Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Young-Cheol Chang.
Polyhydroxyalkanoates (PHAs) are a type of biopolymer developed as intracellular carbon/energy storage materials which have a wide range of material characteristics. PHAs are identified as granular inclusion bodies after extraction from cells, these are becoming popular as prospective replacements for traditional plastics in various applications, including food packaging industries, cultivational fields, scaffold preparation, and biomaterial implants.
- polyhydroxyalkanoates
- Carbohydrates
- production
1. Introduction
Polyhydroxyalkanoates (PHAs) are a type of biopolymer developed as intracellular carbon/energy storage materials which have a wide range of material characteristics. PHAs are identified as granular inclusion bodies after extraction from cells, these are becoming popular as prospective replacements for traditional plastics in various applications, including food packaging industries, cultivational fields, scaffold preparation, and biomaterial implants [1,2][1][2]. PHAs are classified based on the length of the 3-hydroxyalkanoate (3HA) repeat units, the repeat units of PHAs with short chain length (scl-PHAs) are generally 4–5 carbon atoms long (e.g., 3-hydroxybutyrate, 3HB and 3-hydroxyvalerate, 3HV units), medium chain length (mcl-PHAs) are 6–14 carbon atoms long (e.g., 3-hydroxyhexanoate, 3HHx, 3-hydroxyheptanoate, 3HHo), and long chain length (lcl-PHA) are more than 14 carbon atoms (e.g., 3-hydroxyhexadecanoate) [3,4][3][4]. Rakkan et al. (2022) reported the production of mcl-co-lcl PHAs (72 to 75% of DCW) from Enterobacter sp. strains TS3 and TS1L using glucose [5]. Mcl-PHAs are soft, elastomeric, and have less crystallinity, a lower melting point, and lower glass transition temperature [6]. Nutrient limiting conditions are required for PHA generation in Pseudomonas oleovorans, Pseudomonas putida, and Ralstonia eutropha, but not in recombinant Escherichia coli and Alcaligenes latus [7]. High manufacturing costs are a key impediment to the commercialization of PHAs. Carbon conversion yield (g/g), titer or volumetric yield (g/L), and productivity (g/L/h) are crucial in this scenario [8]. Aside from production criteria, low-cost downstream processing techniques and PHA manufacturing that fulfils cost performance standards have remained difficult to achieve. This has prompted researchers to focus on improving PHA fermentation and downstream processing efficiency to lower total costs [9,10][9][10]. The PHA production and degradation cycle is explained in Figure 1.
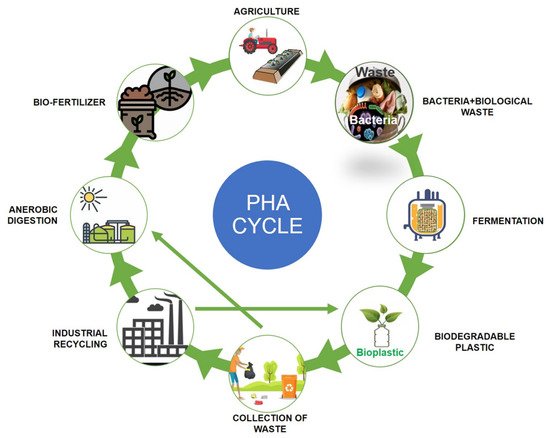
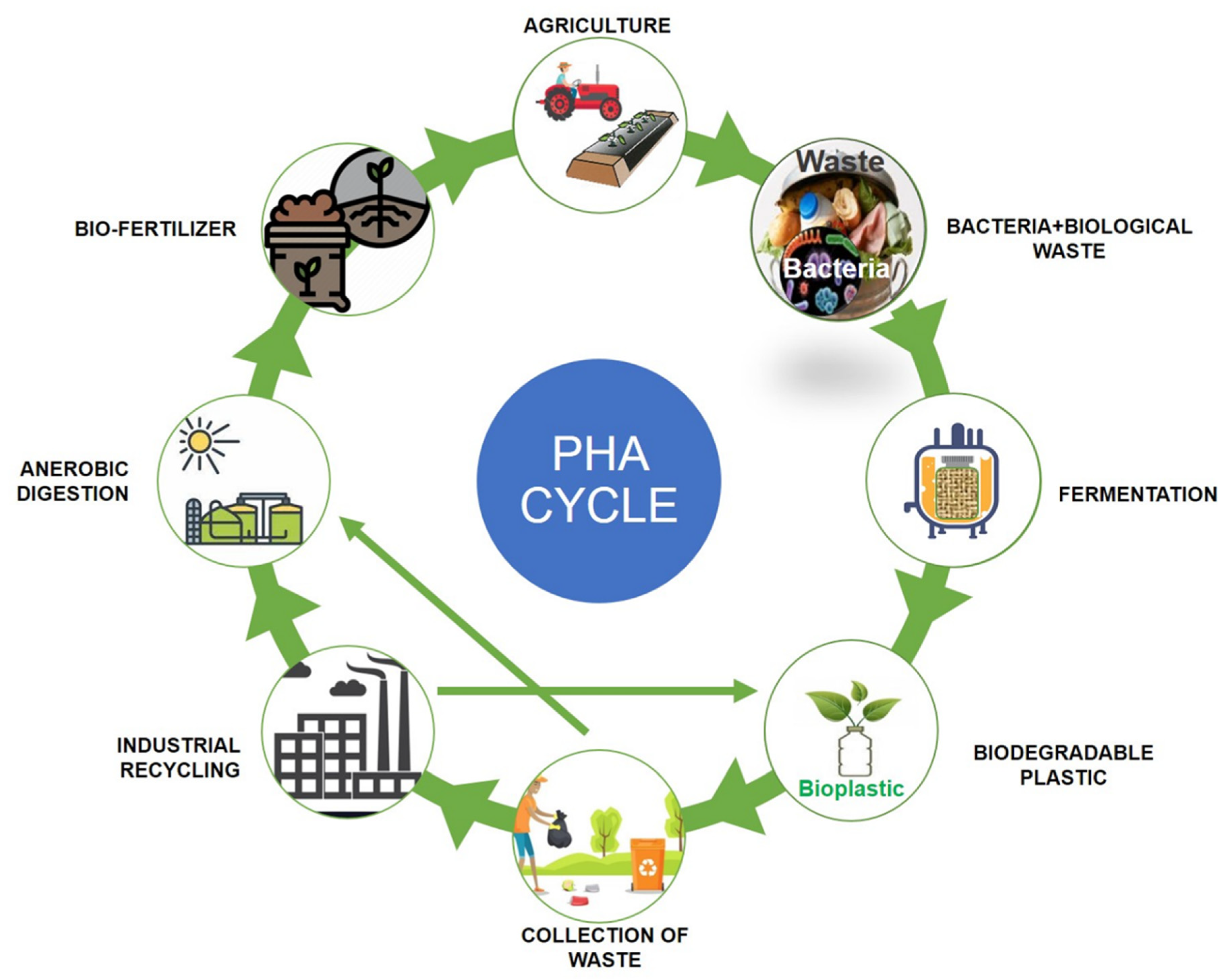
Figure 1. Polyhydroxyalkanoates (PHA) production and degradation cycle.
Polyhydroxyalkanoates (PHA) production and degradation cycle.
