Neurodegenerative disorders including Parkinson’s disease (PD), Huntington’s disease (HD) and the most frequent, Alzheimer’s disease (AD), represent one of the most urgent medical needs worldwide. Despite a significantly developed understanding of disease development and pathology, treatments that stop AD progression are not yet available. The recent approval of sodium oligomannate (GV-971) for AD treatment in China emphasized the potential value of natural products for the treatment of neurodegenerative disorders. Many current clinical studies include the administration of a natural compound as a single and combination treatment. The most prominent mechanisms of action are anti-inflammatory and anti-oxidative activities, thus preserving cellular survival.
- Alzheimer’s disease
- neurodegeneration
- drug development
- clinical studies
1. Introduction
| Agent | Mechanism of Action | Therapeutic Purpose | Trial Identifier and Status | Phase | |
|---|---|---|---|---|---|
| Huperzine A | AChE inhibitor, inhibition of Aβ | improve memory | Not yet recruiting NCT02931136 |
IV | |
| Sodium oligomannate (GV-971) |
neuroinflammation modulators, microbiome modulators, amyloid beta-protein inhibitors; reconditioning the dysbiosis of gut microbiota, preventing peripheral immune cells from invading the brain, inhibiting the inflammatory response in the brain targeting protein folding errors in the brain tissue |
improve the cognitive function of patients with mild to moderate AD | Recruiting NCT05058040 |
IV | |
| Sodium oligomannte capsules (GV-971) |
neuroinflammation modulators, microbiome modulators, amyloid beta-protein inhibitors; reconditioning the dysbiosis of gut microbiota, preventing peripheral immune cells from invading the brain, inhibiting the inflammatory response in the brain targeting protein folding errors in the brain tissue |
improve the cognitive function of patients with mild to moderate AD | Recruiting NCT05181475 |
IV | |
| Ginkgo biloba | metabolism and bioenergetics; plant extract with antioxidant properties | Improve brain blood flow and mitochondrial function (cognitive enhancer) | Recruiting NCT03090516 |
III | |
| Sodium oligomannate (GV-971) |
reconditioning the dysbiosis of gut microbiota, preventing peripheral immune cells from invading the brain, inhibiting the inflammatory response in the brain targeting protein folding errors in the brain tissue | improve the cognitive function of patients with mild to moderate AD; evaluate safety, tolerability and efficacy of GV-971 | Recruiting NCT04520412 |
III | |
| Curcumin + aerobic yoga | herb with antioxidant and anti-inflammatory properties | decrease inflammation and oxidation related neurotoxicity | active, not recruiting NCT01811381 |
II | |
| Elderberry Juice | rich in anthocyanins, has anti-inflammatory and antioxidant activity | improve mitochondrial function | completed NCT02414607 |
II | |
| Grape powder | antioxidant, anti-inflammatory and anticarcinogenic | improves cognitive performance preservation of metabolism in brain regions important to cognitive function | recruiting NCT03361410 |
II | |
| Icosapent ethyl (IPE) | synaptic plasticity, neuroprotection; purified from of the omega-3 fatty acid EPA | improve synaptic function; reduce inflammation | recruiting NCT02719327 |
II | |
