Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Jessie Wu and Version 2 by Jessie Wu.
Optical sensors play an increasingly important role in the development of medical diagnostic devices. They can be very widely used to measure the physiology of the human body. Optical methods include PPG, radiation, biochemical, and optical fiber sensors. Optical sensors offer excellent metrological properties, immunity to electromagnetic interference, electrical safety, simple miniaturization, the ability to capture volumes of nanometers, and non-invasive examination. In addition, they are cheap and resistant to water and corrosion. The use of optical sensors can bring better methods of continuous diagnostics in the comfort of the home and the development of telemedicine in the 21st century.
- wearable
- optical sensors
- medicine
1. Introduction
The development of mankind reflects the efforts of doctors, scientists, and others to maintain and strengthen health, to implement social measures and prevent disease. New and improving diagnostic methods for real-time and long-term health monitoring are constantly being established. Early accurate diagnosis is the key to maintaining a high quality of life [1][2]. Older methodologies based on invasive sampling with the use of heavy equipment are nowadays being transformed into simple scanning methods that do not require demanding manipulation and also make people feel more comfortable [3]. With the advancement of technology, miniaturization, the development of advanced materials, and the advent of the internet, wearable electronics are gaining prominence [2]. As the healthcare regime moves more toward personalized medicine, the wearable medical market is projected to grow by around 26.4% worldwide to $195.57 milliards between 2020 and 2027 [4]. The arrival of intelligent and wirelessly connected wearable monitoring devices brings a revolution in healthcare. The trend began with simple fitness straps and developed rapidly in the form of variable advanced health accessories such as watches, smart clothing, glasses, contact lenses, rings, and various body extensions and inserts [5][6]. These devices can closely monitor life functions, human health, and report long-term a change in the patient’s health indicators. Ideal wearable sensors must be non-invasive, compact, easily portable, easy to manufacture, and low cost [7][8][9]. However, human health monitoring with wearable electronics has its pitfalls, and the sensory principles often differ significantly from conventional laboratory measurements. There are hundreds of these sensory principles and they have been described in many publications [8][9][10], but on many occasions they do not consider their use in real life. Researchers decided to focus more deeply only on a narrow group of promising sensors, specifically those that use optical phenomena for detection. Researchers deal with basic physiological parameters and quantities measurable using optical wearable electronics, and their relationship to human health.