A significant aim for PHA synthesis is to obtain high yields in high cell-density cultures (HCDCs). Bioethanol and yeast that produces single-cell proteins were used to construct HCDC in the beginning. Because of its benefits, such as reduced culture volume and residual liquid, cheaper production costs, and lower capital investment, HCDC technique is favored over low cell density technologies [11,12][11][12]. Dry cell weight (DCW) above 100 g/L is termed HCDC for PHA synthesis, a DCW above 50 g/L is regarded high for recombinant protein production [11,13,14][11][13][14]. More details on PHA production through HCDC were provided in Section 3.2. The species of Bacillus and C. necator are generally known to produce scl-PHAs, whilst Pseudomonas species known to produce mcl-PHAs. PHAs including Scl and Mcl can be developed by the species of Alcaligenes and Rhodococcus. PHAs have been used to construct a variety of drug carriers, cardiac patches, vascular grafts, nerve conduits, heart valves, artificial blood vessels, subcutaneous implants, orthopaedical pins, stents, wound dressings, and slings [15]. In addition, the FDA authorized P4HB for medical usage as absorbable sutures in the year 2007 [16]. Poly-3-hydroxyhexanoate (P3HO) has been used for soft (cardiovascular and neurologic) and hard (bone) tissue engineering [17,18][17][18]. By lowering mitochondrial damage, methyl esters of 3HB were used as drugs to treat Alzheimer’s disease [19]. Memory enhancers have also been shown to be sodium salts of 3HB monomers [20].
Unlike other polymers used before, which degrade through bulk degradation, PHAs degrade through controlled surface erosion [15]. Controlled degradation must be used to preserve implant integrity in vivo. PHAs have been thoroughly tested both inside and outside of the labs to confirm that they are biodegradable. PHAs also degrade far slower than polylactic acid (PLA), making them suited for long-term applications.
2. Mcl-PHA production
2.1. Inorganic Carbon Sources
It is crucial to employ natural substrates for microbial PHA synthesis to reduce manufacturing costs. The ideal feedstock is one that does not compete with human food. Syngas is a carbon monoxide and hydrogen mixture that may be produced by pyrolyzing organic waste. PHAs may be synthesized by a variety of microorganisms using syngas. Heinrich et al. (2016) genetically engineered the Rhodospirillum rubrum S1 to create a heteropolymer of 3-hydroxydecanoic acid and 3-hydroxyoctanoic acid [P(3HD-co-3HO)] from artificial syngas including CO and CO2 [21]. Three genes—3-hydroxyacyl-ACP thioesterase (phaG), mcl-fatty acid CoA ligase (PP 0763), and a PHA synthase (phaC1)—from P. putida KT2440 were chosen for overexpression. To investigate the impact of syngas-mediated gene overexpression, the CO-inducible PcooF promoter was employed. A recombinant mutant produced P(3HD-co-3HO) up to 7.1% (wt/wt) of the DCW. Furthermore, enhanced mcl-PHA synthesis and increased gene expression via the PcooF promoter resulted in a greater molar fraction of 3HO in the generated copolymer than the Plac promoter, which regulated expression on the original vector. The polymer has a molecular mass of 124.3 kDa, melting point of 49.6 °C, and glass transition temperature of 41.1 °C. According to GC analysis, the polymer-isolate fractions were 55.6 mol % 3HD, 44.2 mol % 3HO, and less than 0.2 mol % 3HH. The partial disintegration of the accumulated polymer might be connected to the activity of a lipase identified in R. rubrum, as these enzymes have been shown to digest PHAs with varying chain lengths [22]. Figure 2 depicts the metabolic pathway carried in the production of mcl-PHAs from CO2 in recombinant R. rubrum. After entering into the cytoplasmic membrane, CO2 is fixed via ribulose 1,5-bisphosphate carboxylase through the Calvin cycle and generates glyceraldehyde-3-phosphate, which is subsequently converted to the pyruvate. Pyruvate is further converted into acetyl-CoA by the action of the enzyme pyruvate synthase. Acetyl-CoA is then entered into the fatty acid de novo synthesis cycle and generates 3-hydroxyacyl-ACP. The thiolysis of 3-hydroxyacyl-ACP is catalyzed by the 3-hydroxyacyl-ACP thioesterase and produces 3-hydroxy fatty acid. Activation of 3-hydroxy fatty acids occurred by the addition of CoA, which is catalyzed by the enzyme mcl-fatty acid CoA ligase. The enzyme PHA synthase polymerizes the 3-hydroxyacyl-CoA into mcl-PHA [21].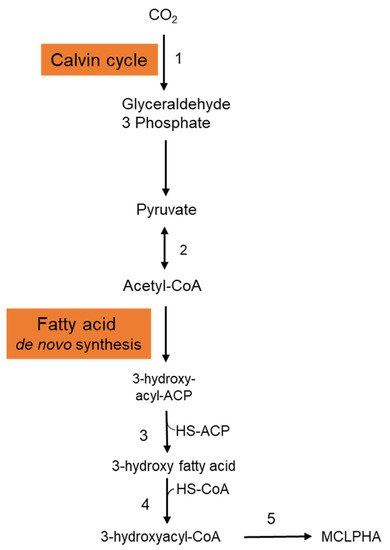
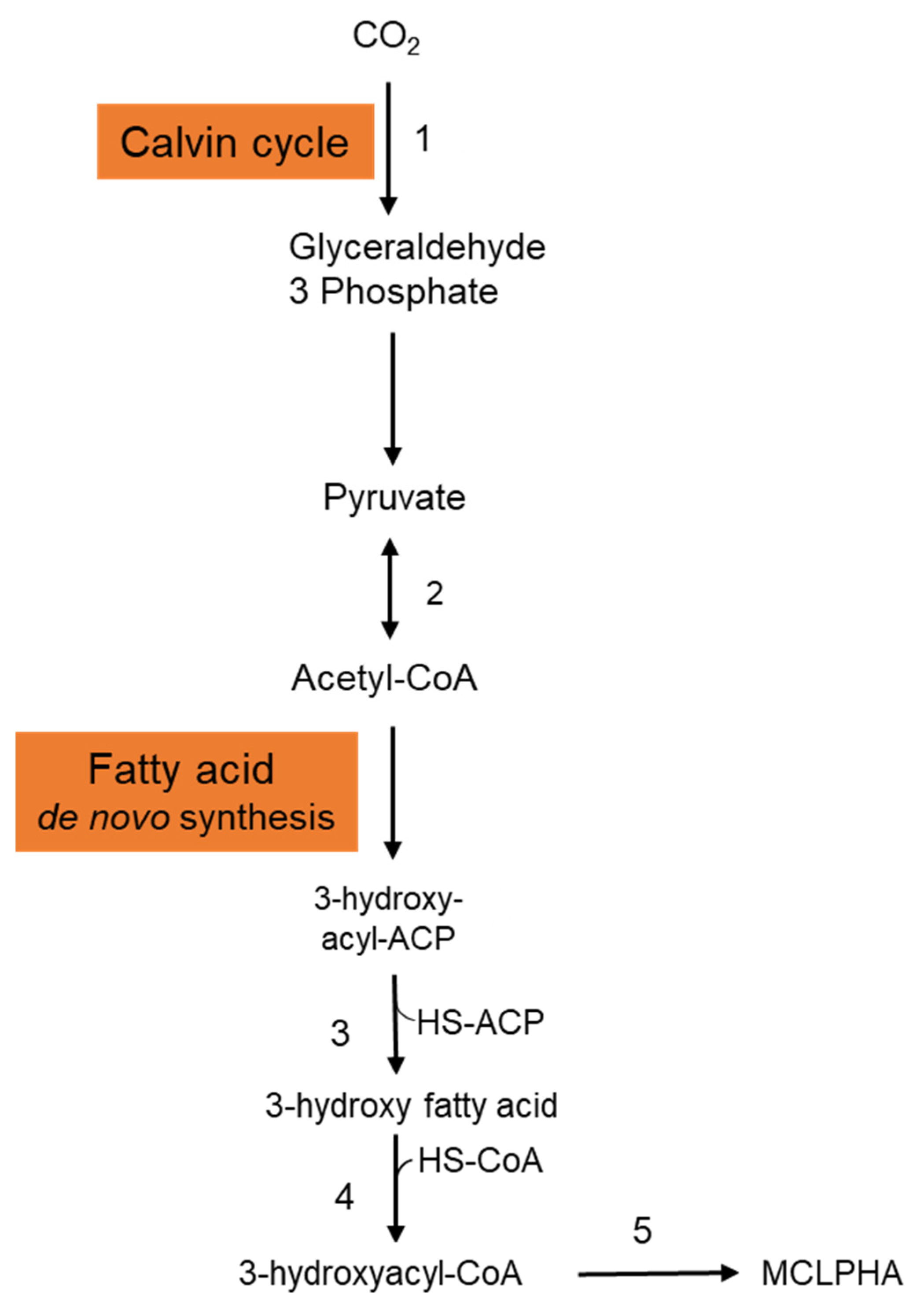 Figure 2. Metabolic pathway involved in the synthesis of mcl-PHA from CO2 in Rhodospirillum rubrum. This figure was generated with the information from Heinrich et al., 2016. 1: Ribulose 1,5-bisphosphate carboxylase; 2: Pyruvate synthase; 3: 3-hydroxyacyl-ACP thioesterase; 4: MCL fatty acid CoA ligase; 5: PHA synthase.