| Meganatrual-Az Grapeseed Extract | polyphenolic extract with antioxidant properties | anti-oligomerization agent; prevents aggregation of amyloid and tau | recruiting NCT02033941 |
II | |
| Omega-3 PUFA | fish oil concentrate standardized to long chain in n-3 PUFA content | reduces inflammation and glial activation; enhances amyloid removal; protect small blood vessels | active, not recruiting NCT01953705 |
II | |
| Rapamycin | anti-inflammatory, antineoplastic; macrolide compound from | Streptomyces hygroscopicus | selectively blocks the transcriptional activation of cytokines | recruiting NCT04629495 |
II |
| Rifaximin | inflammation, infection and immunity; antibiotic | reduce proinflammatory cytokines secreted by harmful gut bacteria | completed NCT03856359 |
II | |
| Tacrolimus | tau proteins; macrolide from culture broth of a strain of | Streptomyces tsukubaensis | reduce pathological changes of tau proteins | withdrawn NCT04263519 |
II |
| THC-free CBD Oil | anti-oxidant and anti-inflammatory; cannabinoids | behavioural and psychological symptoms of dementia (BPSD) decrease with use of cannabinoids | recruiting NCT04436081 |
II | |
| VGH-AD1 | undisclosed; traditional Chinese herbal medicine | undisclosed (cognitive enhancer) | not yet recruiting NCT04249869 |
II | |
| Yangxue Qingnao pills | blood circulation; traditional Chinese medicine, composed of Angelicae Sinensis Radix, Chuanxiong Rhizoma, Paeoniae Radix Alba, Rhemannia glutinosa, Uncaria macrophylla Wall, Caulis spatholobi, Spica Prunellae, Catsia tora Linn, Mater Margarita, Corydalis ambigua and Asarum sieboldii | improve cerebral blood flow and brain nourishment | not yet recruiting NCT04780399 |
II | |
| BDPP (bioactive dietary polyphenol preparation) | metabolism and bioenergetics, amyloid; combination of grape seed polyphenolic extract and resveratrol | prevents amyloid and tau aggregation | recruiting NCT02502253 |
I | |
| Pomace olive oil | prevent inflammation; lipophilic minor components | consumption of olive oil reduces activation of microglia by TRL (triglyceride-rich lipoproteins) | completed NCT04559828 |
not applicable | |
| Extra virgin olive oil “Coratina” | anti-amyloid; biophenol | improve cerebral performance | not yet recruiting NCT04229186 |
not applicable |
2. Natural Products from Non-Algal Sources
2.1. Esterase Inhibitors
| Name | Structure | Source | Characteristics | Ref. | |
|---|---|---|---|---|---|
| galantamine | 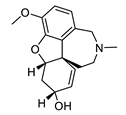 |
Galanthus nivalis | reversible, competitive AChE inhibitor, allosteric modulator of nicotinic acetylcholine receptors, modulates α4β2 and α7 nicotinic receptors | [40][41][42][43] | [40,41,42,43] |
| huperzine A | 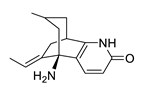 |
Huperzia serrata | specific and reversible AChE inhibitor, protects cells against hydrogen peroxide, β-amyloid toxicity, glutamate, ischemia and staurosporine-induced cytotoxicity and apoptosis | [45][46][47][48][49] | [45,46,47,48,51] |
| physostigmine | 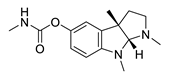 |
Physostigma venenosum, Streptomyces pseudogriseolus | AChE inhibitor | [50] | [57] |
| tolserine |
| Name | Structure | Name | Structure | ||
|---|---|---|---|---|---|
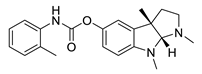 | |||||
| Physostigmine derivative | |||||
| AChE inhibitor | [ | 51 | ] | [66] | |
| eseroline | 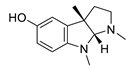 |
