerance. Plant Cell 2016, 28, 1310–1327.
- Sun, Y.; Pri-Tal, O.; Michaeli, D.; Mosquna, A. Evolution of abscisic acid signaling module and its perception. Front. Plant Sci. 2020, 11, 934.
- Umezawa, T.; Nakashima, K.; Miyakawa, T.; Kuromori, T.; Tanokura, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Molecular basis of the core regulatory network in ABA responses: Sensing, signaling and transport. Plant Cell Physiol. 2010, 51, 1821–1839.
- Cominelli, E.; Galbiati, M.; Vavasseur, A.; Conti, L.; Sala, T.; Vuylsteke, M.; Tonelli, C. A guard-cell-specific MYB transcription factor regulates stomatal movements and plant drought tolerance. Curr. Biol. 2005, 15, 1196–1200.
- De Zelicourt, A.; Colcombet, J.; Hirt, H. The role of MAPK modules and ABA during abiotic stress signaling. Trends Plant Sci. 2016, 21, 677–685.
- Brunetti, C.; Sebastiani, F.; Tattini, M. ABA, flavonols, and the evolvability of land plants. Plant Sci. 2019, 280, 448–454.
- Weng, J.K.; Chapple, C. The origin and evolution of lignin biosynthesis. New Phytol. 2010, 187, 273–285.
- Milo, R.; Last, R.L. Achieving diversity in the face of constraints: Lessons from metabolism. Science 2012, 336, 1663–1667.
- Mutwil, M. Computational approaches to unravel the pathways and evolution of specialized metabolism. Curr. Opin. Plant Biol. 2020, 55, 38–46.
- Weng, J.K.; Lynch, J.H.; Matos, J.O.; Dudareva, N. Adaptive mechanisms of plant specialized metabolism connecting chemistry to function. Nat. Chem. Biol. 2021, 17, 1037–1045.
- Jones, C.G.; Firn, R.D. On the evolution of plant secondary chemical diversity. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1991, 333, 273–280.
- Neilson, E.H.; Goodger, J.Q.; Woodrow, I.E.; Møller, B.L. Plant chemical defense: At what cost? Trends Plant Sci. 2013, 18, 250–258.
- Agati, G.; Brunetti, C.; Fini, A.; Gori, A.; Guidi, L.; Landi, M.; Sebastiani, F.; Tattini, M. Are flavonoids effective antioxidants in plants? Twenty years of our investigation. Antioxidants 2020, 9, 1098.
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209.
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 2020, 184, 39–52.
- Hu, L.; Wu, Z.; Robert, C.A.; Ouyang, X.; Züst, T.; Mestrot, A.; Erb, M. Soil chemistry determines whether defensive plant secondary metabolites promote or suppress herbivore growth. Proc. Natl. Acad. Sci. USA 2021, 118, e2109602118.
- Davies, K.M.; Jibran, R.; Zhou, Y.; Albert, N.W.; Brummell, D.A.; Jordan, B.R.; Bowman, J.L.; Schwinn, K.E. The evolution of flavonoid biosynthesis: A bryophyte perspective. Front. Plant Sci. 2020, 11, 7.
- Davies, K.M.; Jibran, R.; Albert, N.W.; Zhou, Y.; Schwinn, K.E. Conservation and divergence between bryophytes and angiosperms in the biosynthesis and regulation of flavonoid production. In Recent Advances Polyphenols Research; Reed, J.D., Freitas, V.A.P., Quideau, S., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; Volume 7, pp. 227–263.
- Saijo, Y.; Loo, E.P.I. Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol. 2020, 225, 87–104.
- Wen, W.; Alseekh, S.; Fernie, A.R. Conservation and diversification of flavonoid metabolism in the plant kingdom. Curr. Opin. Plant Biol. 2020, 55, 100–108.
- Böttner, L.; Grabe, V.; Gablenz, S.; Böhme, N.; Appenroth, K.J.; Gershenzon, J.; Huber, M. Differential localization of flavonoid glucosides in an aquatic plant implicates different functions under abiotic stress. Plant Cell Environ. 2021, 44, 900–914.
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19.
- Tian, B.; Pei, Y.; Huang, W.; Ding, J.; Siemann, E. Increasing flavonoid concentrations in root exudates enhance associations between arbuscular mycorrhizal fungi and an invasive plant. ISME J. 2021, 15, 1919–1930.
- Pollastri, S.; Tattini, M. Flavonols: Old compounds for old roles. Ann. Bot. 2011, 108, 1225–1233.