Figure 2. Metabolic pathway involved in the synthesis of mcl-PHA from CO2 in Rhodospirillum rubrum. This figure was generated with the information from Heinrich et al., 2016. 1: Ribulose 1,5-bisphosphate carboxylase; 2: Pyruvate synthase; 3: 3-hydroxyacyl-ACP thioesterase; 4: MCL fatty acid CoA ligase; 5: PHA synthase.2.2. Organic Carbon Sources
Mcl-PHA production from various organic carbon sources were summarized in Table 1.Table 1.
Mcl-PHA production using various carbon sources from literature reports.

| Bacteria | Carbon Source | DCW (g/L) | PHA Conc. (g/L) |
PHA (%) |
PHA Composition | Productivity (g/L/h) |
Time (h) | References |
|---|---|---|---|---|---|---|---|---|
| P. putida KT2442 | Oleic acid | 141 | 72 | 51 | C6-C8-C10-C12-C14 | 1.91 | 38 | [10] |
| P. putida KT2440 | Glucose + nonanoic acid | 98 | 32 | 33 | C9-C7 | 3.1 | 32 | [13] |
| Recombinant R. eutropha |
Palm oil | 139 | 102 | 74 | Mcl-PHA | 1.2 | 96 | [27] |
| P. putida KT2440 | Waste cooking oil | 159 | 58 | 36 | C6-C8-C10-C12-C14 | 1.93 | 30 | [28] |
| P. putida KT2440 | Glucose + nonanoic acid + acrylic acid | 71 | 53 | 75 | C9-C7 | 1.8 | 30 | [29] |
| P. putida LS46 | Octanoic acid | 29 | 17 | 61 | C6-C8-C10-C12 | 0.66 | 27 | [30] |
| P. putida KT2440 | Glucose + nonanoic acid | 71 | 40 | 56 | C5-C7-C9 | 1.44 | 28 | [31] |
| P. putida IPT 046 | Glucose + fructose | 50 | 31 | 63 | Mcl-PHA | 0.8 | 42 | [32] |
| P. oleovorans | n-octane | 112 | 5.6 | 5 | Mcl-PHA | 0.091 | 61 | [33] |
| P. putida KT2442 | Octanoic acid | 51 | 18 | 35 | C8 | 0.41 | 43 | [34] |
| P. putida KT2442 | Oleic acid | 90 | 18 | 20 | C6-C8-C10-C12-C14 | 0.57 | 32 | [34] |
| P. oleovorans | n-octane | 12 | 3.4 | 28 | Mcl-PHA | 0.58 | 120 | [35] |
| P. oleovorans | n-octane | 37 | 12 | 33 | Mcl-PHA | 0.25 | 48 | [36] |
P. = Pseudomonas; DCW = Dry cell weight; PHA conc. = polyhydroxyalkanoates concentration; R. = Ralstonia.
2.2.1. Fatty Acids
R. eutropha grown on animal fat waste produced 45 g/L biomass which contains 60% PHA [37]. In the R. eutropha recombinant strain, the R. aetherivorans has expressed I24 PHA synthase gene, and PHA with HHx units is produced by P. aeruginosa hydratase gene (phaJ) [27]. The total biomass produced was 139 g/L with 74% of co-polymer, P (3HB-co-19 mol % 3HHx). Sato et al. observed that, in utilizing butyrate and palm kernel oil as carbon and energy sources, recombinant C. necator H16 produced good amount of P (3HB-co-19 mol % 3HHx). Furthermore, the scientists demonstrated that the 3-HHx% in KNK005 phaA-deactivated mutant strains is boosted by the butyrate. This culture environment produces higher biomass (171 g/L) and HHx copolymer in the cells (81%) with PHA titers of 139 g/L [38]. Cai et al. (2009) produced a P. putida KTMQ01 recombinant. The recombinant’s DCW was 86% mcl-PHA [39]. Genetically altered P. putida KT2440 employs inexpensive substrates, such as xylose and octanoic acid, to produce mcl-PHA at a low cost. The cells accumulated mcl-PHA to a level of 20% [40]. A PHA-negative mutant of R. eutropha was created using the phaC gene from Aeromonas caviae, produced 3HB copolymer comprising 5 mol % 3HHx using soybean oil (20 g/L) in fed-batch mode [41]. DCW and % PHA were 133 g/L, 72.5% respectively. A. hydrophila 4AK4 produced P(3HB-co-3HHx), which contains 15% HHx from dodecanoate [42]. The co-expression of phaC with phaP and phaJ resulted in a 3HHx level of 34 mol % in the copolymer. When dodecanoate was used as the only carbon source, 54 g/L DCW and 52.7% PHA were observed [43]. The wild-type strain produced 40.4 g/L of DCW, and 54.6% of P(3HB-co-3HHx) [44]. This modified strain used plant oils successfully, produced 88.3 g/L dry biomass with a polymer content of 57% (3HB-co-3HHx) [45]. Tufail et al. investigated the use of leftover braising liquid as a substrate for PHA production using P. aeruginosa (KF270353) for 72 h. PHAs (53.2%) and DCW (23.7 g/L) were detected in the highest amounts in waste braising liquid [46]. Ruiz et al. (2019) produced 159.4 g/L of DCW, and 57 g/L of mcl-PHA using fatty acids generated from waste cooking oil as the carbon source, and P. putida KT2440 as biocatalyst [28].2.2.2. Carbohydrates