1.1. Advantages of Optical Measurement
Optical sensors are, in principle, detectors that capture the physical amount of light or its variations. Researchers focus on optical sensors that enable continuous and highly sensitive measurement of parameters about our health and the environment for medical diagnostics and physiological health assessment [11]. Such progressive sensors are manufactured applying fundamental optical technologies such as photoplethysmography (PPG), optical fibers with Bragg gratings (FBG), interferometers often woven in smart textiles, various radiation sensors, plasmonic and fluorometric sensors, and colorimetry, as well as the development of prospective new materials and organic components (Figure 1).
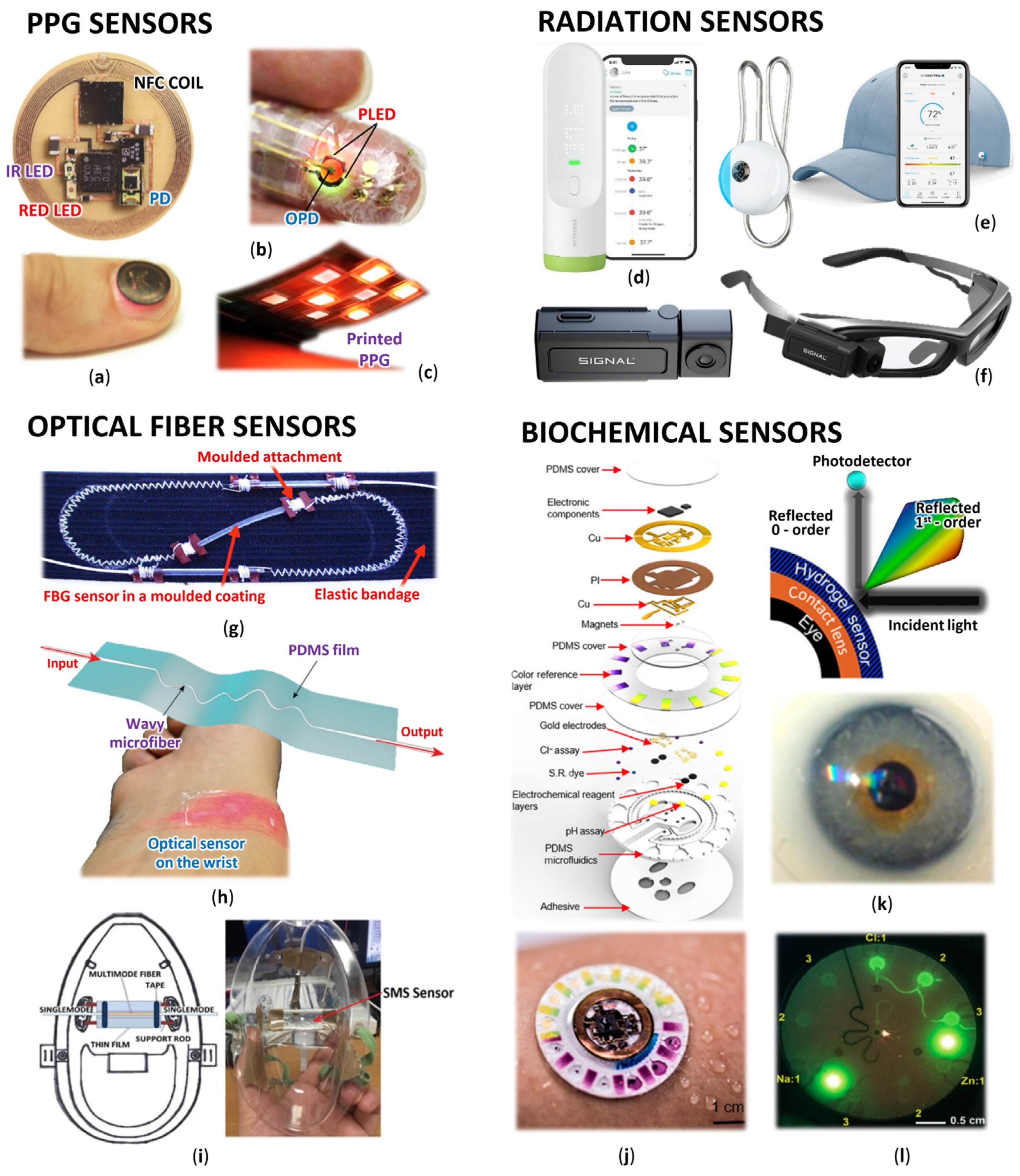
Figure 1. Wearable optical sensors for measuring of human physiology: (a) Miniaturized battery-free NFC enabled wireless systems for wearable pulse oximetry (unencapsulated device and device during operation mounted on a thumbnail) [12]; (b) Ultra-flexible organic PPG sensor attached to finger (smart e-skin system) [13]; (c) Printed reflectance oximeter array composed of four red and IR OLEDs and eight OPDs placed on the forearm for 2D oxygenation mapping [14]; (d) Contactless infrared medical grade thermometer composed from 16 IR sensors for forehead measurement with smartphone connectivity [15]; (e) Battery-free skin UV exposure tracker in form of fashion clip button with smartphone connectivity [16]; (f) Smart glasses with thermal camera for precise temperature measurement and scanning ideal for medical, industrial, and environmental use [17]; (g) Respiration belt with embedded silica fiber optical sensor for thoracic movement analysis [18]; (h) Self-assembled wavy optical microfiber for stretchable wearable sensor (schematic diagram and sensor stuck on the wrist) for monitoring of radial artery pulse wave [19]; (i) Optical fiber interferometer based breathing sensor built into oxygen mask [20]; (j) Skin-interfaced microfluidic battery-free systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat [21]; (k) Contact lens integrated glucose monitoring using smartphones [22]; (l) Skin-wearable fluorometric microfluidic device (emitted by blue light) for measuring of Cl, Na and Zn sweat concentrations [23].