- Remias, D.; Schwaiger, S.; Aigner, S.; Leya, T.; Stuppner, H.; Lütz, C. Characterization of an UV- and VIS-absorbing, purpurogallin-derived secondary pigment new to algae and highly abundant in Mesotaenium berggrenii (Z ygnematophyceae, Chlorophyta), an extremophyte living on glaciers. FEMS Microbiol. Ecol. 2012, 79, 638–648.
- De Vries, J.; Archibald, J.M. Plant evolution: Landmarks on the path to terrestrial life. New Phytol. 2018, 217, 1428–1434.
- Singh, D.K.; Pathak, J.; Pandey, A.; Singh, V.; Ahmed, H.; Kumar, D.; Sinha, R.P. Ultraviolet-screening compound mycosporine-like amino acids in cyanobacteria: Biosynthesis, functions, and applications. In Advances in Cyanobacterial Biology; Singh, P.K., Kumar, A., Singh, V.K., Shrivastava, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 219–233.
- Geraldes, V.; Pinto, E. Mycosporine-Like Amino Acids (MAAs): Biology, Chemistry and Identification Features. Pharmaceuticals 2021, 14, 63.
- Hartmann, A.; Glaser, K.; Holzinger, A.; Ganzera, M.; Karsten, U. Klebsormidin A and B, two new UV-sunscreen compounds in green microalgal interfilum and Klebsormidium Species (Streptophyta) from terrestrial habitats. Front. Microbiol. 2020, 11, 499.
- Bhatia, S.; Garg, A.; Sharma, K.; Kumar, S.; Sharma, A.; Purohit, A.P. Mycosporine and mycosporine-like amino acids: A paramount tool against ultraviolet irradiation. Pharmacogn. Rev. 2011, 5, 138.
- Lüttge, U. Terrestrialization: The Conquest of Dry Land by Plants. In Progress in Botany; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–25.
- Holzinger, A.; Pichrtová, M. Abiotic stress tolerance of charophyte green algae: New challenges for omics techniques. Front. Plant Sci. 2016, 7, 678.
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. Chemical and ecological aspects. Mar. Drugs 2011, 9, 387–446.
- Løvdal, T.; Olsen, K.M.; Slimestad, R.; Verheul, M.; Lillo, C. Synergetic effects of nitrogen depletion, temperature, and light on the content of phenolic compounds and gene expression in leaves of tomato. Phytochemistry 2010, 71, 605–613.
- Wang, Y.; Cheng, X.; Yang, T.; Su, Y.; Lin, S.; Zhang, S.; Zhang, Z. Nitrogen-Regulated Theanine and Flavonoid Biosynthesis in Tea Plant Roots: Protein-Level Regulation Revealed by Multiomics Analyses. J. Agric. Food Chem. 2021, 69, 10002–10016.
- Lu, Y.; Chen, Q.; Bu, Y.; Luo, R.; Hao, S.; Zhang, J.; Yao, Y. Flavonoid accumulation plays an important role in the rust resistance of Malus plant leaves. Front. Plant Sci. 2017, 8, 1286.
- Hernández, I.; Van Breusegem, F. Opinion on the possible role of flavonoids as energy escape valves: Novel tools for nature’s Swiss army knife? Plant Sci. 2010, 179, 297–301.
- Ferreyra, M.L.F.; Serra, P.; Casati, P. Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure. Physiol. Plant 2021, 173, 736–749.
- Becker, B.; Marin, B. Streptophyte algae and the origin of embryophytes. Ann. Bot. 2009, 103, 999–1004.
- Cockell, C.S.; Knowland, J. Ultraviolet radiation screening compounds. Biol. Rev. 1999, 74, 311–345.
- Rensing, S.A. Great moments in evolution: The conquest of land by plants. Curr. Opin. Plant Biol. 2018, 42, 49–54.
- Rensing, S.A. How plants conquered land. Cell 2020, 181, 964–966.
- Berbee, M.L.; Strullu-Derrien, C.; Delaux, P.M.; Strother, P.K.; Kenrick, P.; Selosse, M.A.; Taylor, J.W. Genomic and fossil windows into the secret lives of the most ancient fungi. Nat. Rev. Microbiol. 2020, 18, 717–730.
- Laliberté, E.; Grace, J.B.; Huston, M.A.; Lambers, H.; Teste, F.P.; Turner, B.L.; Wardle, D.A. How does pedogenesis drive plant diversity? Trends Ecol. Evol. 2013, 28, 331–340.
- Zemunik, G.; Turner, B.L.; Lambers, H.; Laliberté, E. Increasing plant species diversity and extreme species turnover accompany declining soil fertility along a long-term chronosequence in a biodiversity hotspot. J. Ecol. 2016, 104, 792–805.