P(3HB-co-3HHx) with 14 mol % HHx units was produced with gluconate using recombinant A. hydrophila 4AK4 strain [47]. Poblete-Castro et al. (2014) produced PHA using gad (gluconate dehydrogenase) deleted mutant, P. putida KT2440 [48] under fed-batch mode. The DO-stat feeding method generated the 67% of mcl-PHA with 62 g/L DCW [46]. Fed-batch cultivation of P. putida KT2440 on mixed substrates of acrylic acid, nonanoic acid, and glucose (0.05: 1.25: 1) generated 71 g/L of DCW with 75% of PHA which contains the 89 mol % 3HN [29]. However, the concentration of 3HN was decreased to 65 mol % in the absence of acrylic acid. The amount of mcl-PHA produced increased 10-fold when dodecanoic acid was utilized as the feedstock by Zhao et al. (2020) [49]. Liu et al. (2019) reported that Pseudomonas-Saccharomyces produced up to 152.3 mg/L mcl-PHA using xylose as a carbon root [50]. The inclusion of S. cerevisiae in the consortium produced cell mass sedimentation. Sugarcane biorefinery derived sucrose hydrolysate and decanoic acid used in fed-batch cell cultivations produced 33% PHA in 53.4 g/L of DCW using P. putida KT2440 [51]. A β-oxidation metabolism in the R. eutropha mutant was studied for P(3HB-co-3-HHx) development from soybean oil [52]. The removal of fadB1 (enoyl-CoA hydratase/3HACoA dehydrogenase) in R. eutropha recombinant strains with additional genes generating (R)-enoyl-CoA hydratases resulted in a 6–21% 3-HHx content in the copolymer [52]. Figure 3 depicts how the metabolic pathway operates in the production of mcl-PHAs from sugars.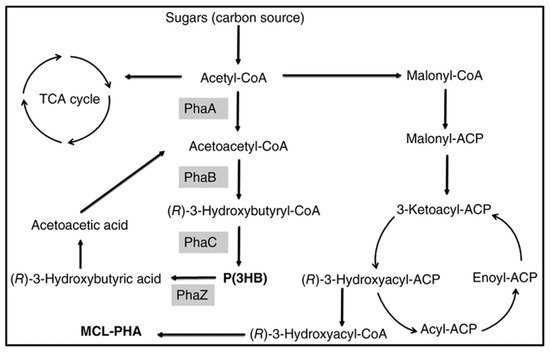
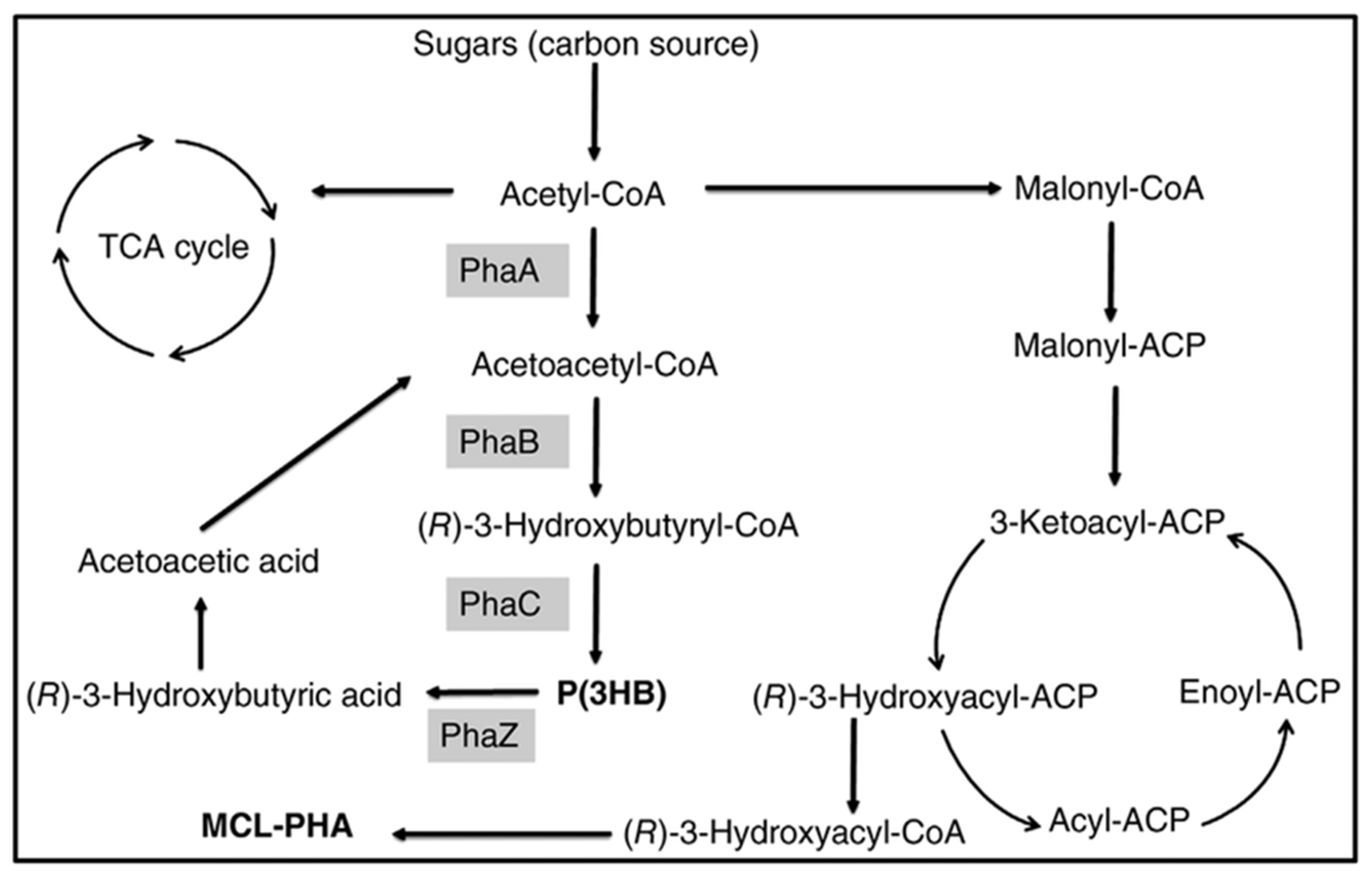 Figure 3. Metabolic pathway involved in the synthesis of PHAs from sugars. PhaA: β-ketothiolase; PhaB: β-ketoacyl-CoA reductase; PhaC: PHA synthase; PhaZ: PHA depolymerase.
Figure 3. Metabolic pathway involved in the synthesis of PHAs from sugars. PhaA: β-ketothiolase; PhaB: β-ketoacyl-CoA reductase; PhaC: PHA synthase; PhaZ: PHA depolymerase.2.2.3. Organic Residues/Wastes and Others
PHA production was carried out using organic acids/or sugar-based compounds derived from renewable waste materials [53]. Davis et al. (2013) produced mcl-PHA (25–34%) from perennial ryegrass biomass using P. fluorescens 555 and P. putida W619 [54]. Awasthi et al. (2021) reported about the mcl-PHA production using watermelon waste residues [55]. Muhr et al. (2013 a,b) produced mcl-PHA (22–30% DCW) from P. chlororaphis DSM 50083 and animal-derived waste [6,56][6][56]. Grape pulp was used to produce mcl-PHA from P. resinovorans in a 3.7 L bioreactor [57]. Chicken feathers were used to produce mcl-PHA with P. putida KT2440, the polymer consisted of 3-HHx (27.2 mol %) and 3-HHo (72.8 mol %) monomer units [58]. Apple pomace, the residue which is left out after processing of apple serves as a potential carbon source to produce PHAs [59]. Blanco et al. (2021) reviewed the PHA production using various substrates [60]. Apart from the above-mentioned carbon sources, there is a scope to produce mcl-PHAs from waste materials such as food waste, molasses, lingo-cellulosic biomass, cannery waste, biodiesel industry waste, paper-mill wastewater, coffee waste, and cheese whey [61]. As an example, production from cheese whey shown in Figure 4. PHAs were produced using synthetic substrates, waste materials, and toxic compounds [62,63,64,65,66,67,68,69,70,71,72][62][63][64][65][66][67][68][69][70][71][72].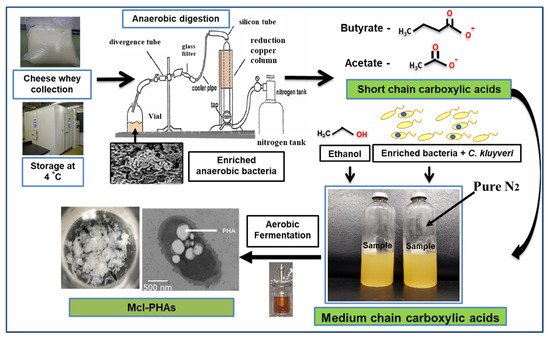
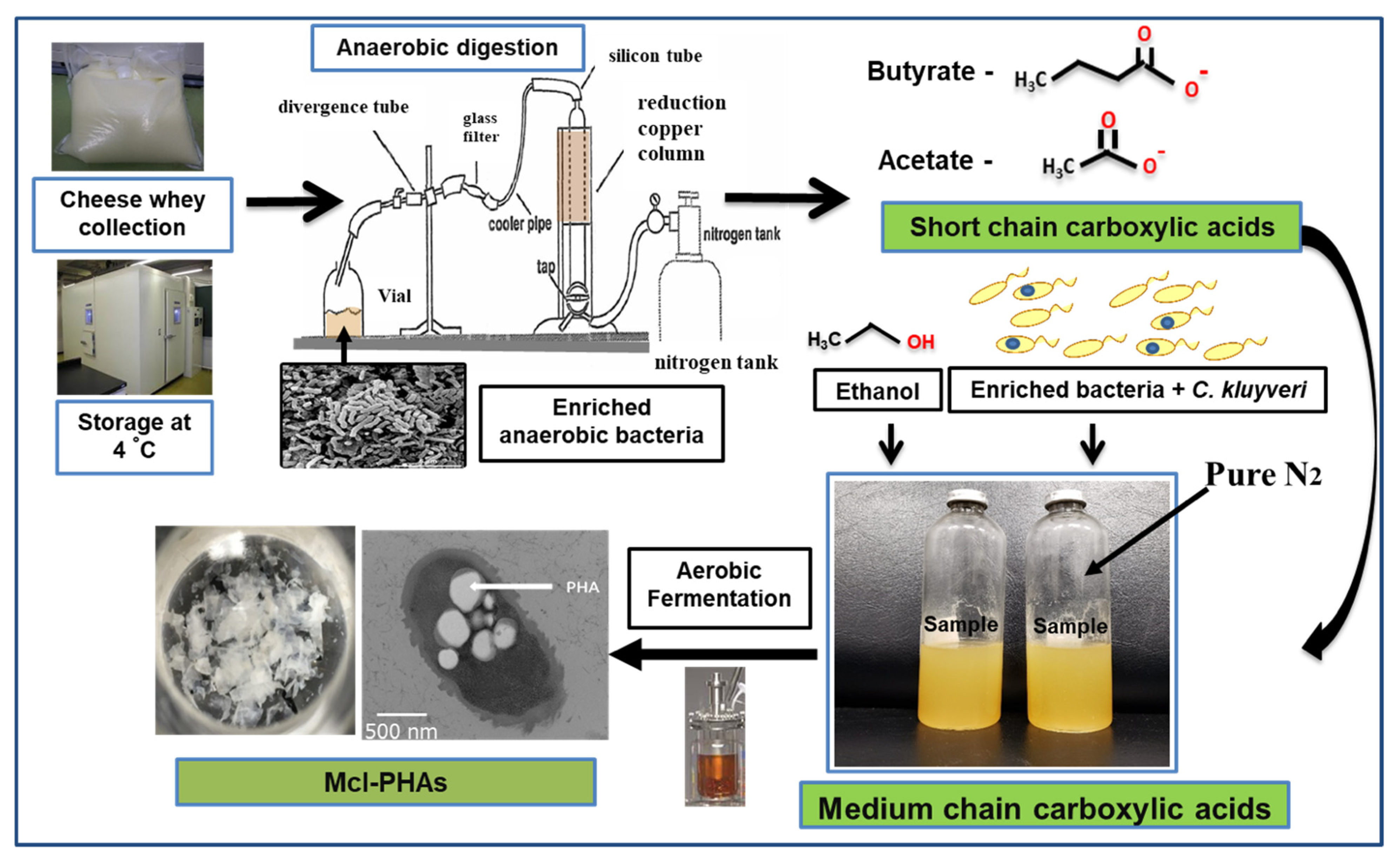
Figure 4. Hypothetical flow chart describing steps involved in mcl-PHA production from cheese whey.
Hypothetical flow chart describing steps involved in mcl-PHA production from cheese whey.
2.2.4. Vegetable Oils