Optical sensors are currently gaining great recognition and are becoming an increasing alternative to traditional electrical or mechanical sensors. Overall, they offer unique advantages in recording human health compared to electrical sensors. They propose excellent metrological properties such as low zero and low sensitivity drift, good accuracy, sensitivity, and large usable bandwidth [24]. They are immune to electromagnetic interference, electrically safe, can achieve outstanding miniaturization, and they are capable of capturing nanoscale volumes, allowing the non-invasive examination of biological matter with relatively large penetration depths. Sensing elements, whether in the form of optical fibers or optically transparent encapsulated photoplethysmographic and biochemical sensors, are often inexpensive, water- and corrosion-resistant [25]. As microelectronic technologies are driven by requirements for wearable devices towards higher sensitivity, faster response, better robustness, and higher integration, they may ultimately reach their limits, which are inherent in the very nature of low-frequency electromagnetic fields [26][27][28][29][30][31]. The response time is limited by parasitic effects and in high-density electronic circuits by signal crosstalk. These limitations can be avoided by using photons as a signal carrier [32]. In many cases, the optical sensors do not even have to be in direct contact with the human body or do not require a high quality of contact. Other significant advantages include the possibility of implementing distributed sensors, which allow quantities not only to be read but also to be transmitted directly. Due to these features, optical sensors become a great and advanced solution for monitoring physiological parameters with wearable devices and for medicine in general. Today, about 15% of the market for wearable devices is based on optical sensors and this number is constantly growing [24]. Another not negligible benefit is the great progress of flexible technologies in the field of optical sensors, thus the newly discovered highly flexible and soft optical sensors are expected to provide a reliable and safe alternative for the next generation of intelligent wearable medical devices. Organic semiconductor devices have many attractive properties. These include simple production on flexible substrates, the possibility of miniaturization, the simultaneous ability to generate and detect an optical signal, and tunable light emission in a wide range of values [33][34][35]. The potential of optical sensors in medicine has even begun to be explored. They serve in this field not only as sensors but also as manipulators of biological activity, for example, biosensors such as lab-on-chip spectrometers [36][37], plasmonic spectrometers [38], and flexible e-skin [39][40][41].
1.2. Health and Optical Wearables
Optical sensors in medicine have a broad spectrum of capabilities. They can be used to increase the intelligence of medical equipment, implants, and to monitor human physiology remotely even without direct contact with the patient. Monitoring the processes in the human body allows easier detection of vital signs such as heart rate (HR), respiratory rate (RR), blood pressure, etc., allowing early rapid diagnosis and prevention to be performed, and generally helping people to monitor their physiological parameters and inform the doctor in case of change.
HR measurement is a common method for determining the physical activity and condition of the body. It can predict cardiovascular morbidity and mortality in a very reliable and easily accessible way. HR reflects the overall activity of the autonomic nervous system and provides a suitable indicator of a person’s condition and mood. HR variability (HRV) is derived from HR and is a time variance between heartbeats. It is a good sign of physical fitness. When the HRV is high, the nervous system is balanced, and the body can adapt to the environment and function well. Low HRV indicates that the body is working hard, it is tired, dehydrated, stressed, or sick [42][43]. As the HR arises as a wave in the blood vessel walls caused by tensioning and accelerating blood flow, it then spreads from there through other arteries throughout the body. These changes can be easily and reliably detected by optical sensors, mostly PPG or optical fiber.
Respiration monitoring is also a crucial physiologic parameter in inpatient examination. Breathing, along with pulse, blood pressure, and body temperature, is one of the vital signs. Healthy, normal breathing is regular, evenly deep, soundless, and odorless. The respiratory impulse increases with decreasing oxygen content, increasing carbon dioxide content, and decreasing pH. Deviations may indicate certain diseases, such as anxiety and potential hypoxia. Respiratory disorders occur not only in respiratory diseases but also in cardiovascular diseases and metabolic disorders. RR monitoring is important in detecting symptoms of sleep apnea, chronic obstructive pulmonary disease, asthma, or children’s pulmonary diseases [44]. RR can be measured using various devices and physiological principles such as spirometry, capnometry, impedance pneumography, acceleration sensors, etc. Today, a very progressive method is the algorithms’ extraction from the captured photoplethysmography signal.
Blood pressure (BP) demonstrates the pressure exerted by the blood on the arterial wall, which provides information on blood flow during heart contraction (systole) and relaxation (diastole), and may also indicate cellular oxygen supply. Its value is affected by cardiac output, blood viscosity, vascular elasticity, and resistance. Hypertension is a modern epidemic and is the most important risk factor for cardiovascular disease, leading to an increase in overall mortality. BP is traditionally measured using inflatable pressure cuffs, but this is completely impractical in wearable electronics. Thus, great efforts are made to measure BP based on the pulse wave transition time (PTT), whether from the shape of the PPG curve or the time shift between ECG and PPG in the periphery [44].