Physostigmine derivative | AChE inhibitor | [51] | [66] |
| phenserine | 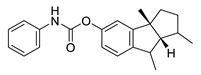 |
Physostigmine derivative | AChE inhibitor | [51] | [66] |
2.2. Plant Natural Products with Antioxidant and Anti-Inflammatory Efficacy
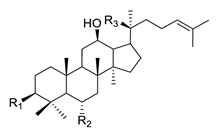
Ginseng. Extracts of the rhizome of the plant Panax ginseng have been used in Asia for thousands of years to treat different diseases including neurological disorders [59][67].The extract of the plant has several active compounds, ginsenosides, ginseng polysaccharides, volatile oils, peptides and amino acids [60][61][68,69]. There are several ginsenosides identified as useful in the treatment of neurodegenerative disease such as AD, PD and HD. The ginsenoside Rb1, Rg1, Rg2, Rg3, Re and Rh2 and Gintonin showed a beneficial effect on AD symptomatology; Rg1, Re and Rd in PD and Ginseng total saponins and Ginsenosides in HD [62][63][64][70,71,72]. The ginsenosides are classified in two groups: the 20(S)-protopanaxadiol (PPD) group and the 20(S)-protopanaxtriol (PPT) group. Rb1, Rc, Rb2, Rd and Rg3 belong to the 20(S)-protopanaxadiol group, while Rg1, Re, Rg2 and Rh1 belong to the 20(S)-protopanaxtriol group [65][73]. The chemical structure of the ginsenosides is shown in Table 3. Ginsenosides prevent neuroinflammation and oxidative stress. They also have a positive influence on the brain function by apparently diverse mechanisms [66][67][68][69][74,75,76,77].| Name | Structure | |||
|---|---|---|---|---|
| ginkgolide A | 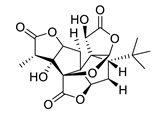 |
ginkgolide B | 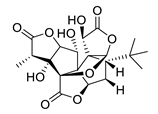 |
|
| ginkgolide C | 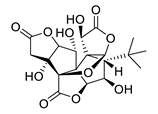 |
ginkgolide J | ||
| Rd | -O-Glc-Glc | -H | -O-Glc | |
| Rg3 | -O-Glc-Glc | -H | -OH | |
| F2 | -O-Glc | -H | -O-Glc | |
| Rh2 | -O-Glc | -H | -OH | |
| Compound K | -OH | -H | -O-Glc | |
| PPD | -OH | -H | -OH | |
| PPT-type | Re | -OH | -O-Glc-Rha | -O-Glc |
| Rf | -OH | -O-Glc-Glc | -OH | |
| Rg1 | -OH | -O-Glc | -O-Glc | |
| Rg2 | -OH | -O-Glc-Rha | -OH | |
| Rh1 | -OH | -O-Glc | -OH | |
| F1 | -OH | -OH | -O-Glc | |
| PPT | -OH | -OH | -OH |
3.1. Carbohydrates
Sodium oligomannate is a mixture of oligosaccharides obtained by the depolymerization of alginate from marine brown algae, followed by its oxidation to oligosaccharides [86][87][123,124]| Source |
|---|
| Characteristics | Ref. | ||||
|---|---|---|---|---|---|
| GV971 (Sodium oligomannate) |
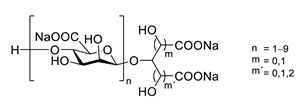 |
marine brown algae | might act via decreasing neuroinflammation by remodeling gut microbiota and balancing the amino acid metabolism, especially phenylalanine and isoleucine | [87] | [124] |
| porphyran | 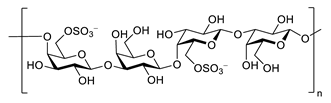 |
Porphyra yezoensis | superoxide anion and hydroxyl radical scavenging activity | [90] | [130] |
| floridoside | 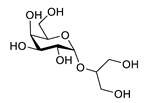 |
Laurencia undulata | anti-inflammatory activity, inhibits the production of NO and ROS, downregulates iNOS and COX-2 | [91] | [132] |
| fucoidan | 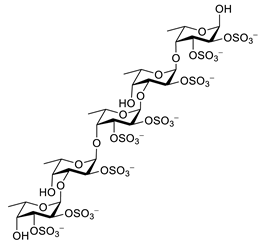 |
Ascophyllum nodosum | inhibits ROS and TNF-α release, reduces NO, PGE2, COX-2, iNOS, MCP-1, TNF-α and IL-1β | [92][93] | [134,135] |
| κ-carrageenan | 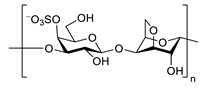 |
inhibits TNF-α secretion | [94] | [137] |