Coconut oil containing both medium (C6–C14) and long (>C14) chain length fatty acids, lauric acid (C12:0) and myristic acid (C14:0) (up to 55%) are the main fatty acids [73]. Pseudomonas sp. is recognized to produce PHAs through fatty acids. Fatty acids are shortened by basically 2, 4, or 6 carbon atoms after each cycle of β-oxidation. In nature, Pseudomonas sp. is known for its adaptability, and it can make mcl-PHAs from a range of carbon feedstocks. PHAs (58% DCW) were produced in 20 L bioreactor in a batch mode by P. mendocina CH50 (NCIMB 10541) using coconut oil at a concentration of 20 g/L [74]. The optical density increased to 31 after 48 h, and the pH decreased slightly during fermentation, nitrogen content declined from 0.12 g/L to 0.015 g/L shows that the fermentation took place in a nitrogen-limiting environment. The mcl-PHAs terpolymer was composed of 3HO-3HD-3HDD. P(3HO-3HD-3HDD) has a molecular weight of 333 kDa and a PDI (polydispersity index) of 2.37, whereas mcl-PHAs with both unsaturated and saturated groups have an average molecular weight of 60–410 kDa [75]. Pseudomonas sp. Gl01 was used to produce mcl-PHA using palm oil and waste rapeseed oil. The purified polymers consisted of monomers ranging from C6 to C16 [76,77][76][77]. Song et al. (2008) used Pseudomonas sp. strain DR2 and the waste vegetable oil to produce mcl-PHA in the range of 24–38% [78]. Previous studies find out the importance of C/N balance for PHA production [79,80,81][79][80][81]. Carbon and nitrogen concentrations play a major role in PHA production. Mohan and Reddy (2013) applied design of experimental methodology using Taguchi orthogonal array to evaluate the influence and specific function of eight important factors and mentioned that glucose at 6 g/L and NH4Cl at 100 mg/L concentrations are best for higher PHA accumulation [80]. Reddy et al. (2015) reported that phaC gene expression was 5.37 folds higher at 100 mg/l nitrogen concentration than other concentrations [63]. The precursor molecules required for mcl-PHA synthesis in bacteria are generated through three different metabolic routes, which depend on the type of carbon source that existed in the medium. If carbohydrates are the main carbon source, the de novo fatty acid pathway is dominant, and fatty acids are the main carbon source, the β-oxidation pathway is dominant. The third pathway is chain elongation, and this pathway utilizes the precursors produced from both carbohydrates (acetyl-CoA) and fatty acids (acyl-CoA). All the three metabolic pathways generate different intermediate precursors—such as (R)-3-hydroxyacyl-acyl carrier protein, 2-trans-enoyl-CoA, (S)-3-hydroxyacyl-coA, and 3-ketoacyl-CoA—which are involved in the mcl-PHA synthesis. The hypothesis is that the (S)-3-hydroxyacyl-CoA, and 3-ketoacyl-CoA are subsequently converted to (R)-3-hydroxyacyl-CoA by the action of enzymes, 3-hydroxyacyl-coA epimerase, and 3-ketoacyl-ACP reductase respectively. The enzyme, (R)-specific enoyl-CoA hydratase (PhaJ)—which catalyzes the 2-trans-enoyl-CoA to (R)-3-hydroxyacyl-coA—plays a critical role in supplying monomer units from β-oxidation to PHA synthesis. (R)-3-hydroxyacyl-ACP-CoA transferase (PhaG), which has been identified in P. putida and P. aeruginosa plays, an important role in the metabolic connection of de novo fatty acid biosynthesis with mcl-PHA synthesis. PhaG catalyzes the conversion of (R)-3-hydroxyacyl-ACP to (R)-3-hydroxyacyl-CoA and contribute to mcl-PHA synthesis from gluconate or other carbohydrate sources. In the final step of mcl-PHA synthesis, the enzyme PHA synthase (PhaC) catalyzes the conversion of (R)-3-hydroxyacyl-CoA molecules into mcl-PHA [6,28,40,48,52,54,56][6][28][40][48][52][54][56].References
- Lemoigne, M. Dehydration and polymerization product of β-oxybutyric acid. Bull. Soc. Chim. Biol. 1926, 8, 770–782.
- Muhammadi, S.; Muhammad, A.; Shafqat, H. Bacterial polyhydroxyalkanoates-eco-friendly next generation plastic: Production, biocompatibility, biodegradation, physical properties and applications. Green Chem. Lett. Rev. 2015, 8, 56–77.