Body temperature (BT) is essential for maintaining all vital functions and metabolic processes. In humans, BT is usually constant, but various external and internal influences, especially inflammation, can affect it. From a medical point of view, the measurement of BT is very important, because many diseases are accompanied by characteristic changes. Different values of the temperature control center are related to the immune response of the organism defending itself against the progression of the disease. BT is divided into body core and peripheral temperature, where the peripheral temperature is more variable. The temperature is affected by blood circulation, HR, stress, metabolism, and external microclimatic factors [44]. Among the innovative methods of measuring BT is the use of radiation sensors or optical fibers.
Human fluids also offer an important source of information about the human body. Non-invasive and continuous measurement of biomarkers such as sodium, chlorine, potassium, lactate, calcium, glucose, ammonia, ethanol, urea, cortisol, and various neuropeptides and cytokinesis is possible from sweat or saliva. For example, excessive drinking leads to hyponatremia (low serum sodium) and conversely, hypohydration leads to a higher risk of disease (e.g., dementia) and body failure (e.g., sunburn). Proper hydration is important and therefore measurements and warnings of non-compliance can help prevent the associated problems [45]. Lack of drinking can be easily detected from urine or blood, but collection cannot be performed while moving, but this measurement problem can be solved with the help of optical sensors together with a set of sensors that allow to measure the indirect method of hydration [46]. Various biochemical optical sensors are most useful in this area.
The incidence of diabetes mellitus is growing to epidemic proportions in today’s developed world. Continuous monitoring of blood glucose levels is important for people with this disease. In this case, the sensors offer a non-invasive method of continuous measurement, unlike current glucometers, which require blood collection from a finger. The accuracy of home glucometers also varies ±15%. The development of optic sensors today focuses on the rapid, continuous and simple determination of glucose in surrounding body fluids, for example, the use of a photonic crystal that responds to glucose levels has been described, the sensor bends light, and the diffracted band shifts as the glucose concentration changes [47].
In today’s modern age, the total physical activity of the population is falling below the recommended levels, so the incidence of population-related diseases such as obesity and diabetes is increasing. Monitoring of childhood obesity is known, where accelerometers offer objective measurement of normal activity independently of self-report [48][49], or the monitoring of seniors in in-home care, where the system records their activities, events, and potentially important health symptoms [50][51]. Nowadays, sensors based on optical sensors are coming to the forefront that help to better monitor patients, which helps to improve the quality of life.
2. Photoplethysmography
In healthcare, one of the most widely used optical imaging methods is photoplethysmography (PPG) (Table 1), which is used to monitor blood flow in real-time. It is used to determine the physiological parameters such as oxygen saturation, blood pressure, cardiac output, respiration but also to evaluate autonomic function, depth of anesthesia, as well as detection of peripheral vascular disease [52]. In essence, this method can measure changes in volume throughout the body. It measures the amount of light that is absorbed or reflected by blood vessels in living tissue. As blood flows, a cardiovascular pulse wave emanates from the heart, propagating through the body and periodically dilating the arteries and arterioles in the subcutaneous tissue. The PPG signal is a mixture of blood flow in the veins and arteries and generally consists of a pulsating and non-pulsating component of the blood volume [53]. The pulsating component is related to changes in arterial blood volume and is synchronous with the heartbeat, while the non-pulsating component is a function of basal blood volume, respiration, sympathetic nervous system activity, and thermoregulation [54]. Light in the spectrum from the visible to the near-infrared (NIR) region can only reach human tissues to a depth of a few millimeters, and its penetration is limited due to the absorption of light by blood, melanin, fat, water, and light scattering. The actual interaction of light with the tissue depends not only on the composition of the tissue but also on the wavelength. These differences allow the detection of different information from the PPG signal. For example, green light is immediately absorbed by the body, so it is only suitable for measuring in places where a lot of blood is in the tissue, but it is less affected by ambient light interference. On the contrary, red light and NIR penetrate deeper into the human body and thus provide a wider range of information about the physiological signal [55][56]. This phenomenon is also related to the composition of the skin and evolutionary development, where the epidermis performs a protective function of the underlying soft tissue against harmful UV radiation. Skin pigmentation absorbs shorter wavelengths, UV, and to some degree also visible light. Water in tissues, on the contrary, absorbs in areas with long wavelengths. In the literature is also mentioned an “optical window” for wavelengths of 600 to 1300 nm [57][58][59].