- Dievart, A.; Gottin, C.; Périn, C.; Ranwez, V.; Chantret, N. Origin and diversity of plant receptor-like kinases. Ann. Rev. Plant Biol. 2020, 71, 131–156.
- Montero, H.; Lee, T.; Pucker, B.; Ferreras-Garrucho, G.; Oldroyd, G.; Brockington, S.F.; Paszkowski, U. A mycorrhiza-associated receptor-like kinase with an ancient origin in the green lineage. Proc. Natl. Acad. Sci. USA 2021, 118, e2105281118.
- Delaux, P.M.; Radhakrishnan, G.V.; Jayaraman, D.; Cheema, J.; Malbreil, M.; Volkening, J.D.; Ané, J.M. Algal ancestor of land plants was preadapted for symbiosis. Proc. Natl. Acad. Sci. USA 2015, 112, 13390–13395.
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775.
- Wang, B.; Yeun, L.H.; Xue, J.Y.; Liu, Y.; Ané, J.M.; Qiu, Y.L. Presence of three mycorrhizal genes in the common ancestor of land plants suggests a key role of mycorrhizas in the colonization of land by plants. New Phytol. 2010, 186, 514–525.
- Vigneron, N.; Radhakrishnan, G.V.; Delaux, P.M. What have we learnt from studying the evolution of the arbuscular mycorrhizal symbiosis? Curr. Opin. Plant Biol. 2018, 44, 49–56.
- Bonfante, P.; Genre, A. Plants and arbuscular mycorrhizal fungi: An evolutionary-developmental perspective. Trends Plant Sci. 2008, 13, 492–498.
- Field, K.J.; Pressel, S.; Duckett, J.G.; Rimington, W.R.; Bidartondo, M.I. Symbiotic options for the conquest of land. Trends Ecol. Evol. 2015, 30, 477–486.
- Hoysted, G.A.; Kowal, J.; Jacob, A.; Rimington, W.R.; Duckett, J.G.; Pressel, S.; Bidartondo, M.I. A mycorrhizal revolution. Curr. Opin. Plant Biol. 2018, 44, 1–6.
- Rich, M.K.; Vigneron, N.; Libourel, C.; Keller, J.; Xue, L.; Hajheidari, M.; Delaux, P.M. Lipid exchanges drove the evolution of mutualism during plant terrestrialization. Science 2021, 372, 864–868.
- Field, K.J.; Bidartondo, M.I.; Rimington, W.R.; Hoysted, G.A.; Beerling, D.; Cameron, D.D.; Pressel, S. Functional complementarity of ancient plant–fungal mutualisms: Contrasting nitrogen, phosphorus and carbon exchanges between Mucoromycotina and Glomeromycotina fungal symbionts of liverworts. New Phytol. 2019, 223, 908–921.
- Rimington, W.R.; Duckett, J.G.; Field, K.J.; Bidartondo, M.I.; Pressel, S. The distribution and evolution of fungal symbioses in ancient lineages of land plants. Mycorrhiza 2020, 30, 23–49.
- Genre, A.; Lanfranco, L.; Perotto, S.; Bonfante, P. Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 2020, 18, 649–660.
- Rimington, W.R.; Pressel, S.; Duckett, J.G.; Field, K.J.; Read, D.J.; Bidartondo, M.I. Ancient plants with ancient fungi: Liverworts associate with early-diverging arbuscular mycorrhizal fungi. Proc. R. Soc. B 2018, 285, 20181600.
- MacLean, A.M.; Bravo, A.; Harrison, M.J. Plant signaling and metabolic pathways enabling arbuscular mycorrhizal symbiosis. Plant Cell 2017, 29, 2319–2335.
- Cosme, M.; Fernández, I.; Van der Heijden, M.G.; Pieterse, C.M. Non-mycorrhizal plants: The exceptions that prove the rule. Trends Plant Sci. 2018, 23, 577–587.
- Wang, B.; Qiu, Y.L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363.
- Martin, F.M.; Uroz, S.; Barker, D.G. Ancestral alliances: Plant mutualistic symbioses with fungi and bacteria. Science 2017, 356, eaad4501.
- Fusconi, A. Regulation of root morphogenesis in arbuscular mycorrhizae: What role do fungal exudates, phosphate, sugars and hormones play in lateral root formation? Ann. Bot. 2014, 113, 19–33.
- Etemadi, M.; Gutjahr, C.; Couzigou, J.M.; Zouine, M.; Lauressergues, D.; Timmers, A.; Combier, J.P. Auxin perception is required for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Physiol. 2014, 166, 281–292.