- Lee, S.Y.; Chang, H.N. Production of poly (hydroxy alkanoic acid). Adv. Biochem. Eng. Biotechnol. 1995, 52, 27–58.
- Choonut, A.; Prasertsan, P.; Klomklao, S.; Sangkharak, K. A Novel Green Process for Synthesis of 3-Hydroxyalkanoate Methyl Ester Using Lipase and Novel mcl-co-lcl PHA as Catalyst and Substrate. J. Polym. Environ. 2021, 30, 1423–1434.
- Rakkan, T.; Chana, N.; Chirapongsatonkul, N.; Sangkharak, K. Screening and identification of newly isolated basic red 9-degrading bacteria from textile wastewater and their ability to produce medium-co-long-chain-length polyhydroxyalkanoates. J. Polym. Environ. 2022, 30, 415–423.
- Muhr, A.; Rechberger, E.M.; Salerno, A.; Reiterer, A.; Schiller, M.; Kwicien, M.; Adamus, G.; Kowalczuk, M.; Strohmeier, K.; Schober, S.; et al. Biodegradable latexes from animal-derived waste: Biosynthesis and characterization of mcl-PHA accumulated by P. citronellolis. React. Funct. Polym. 2013, 73, 1391–1398.
- Nitschke, M.; Costa, S.G.V.A.O.; Contiero, J. Rhamnolipids and PHAs: Recent reports on Pseudomonas-derived molecules of increasing industrial interest. Process Biochem. 2011, 46, 621–630.
- Chen, G.-Q.; Jiang, X.-R. Engineering bacteria for enhanced polyhydroxyalkanoates (PHA) biosynthesis. Synth. Syst. Biotechnol. 2017, 2, 192–197.
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering 2017, 4, 55.
- Lee, S.Y.; Wong, H.H.; Choi, J.; Lee, S.H.; Lee, S.C.; Han, C.S. Production of Medium-Chain-Length Polyhydroxyalkanoates by High-Cell-Density Cultivation of Pseudomonas putida Under Phosphorus Limitation. Biotechnol. Bioeng. 2000, 68, 466–470.
- Riesenberg, D.; Guthke, R. High-cell-density cultivation of microorganisms. Appl. Microbiol. Biotechnol. 1999, 51, 422–430.
- Cavalheiro, J.M.B.T.; Raposo, S.R.; Almeida, M.C.M.D.; Sevrin, M.T.C.C.; Grandfils, C.; Fonseca, M.M.R. Effect of cultivation parameters on the production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) and poly (3-hydroxybutyrate-4-hydroxybutyrate-3-hydroxyvalerate) by Cupriavidus necator using waste glycerol. Bioresour. Technol. 2012, 111, 391–397.
- Davis, R.; Duane, G.; Kenny, S.T.; Cerrone, F.; Guzik, M.W.; Babu, R.P.; Casey, E.; O’Connor, K.E. High cell density cultivation of Pseudomonas putida KT2440 using glucose without the need for oxygen enriched air supply. Biotechnol. Bioeng. 2015, 112, 725–733.
- Ryu, H.W.; Hahn, S.K.; Chang, Y.K.; Chang, H.N. Production of poly(3-hydroxybutyrate) by high cell density fed-batch culture of Alcaligenes eutrophus with phosphate limitation. Biotechnol. Bioeng. 1997, 55, 25–32.
- Chen, G.-O.; Zhang, J. Microbial polyhydroxyalkanoates as medical implant biomaterials. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1–18.
- Williams, S.F.; Rizk, S.; Martin, D.P. Poly-4-hydroxybutyrate (P4HB): A new generation of resorbable medical devices for tissue repair and regeneration. Biomed. Eng. 2013, 58, 439–452.
- Skibiński, S.; Cichoń, E.; Haraźna, K.; Marcello, E.; Roy, I.; Witko, M.; Ślósarczyk, A.; Czechowska, J.; Guzik, M.; Zima, A. Functionalized tricalcium phosphate and poly (3-hydroxyoctanoate) derived composite scaffolds as platforms for the controlled release of diclofenac. Ceram. Int. 2021, 47, 3876–3883.
- Cichoń, E.; Haraźna, K.; Skibiński, S.; Witko, T.; Zima, A.; Ślósarczyk, A.; Zimowska, M.; Witko, M.; Leszczyński, B.; Wróbel, A.; et al. Novel bioresorbable tricalcium phosphate/polyhydroxyoctanoate (TCP/PHO) composites as scaffolds for bone tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2019, 98, 235–245.
- Zhang, J.; Cao, Q.; Li, S.; Lu, X.; Zhao, Y.; Guan, J.-S.; Chen, J.-C.; Wu, Q.; Chen, G.-Q. 3-Hydroxybutyrate methyl ester as a potential drug against Alzheimer’s disease via mitochondria protection mechanism. Biomaterials 2013, 34, 7552–7562.
- Xiao, X.-Q.; Zhao, Y.; Chen, G.-Q. The effect of 3-hydroxybutyrate and its derivatives on the growth of glial cells. Biomaterials 2007, 28, 3608–3616.
- Heinrich, D.; Raberg, M.; Fricke, P.; Kenny, S.T.; Gamez, L.M.; Babu, R.P.; O’Connor, K.; Steinbüchel, A. Synthesis gas (syngas)-derived medium-chain-length polyhydroxyalkanoate synthesis in engineered Rhodospirillum Rubrum. Appl. Environ. Microbiol. 2016, 82, 6132–6140.
- Jaeger, K.E.; Steinbüchel, A.; Jendrossek, D. Substrate specificities of bacterial polyhydroxyalkanoate depolymerases and lipases: Bacterial lipases hydrolyze poly(ω-hydroxyalkanoates). Appl. Environ. Microbiol. 1995, 61, 3113–3118.
- Volova, T.G.; Kiselev, E.G.; Shishatskaya, E.I.; Zhila, N.O.; Boyandin, A.N.; Syrvacheva, D.A.; Vinogradova, O.N.; Kalacheva, G.S.; Vasiliev, A.D.; Peterson, I.V. Cell growth and accumulation of polyhydroxyalkanoates from CO2 and H2 of a hydrogen-oxidizing bacterium, Cupriavidus eutrophus B-10646. Bioresour. Technol. 2013, 146, 215–222.
- Volova, T.G.; Shishatskaya, E.I.; Zhila, N.O.; Shishatskii, O.N.; Kiselev, Y.G.; Mironov, P.V.; Vasiliev, A.D.; Peterson, I.V.; Sinskey, A.J. Fundamental basis of production and application of biodegradable polyhydroxyalkanoates. J. Sib. Fed. Univ. Biol. 2012, 3, 280–299.
- Tanaka, K.; Yoshida, K.; Orita, I.; Fukui, T. Biosynthesis of Poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) from CO2 by a recombinant Cupriavidusnecator. Bioengineering 2021, 8, 179.
- Löwe, H.; Hobmeier, K.; Moos, M.; Kremling, A.; Pflüger-Grau, K. Photoautotrophic production of polyhydroxyalkanoates in a synthetic mixed culture of Synechococcus elongatus cscB and Pseudomonas putida cscAB. Biotechnol. Biofuels 2017, 10, 1–14.
- Riedel, S.L.; Bader, J.; Brigham, C.J.; Budde, C.F.; Yusof, Z.A.; Rha, C.; Sinskey, A.J. Production of poly (3-hydroxybutyrate- co-3-hydroxyhexanoate) by Ralstonia eutropha in high cell density palm oil fermentations. Biotechnol. Bioeng. 2012, 109, 74–83.
- Ruiz, C.; Kenny, S.T.; Babu, P.R.; Walsh, M.; Narancic, T.; O’Connor, K.E. High cell density conversion of hydrolyzed waste cooking oil fatty acids into medium chain length polyhydroxyalkanoate using Pseudomonas putida KT2440. Catalysts 2019, 9, 468.