Table 1. Photoplethysmography sensors.
| Sensor type | Application | Sensing Element | Key Parameters | Ref. |
|---|---|---|---|---|
| Wrist-worn reflectance LED |
Motion artifact reduction | PP: Four x OSRAM SFH7050, Motion Sensor: InvenSense MPU9250 | Wavelengths 530, 660, 940 nm | [60] |
| Chest reflectance LED PPG sensor | HR, BP from PPG and PCG | PPG: Osram SFH 7060, PCG 1 NXP Semiconductors MPXV7002 | Wavelengths 3 × 530, 660, 950 nm | [56] |
| Body-worn reflectance LED PPG | Skin and muscle perfusion | Eight x LED, Three x PD line/circle configuration |
Wavelengths 560, 880 nm | [61] |
| PPG hands and legs measurement | PPG and ECG system for cardiovascular | Seven PPG probes: STMicroelectronics SiPM detector, Roither LasetTechnick SMC940 LED | Wavelength 940 nm | [62] |
| Finger-worn organic pulse meter | HR | OLED 2, OPD 3 | Wavelength 625 nm, 46 dB SNR, a constant current of 93.6 µA | [63] |
| Wrist-worn watch with LED PPG | HR, SpO2 | PPG: Analog Devices ADPD144RI, Accelerometer: Analog Devices ADXL362 | Wavelengths 660, 880 nm, Power consumption 30 μW for a 75 dB, 25 Hz output | [64] |
| Wrist-worn printed organic | HR, SpO2 | OLED 2, OPD 3 multichannel PPG |
Wavelengths: Red and IR | [65] |
| PPG probe | PPG and ECG pattern | OSRAM LT M673 LEDs, STMicroelectronics SiPM detector | Wavelength 529 nm | [66] |
| PPG reflection sensors | HR, SpO2 | Maxim Integrated MAX86140/MAX86141 | Wavelengths 530, 560, 570, 590 nm | [67] |
| Ear-worn reflectance LED sensor | PPG during hypothermia | Excelitas technologies CR 50 IRH and CR 50 1M LEDs, 10 BP-BH PD | Wavelengths 658, 870 nm | [68] |
| Glasses | HR | Reflectance PPG on the nose | - | [69] |
| Flexible body attachment |
HR, BT | Reflectance PPG with thermistor | NFC, placed on wrist, fingertip, temple, or neck | [70] |
| Flexible body attachment |
HR, SpO2 | Reflectance PPG | Wavelengths 625, 950 nm, NFC, placed on thumbnail, or ear lobe | [12] |
| Flexible plaster | HR, SpO2, RR | Reflectance PPG | Wavelengths 633, 940 nm NFC, graphene PDs | [71] |
| Mobile phone | HR, RR, SpO2 | Motorola Droid phone camera | Advanced signal processing | [72] |
| Laboratory device | HR, ECG, SpO2, BP | PPG: HKG-07B, ECG: HKD-10C: Pressure HK-2000 (all Hefei Huake Information Technology) | Integrated PPG, ECG, and pressure pulse wave for cardiovascular disease | [73] |
| MW 4-PPG pad | MW 4-PPG spectrometer | 15 channels MW 4-PPG sensor, plasmonic filters integrated CMOS imager |
Wavelengths 505, 510, 515, 520, 525, 620, 625, 630, 635, 640, 930, 935, 940, 945, 950 nm | [74] |
| Finger-worn reflectance LED | MW 4-PPG spectrometer | PPG: plasmonic filters integrated onto a regular photodetector | Wavelengths 515, 630, 940 nm | [74] |
| Opto-electronic patch sensor | MW 4 -PPG movement |
PPG: Four channel board Dialog Devices DISCO4 | Wavelengths 525, 590, 650, 870 nm | [75][76][77] |
| MW 4-PPG sensor | HR, BP | Four channels MW 4-PPG sensor | Wavelengths 470, 570, 590, 940 nm, Sampling frequency 1 kHz | [78] |
| Flexible wearables | HR, RR, SpO2 | OLED 2, OPD 3, POF 5 for signal transport | PD current 0.05–4 μA, Signal frequency 0.05–30 Hz | [1][6] |
| Electrooptical muscle sensor | Muscle contraction | One x LEDs Rodan HIRL 8810, 4x PD Siemens BPW34 | Wavelength 880 nm, 4 PDs around LED | [79] |
| Wearable Organic Optoelectronic | Muscle contraction, SpO2 | OLED 2, PDs | Wavelengths 610, 700 nm | [39] |
| Sensor for a skin evaluation | Glucose level | C8 MediSensors | Raman spectroscopy sensor | [7] |
| Finger-worn | Glucose level, HR, SpO2 | Two x LED, PD, Arduino transmission and reflectance |
Wavelengths 525, 615 nm, 22 features from 29 s frames, Random Forest regression algorithm | [80] |