- Chen, X.; Chen, J.; Liao, D.; Ye, H.; Li, C.; Luo, Z.; Xu, G. Auxin-mediated regulation of arbuscular mycorrhizal symbiosis: A role of SlGH3. 4 in tomato. Plant Cell Environ. 2021, 45, 955–968.
- Hanlon, M.T.; Coenen, C. Genetic evidence for auxin involvement in arbuscular mycorrhiza initiation. New Phytol. 2011, 189, 701–709.
- Foo, E.; Ross, J.J.; Jones, W.T.; Reid, J.B. Plant hormones in arbuscular mycorrhizal symbioses: An emerging role for gibberellins. Ann. Bot. 2013, 111, 769–779.
- Ludwig-Müller, J.; Güther, M. Auxins as signals in arbuscular mycorrhiza formation. Plant Sign. Behav. 2007, 2, 194–196.
- Peer, W.A.; Murphy, A.S. Flavonoids and auxin transport: Modulators or regulators? Trends Plant Sci. 2007, 12, 556–563.
- Zhang, J.; Peer, W.A. Auxin homeostasis: The DAO of catabolism. J. Exp. Bot. 2017, 68, 3145–3154.
- Gianinazzi-Pearson, V.; Branzanti, B.; Gianinazzi, S. In vitro enhancement of spore germination and early hyphal growth of a vesicular-arbuscular mycorrhizal fungus by host root exudates and plant flavonoids. Symbiosis 1989, 7, 243–255.
- Akiyama, K.; Matsuoka, H.; Hayashi, H. Isolation and identification of a phosphate deficiency-induced C-glycosyl flavonoid that stimulates arbuscular mycorrhiza formation in melon roots. Mol. Plant-Microbe Inter. 2002, 15, 334–340.
- Cesco, S.; Neumann, G.; Tomasi, N.; Pinton, R.; Weisskopf, L. Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 2010, 329, 1–25.
- Salloum, M.S.; Menduni, M.F.; Luna, C.M. A differential capacity of arbuscular mycorrhizal fungal colonization under well-watered conditions and its relationship with drought stress mitigation in unimproved vs. improved soybean genotypes. Botany 2018, 96, 135–144.
- Dong, W.; Song, Y. The Significance of Flavonoids in the Process of Biological Nitrogen Fixation. Int. J. Mol. Sci. 2020, 21, 5926.
- Pei, Y.; Siemann, E.; Tian, B.; Ding, J. Root of an invasive flavonoids are related to enhanced AMF colonization tree. AoB Plants 2020, 12, plaa002.
- Subramanian, S.; Stacey, G.; Yu, O. Distinct, crucial roles of flavonoids during legume nodulation. Trends Plant Sci. 2007, 12, 282–285.
- Stafford, H.A. Roles of flavonoids in symbiotic and defense functions in legume roots. Bot. Rev. 1997, 63, 27–39.
- Ng, J.L.P.; Hassan, S.; Truong, T.T.; Hocart, C.H.; Laffont, C.; Frugier, F.; Mathesius, U. Flavonoids and auxin transport inhibitors rescue symbiotic nodulation in the Medicago truncatula cytokinin perception mutant cre1. Plant Cell 2015, 27, 2210–2226.
- Soltis, D.E.; Soltis, P.S.; Morgan, D.R.; Swensen, S.M.; Mullin, B.C.; Dowd, J.M.; Martin, P.G. Chloroplast gene sequence data suggest a single origin of the predisposition for symbiotic nitrogen fixation in angiosperms. Proc. Nat. Acad. Sci. USA 1995, 92, 2647–2651.
- Kistner, C.; Parniske, M. Evolution of signal transduction in intracellular symbiosis. Trends Plant Sci. 2002, 7, 511–518.
- Taylor, L.P.; Grotewold, E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323.
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555.
- Watkins, J.M.; Hechler, P.J.; Muday, G.K. Ethylene-induced flavonol accumulation in guard cells suppresses reactive oxygen species and moderates stomatal aperture. Plant Physiol. 2014, 164, 1707–1717.
- Watkins, J.M.; Chapman, J.M.; Muday, G.K. Abscisic acid-induced reactive oxygen species are modulated by flavonols to control stomata aperture. Plant Physiol. 2017, 175, 1807–1825.
- Watkins, J.M.; Hechler, P.J.; Muday, G.K. Ethylene-induced flavonol accumulation in guard cells suppresses reactive oxygen species and moderates stomatal aperture. Plant Physiol. 2014, 164, 1707–1717.
- Watkins, J.M.; Chapman, J.M.; Muday, G.K. Abscisic acid-induced reactive oxygen species are modulated by flavonols to control stomata aperture. Plant Physiol. 2017, 175, 1807–1825.
More