- Jiang, X.J.; Sun, Z.; Ramsay, J.A.; Ramsay, B.A. Fed-batch production of MCL-PHA with elevated 3-hydroxynonanoate content. AMB Express 2013, 50, 1–18.
- Blunt, W.; Dartiailh, C.; Sparling, R.; Gapes, D.J.; Levin, D.B.; Cicek, N. Development of high cell density cultivation strategies for improved medium chain length polyhydroxyalkanoate productivity using Pseudomonas putida LS46. Bioengineering 2019, 6, 89.
- Sun, Z.; Ramsay, J.A.; Guay, M.; Ramsay, B.A. Enhanced yield of medium-chain-length polyhydroxyalkanoates from nonanoic acid by co-feeding glucose in carbon-limited, fed-batch culture. J. Biotechnol. 2009, 143, 262–267.
- Diniz, S.C.; Taciro, M.K.; Gomez, J.G.C.; Pradella, J.G. High-cell-density cultivation of Pseudomonas putida IPT 046 and medium-chain-length polyhydroxyalkanoate production from sugarcane carbohydrates. Appl. Biochem. Biotech. 2004, 119, 1–69.
- Kellerhals, M.B.; Hazenberg, W.; Witholt, B. High cell density fermentations of Pseudomonas oleovorans for the production of mcl-PHAs in two-liquid phase media. Enz. Microbiol. Technol. 1999, 24, 111–116.
- Kellerhals, M.B.; Kessler, B.; Witholt, B.; Tchouboukov, A.; Brand, H. Renewable long-chain fatty acids for production of biodegradable medium-chain-length polyhydroxyalkanoates (mcl-PHAs) at laboratory and pilot plant scales. Macromolecules 2000, 33, 4690–4698.
- Preusting, H.; Hazenberg, W.; Witholt, B. Continuous production of poly(3-hydroxyalkanoates) by Pseudomonas oleovorans in a high-ceil-density, two-liquid-phase chemostat. Enzym. Microb. Technol. 1993, 15, 311–316.
- Preusting, H.; Houten, R.; Hoefs, A.; Langenberghe, E.K.; Favre-Bulle, O.; Witholt, B. High cell density cultivation of Pseudomonas oleovorans: Growth and production of poly (3-hydroxyalkanoates) in two-liquid phase batch and fed-batch systems. Biotechnol. Bioeng. 1993, 41, 550–556.
- Riedel, S.L.; Jahns, S.; Koenig, S.; Bock, M.C.; Brigham, C.J.; Bader, J.; Stahl, U. Polyhydroxyalkanoates production with Ralstonia eutropha from low quality waste animal fats. J. Biotechnol. 2015, 214, 119–127.
- Sato, S.; Maruyama, H.; Fujiki, T.; Matsumoto, K. Regulation of 3-hydroxyhexanoate composition in PHBH synthesized by recombinant Cupriavidus necator H16 from plant oil by using butyrate as a co-substrate. J. Biosci. Bioeng. 2015, 120, 246–251.
- Cai, L.; Yuan, M.Q.; Liu, F.; Jian, J.; Chen, G.Q. Enhanced production of medium-chain-length polyhydroxyalkanoates (PHA) by PHA depolymerase knockout mutant of Pseudomonas putida KT2442. Bioresour. Technol. 2009, 100, 2265–2270.
- LeMeur, S.; Zinn, M.; Egli, T.; Thöny-Meyer, L.; Ren, Q. Production of medium-chain-length polyhydroxyalkanoates by sequential feeding of xylose and octanoic acid in engineered Pseudomonas putida KT2440. BMC Biotechnol. 2012, 12, 53.
- Kahar, P.; Tsuge, T.; Taguchi, K.; Doi, Y. High yield production of polyhydroxyalkanoates from soybean oil by Ralstonia eutropha and its recombinant strain. Polym. Degrad. Stab. 2004, 83, 79–86.
- Jing, H.; Yuan-Zheng, Q.; Dai-Cheng, L.; Guo-Qiang, C. Engineered Aeromonas hydrophila for enhanced production of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) with alterable monomers composition. FEMS Microbiol. Lett. 2004, 239, 195–201.
- Ouyang, S.; Han, J.; Qiu, Y.; Qin, L.; Chen, S.; Wu, Q.; Leski, M.L.; Chen, G. Poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) production in recombinant Aeromonas hydrophila 4ak4 harboring phba, phbb and vgb genes. Macromol. Symp. 2005, 224, 21–34.
- Budde, C.F.; Riedel, S.L.; Willis, L.B.; Rha, C.; Sinskey, A.J. Production of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) from plant oil by engineered Ralstonia eutropha strains. Appl. Environ. Microbiol. 2011, 77, 2847–2854.
- Thinagaran, L.; Sudesh, K. Evaluation of sludge palm oil as feedstock and development to efficient method for its utilization to produce polyhydroxyalkanoate. Waste Biomass Valoriz. 2017, 10, 709–720.
- Tufail, S.; Munir, S.; Jamil, N. Variation analysis of bacterial polyhydroxyalkanoates production using saturated and unsaturated hydrocarbons. Braz. J. Microbiol. 2017, 48, 629–636.
- Qiu, Y.Z.; Han, J.; Guo, J.J.; Chen, G.Q. Production of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) from gluconate and glucose by recombinant Aeromonas hydrophila and Pseudomonas putida. Biotechnol. Lett. 2005, 27, 1381–1386.
- Poblete-Castro, I.; Rodriguez, A.L.; Lam, C.M.; Kessler, W. Improved production of medium-chain-length polyhydroxyalkanoates in glucose-based fed-batch cultivations of metabolically engineered Pseudomonas putida strains. J. Microbiol. Biotechnol. 2014, 24, 59–69.
- Zhao, F.; He, F.; Liu, X.; Shi, J.; Liang, J.; Wang, S.; Yang, C.; Liu, R. Metabolic engineering of Pseudomonas mendocina NK-01 for enhanced production of medium-chain-length polyhydroxyalkanoates with enriched content of the dominant monomer. Int. J. Biol. Macromol. 2020, 154, 1596–1605.
- Liu, C.; Qi, L.; Yang, S.; He, Y.; Jia, X. Increased sedimentation of a Pseudomonas–Saccharomyces microbial consortium producing medium chain length polyhydroxyalkanoates. Chin. J. Chem. Eng. 2019, 27, 1659–1665.
- Oliveira, G.H.D.; Zaiat, M.; Rodrigues, J.A.D.; Ramsay, J.A.; Ramsay, B.A. Towards the production of mcl-pha with enriched dominant monomer content: Process development for the sugarcane biorefinery context. J. Polym. Environ. 2020, 28, 844–853.
- Insomphun, C.; Mifune, J.; Orita, I.; Numata, K.; Nakamura, S.; Fukui, T. Modification of β-oxidation pathway in Ralstonia eutropha for production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from soybean oil. J. Biosci. Bioeng. 2014, 117, 184–190.
- Serafim, L.S.; Lemos, P.C.; Albuquerque, M.G.; Reis, M.A. Strategies for PHA production by mixed cultures and renewable waste materials. Appl. Microbiol. Biotechnol. 2008, 81, 615–628.
- Davis, R.; Kataria, R.; Cerrone, F.; Woods, T.; Kenny, S.; O’Donovan, A.; Guzik, M.; Shaikh, H.; Duane, G.; Gupta, V.K.; et al. Conversion of grass biomass into fermentable sugars and its utilization for medium chain length polyhydroxyalkanoate (mcl-PHA) production by Pseudomonas strains. Bioresour. Technol. 2013, 150, 202–209.
- Awasthi, M.K.; Kumar, V.; Yadav, V.; Sarsaiya, S.; Awasthi, S.K.; Sindhu, R.; Zhang, Z. Current state of the art biotechnological strategies for conversion of watermelon wastes residues to biopolymers production: A review. Chemosphere 2022, 290, 133310.