| Wrist-worn | Glucose level, HR | Four x LED (OSRAM SFH7060 and Vishay VSMY2853), PD (400–1000 nm) | Wavelengths 530, 660, 850, 950 nm, 24 features from 10 s frames, Partial least squares calibration algorithm | [81] |
1 Phonocardiology; 2 Organic LED; 3 Organic photodiodes; 4 Multi-wavelength; 5 Polymer optical fiber.
The PPG signal can be measured in two geometric configurations. If the light-emitting diodes (LEDs) and the photodiode (PD) are facing each other and the light passes through the tissue, where researchers measure the non-absorbed light, researchers speak of the transmission principle. This configuration is most suitable for areas with high capillary density, such as fingers or the earlobe. Red (680 nm) or near-infrared (810 nm) lights are usually used and deep penetration is required [61][82]. Such measurements are more stable, repetitive, and less sensitive to position changes. Transmission PPG configurations increase the perfusion index by 40 dB to 60 dB [45][83]. If the LED and PD are on the same side and use reflection from the internal structures of the skin, researchers speak of reflective PPG [62][84][85][86][87]. This configuration has a lower signal quality, but it is more suitable for nonstop wearing. Since the maxima of the pulsating component of the reflected light occurs in the range between 510 and 590 nm [88], the green (565 nm) or yellow (590 nm) light is usually used [89].
The PPG measurement can be performed in all parts of the human body, which provides sufficient availability of blood vessels and a sufficient pulsating component, especially when using the reflective method. Very common used areas for the reflective method are around the wrist [3][63], mostly in the form of various smart bracelets and watches, at the forehead [90], areas around the biceps, and the calf muscles or chest [64], often in the form of patches. In medicine, the fingers and ear lobes are very often used, where devices in the form of pliers working on the transmission principle are used. When designing the sensor, researchers must consider that the skin structure may slightly differ depending on the person and location on the body. Age, gender, and pathological conditions can cause various reflections, scattering, and light absorption [91]. For example, if you try to use a PPG sensor optimized for measurement on the wrist (in a smartwatch) and place it on the chest, you will find that the optical performance is insufficient and the sensor does not reach the required penetration. Some progressive PPG sensors are introduced to the use of organic materials. There is a systematic study of the reflective PPG sensor, where different printed OLEDs (RED and NIR) were utilized and compared, and the organic photodetector geometries, spacing, and barriers to maximize sensor performance. Finally, they also used inverse-variance weighting and template matching algorithms to improve the HR detection from the multichannel PPG signals [65]. A comprehensive review of the most up-to-date wearable PPGs was described by Daniel Ray et al. [45], who focused on multi-wavelength PPG sensing, technology, physiological parameter estimation, motion artifact reduction [60], and recommendations for standardization and overall theoretical details. Tarara et al. [91] described their challenges, and Bent et al. [92] examined the sources of inaccuracy such as different skin types, movement, and signal crossover. PPG technology is very successful but still not ideal. There are still challenges and improvements to be made. PPG is not fully reliable, the limitations are in the measurement’s standardization, the setting up of regularizing ranges of data to correlate with patients, and the evaluation of different treatment actions.