- Muhr, A.; Rechberger, E.M.; Salerno, A.; Reiterer, A.; Malli, K.; Strohmeier, K.; Koller, M. Novel description of mcl-PHA biosynthesis by Pseudomonas chlororaphis from animal-derived waste. J. Biotechnol. 2013, 165, 45–51.
- Andler, R.; Valdés, C.; Urtuvia, V.; Andreeßen, C.; Díaz-Barrera, A. Fruit residues as a sustainable feedstock for the production of bacterial polyhydroxyalkanoates. J. Clean. Prod. 2021, 307, 127236.
- Pernicova, I.; Enev, V.; Marova, I.; Obruca, S. Interconnection of waste chicken feather biodegradation and keratinase and mcl-PHA production employing Pseudomonas putida KT2440. Appl. Food Biotechnol. 2019, 6, 83–90.
- Liu, H.; Kumar, V.; Jia, L.; Sarsaiya, S.; Kumar, D.; Juneja, A.; Awasthi, M.K. Biopolymer poly-hydroxyalkanoates (PHA) production from apple industrial waste residues: A review. Chemosphere 2021, 284, 131427.
- Blanco, F.G.; Hernández, N.; Rivero-Buceta, V.; Maestro, B.; Sanz, J.M.; Mato, A.; Prieto, M.A. From residues to added-value bacterial biopolymers as nanomaterials for biomedical applications. Nanomaterials 2021, 11, 1492.
- Yadav, B.; Pandey, A.; Kumar, L.R.; Tyagi, R.D. Bioconversion of waste (water)/residues to bioplastics-A circular bioeconomy approach. Bioresour. Technol. 2020, 298, 122584.
- Chang, Y.C.; Venkateswar Reddy, M.; Imura, K.; Onodera, R.; Kamada, N.; Sano, Y. Two-Stage polyhydroxyalkanoates (PHA) production from cheese whey using Acetobacter pasteurianus C1 and Bacillus sp. CYR1. Bioengineering 2021, 8, 157.
- Chang, Y.C.; Venkateswar Reddy, M.; Choi, D.B. Cometabolic degradation of toxic trichloroethene or cis-1,2-dichloroethene with phenol and production of poly-β-hydroxybutyrate (PHB). Green Chem. 2021, 23, 2729–2737.
- Reddy, M.V.; Watanabe, A.; Onodera, R.; Mawatari, Y.; Tsukiori, Y.; Watanabe, A.; Kudou, M.; Chang, Y.C. Polyhydroxyalkanoates (PHA) production using single or mixture of fatty acids with Bacillus sp. CYR1: Identification of PHA synthesis genes. Bioresour. Technol. Rep. 2020, 11, 100483–100491.
- Reddy, M.V.; Mawatari, S.; Onodera, R.; Nakamura, Y.; Yajima, Y.; Chang, Y.C. Bacterial conversion of waste into polyhydroxybutyrate (PHB): A new approach of bio-circular economy for treating waste and energy generation. Bioresour. Technol. Rep. 2019, 7, 100246–100254.
- Reddy, M.V.; Mawatari, Y.; Onodera, R.; Nakamura, Y.; Yajima, Y.; Chang, Y.C. Polyhydroxyalkanoates (PHA) production from synthetic waste using Pseudomonas pseudoflava: PHA synthase enzyme activity analysis from P. pseudoflava and P. palleronii. Bioresour. Technol. 2017, 234, 99–105.
- Reddy, M.V.; Mawatari, Y.; Yajima, Y.; Satoh, K.; Venkata Mohan, S.; Chang, Y.C. Production of poly-3-hydroxybutyrate (P3HB) and poly-3-(hydroxybutyrate-co-hydroxyvalerate) P(3HB-co-3HV) from synthetic wastewater using Hydrogenophaga palleronii. Bioresour. Technol. 2016, 215, 155–162.
- Amulya, K.; Venkateswar Reddy, M.; Rohit, M.V.; Mohan, S.V. Wastewater as renewable feedstock for polyhydroxyalkanoates (PHA) production: Understanding the role of reactor microenvironment and system pH. J. Clean. Prod. 2016, 112, 4618–4627.
- Amulya, K.; Venkateswar Reddy, M.; Mohan, S.V. Acidogenic spent wash valorization through polyhydroxyalkanoate (PHA) synthesis coupled with fermentative biohydrogen production. Bioresour. Technol. 2014, 158, 336–342.
- Reddy, M.V.; Mohan, S.V. Influence of aerobic and anoxic microenvironments on polyhydroxyalkanoates (PHA) production from food waste and acidogenic effluents using aerobic consortia. Bioresour. Technol. 2012, 103, 313–321.
- Srikanth, S.; Venkateswar Reddy, M.; Mohan, S.V. Microaerophilic microenvironment at biocathode enhances electrogenesis with simultaneous synthesis of polyhydroxyalkanoates (PHA) in bioelectrochemical system (BES). Bioresour. Technol. 2012, 125, 291–299.
- Mohan, S.V.; Venkateswar Reddy, M.; Subhash, G.V.; Sarma, P.N. Fermentative effluents from hydrogen producing bioreactor as substrate for poly (β-OH) butyrate production with simultaneous treatment: An integrated approach. Bioresour. Technol. 2010, 101, 9382–9386.
- Appaiah, P.; Sunil, L.; Prasanth Kumar, P.K.; Gopala Krishna, A.G. Composition of Coconut Testa, Coconut Kernel and its Oil. J. Am. Oil Chem. Soc. 2014, 91, 917–924.
- Basnett, P.; Marcello, E.; Lukasiewicz, B.; Panchal, B.; Nigmatullin, B.; Knowles, J.C.; Roy, I. Biosynthesis and characterization of a novel, biocompatible medium chain length polyhydroxyalkanoate by Pseudomonas mendocina CH50 using coconut oil as the carbon source. J. Mater. Sci. Mater. Med. 2018, 29, 1–11.
- Valappil, S.P.; Misra, S.K.; Boccaccini, A.R.; Roy, I. Biomedical applications of polyhydroxyalkanoates (PHAs), an overview of animal testing and in vivo responses. Expert Rev. Med. Devices 2006, 3, 853–868.
- Możejko, J.; Ciesielski, S. Saponified waste palm oil as an attractive renewable resource for mcl-polyhydroxyalkanoate synthesis. J. Biosci. Bioeng. 2013, 116, 485–492.
- Mozejko, J.; Wilke, A.; Przybylek, G.; Ciesielski, S. Mcl-PHAs produced by Pseudomonas sp. Gl01 using fed-batch cultivation with waste rapeseed oil as carbon source. J. Microbiol. Biotechnol. 2012, 22, 371–377.
- Song, J.H.; Jeon, C.O.; Choi, M.H.; Yoon, S.C.; Park, W.J. Polyhydroxyalkanoate (PHA) production using waste vegetable oil by Pseudomonas sp. strain DR2. J. Microbiol. Biotechnol. 2008, 18, 1408–1415.
- Reddy, M.V.; Yajima, Y.; Mawatari, Y.; Hoshino, T.; Chang, Y.C. Degradation and conversion of toxic compounds into useful bioplastics by Cupriavidus sp. CY-1: Relative expression of PhaC gene under phenol and nitrogen stress. Green Chem. 2015, 17, 4560–4569.
- Mohan, S.V.; Venkateswar Reddy, M. Optimization of critical factors to enhance polyhydroxyalkanoates (PHA) synthesis by mixed culture using Taguchi design of experimental methodology. Bioresour. Technol. 2013, 128, 409–416.
- Reddy, M.V.; Mohan, S.V. Effect of substrate load and nutrients concentration on the Polyhydroxyalkanoates (PHA) production using mixed consortia through wastewater treatment. Bioresour. Technol. 2012, 114, 573–582.
More