2.1. Heart Pulse Measurement
The skin consists of seven main layers, and each layer exhibits a different thickness, absorption, and scattering coefficients. The layer that determines pulse detection is the sixth layer called the inferior blood net dermis layer. For reliable detection in these skin layers, the used LEDs (respectively, light sources) and photodiodes PDs must ideally operate in the wavelength range of the absorption peaks of blood hemoglobin and deoxyhemoglobin, i.e., between 540 and 570 nm [66]. PDs and the LEDs must be optically isolated [93], for example, using a raised mesa, and the whole optical system must be surrounded by optical separation techniques to reduce the impact of ambient light [94]. For a more reliable result, modern chips also include an ambient light sensor. The PPG signal, when recorded in a real environment, often contains movement artifacts and is also affected by the changing pressure of the sensor on the skin [95]. To minimize these artifacts, accelerometric sensors or multiple optical paths using two photodiodes are therefore included in modern PPG chips. The output of the accelerometer and the two PPG signals are then processed using motion compensation algorithms, a Kalman filter, and an adaptive notch filter [67]. HR measuring using PPG is well characterized and works seamlessly; however, researchers must take into account that the amount of data processing for healthcare applications is large and requires considerable computing power and continuous energy supply [11].
Ishikawa et al. [96] introduced the PPG HR sensor, which overcame motion artifacts, in the form of a wristband that can be worn daily during various activities. A FIR filter was used to cancel arm-related motion artefact, and for finger and wrist-related motion a band-pass filter based on the body tissue HR was used. Finally, pulse noise-free detection signals were obtained using peak detection and autocorrelation methods. This calibration achieved noise-free HR detection [93]. Tison’s group has made progress in the diagnosis of atrial fibrillation (AF). They used a PPG sensor and an accelerometer of a commercial smartwatch to obtain HR data and the number of steps. Thanks to the algorithm based on heuristic pretraining and deep neural network training, their device can detect the fibrillation with sensitivity of 98% and specificity of 90.2% [97]. An interesting project was the smart pulse sensing glasses with Bluetooth low energy connectivity designed by Nicholas Constant et al. This device used a PPG sensor located on the nose for unobtrusive and continuous monitoring of HR [69]. Devices using NFC communication represent a very promising version of HR monitors [70]. In this particular case, it can also measure temperature via a thermistor. The sensing device is activated only when a reader-device, such as a smartphone or tablet, is approaching, for example during a call. The reader-devices supply all the necessary energy for measurement via a coil at the edge of the sensing-device. It resembles contactless ATM cards, only instead of sending data about your identity and bank transaction, it performs a quick physiology measurement [98]. Such devices represent an interesting alternative to conventional battery powered devices. Optical sensors for HR detection can be in the form of wide variety of daily wear items that do not interfere with humans and fulfill their diagnostic role.
2.2. Detection of Blood Oxygen and Glucose
Arterial oxygen saturation (SpO2) indicates the percentage of the total hemoglobin in the blood that is saturated with oxygen. The SpO2 provides information on lung efficiency and is an important indicator of overall human health. These measurements are similar to those using LEDs and PDs, but the estimation of SpO2 level is based on the different spectral characteristics of hemoglobin and deoxyhemoglobin, thus two LEDs are used. Deoxyhemoglobin absorbs red light better than oxyhemoglobin, while oxyhemoglobin absorbs infrared (IR) radiation better. Blood oxygenation is proportional to reflected red light (622–780 nm) and IR (780–2400 nm) [74]. However, some researchers have identified that orange and green lights perform better because of their resistance to motion artifacts [75][76]. A very promising design is the flexible concept (Figure 1a) [12], which incorporates advanced optoelectronic functions for PPG wireless NFC monitoring applications, including SpO2, HR, and HRV. The device works on the reflectance principle and since it is battery-free, it was possible to achieve a miniature, thin, and flexible construction that can be mounted on a thumbnail or ear lobe. In particular, it uses the reflectance principle in conjunction with near-field communication (NFC) capabilities, which allows operation in a thin, miniaturized, flexible device. A similar flexible device was presented by Polat et al. [71], who designed a patch to record HR, SpO2, and respiration. Again, the communication is via NFC, but the photodiode in this case is based on graphene sensitized with semiconducting quantum dots.
Another very important component in the bloodstream indicating health is glucose. The researchers tried to estimate blood glucose levels [80] from green and red light on fingers using machine learning and a random forest regression algorithm. The next approach extracted blood glucose levels by applying four wavelengths of light (green, red, and two IRs) on the wrist using a partial least squares algorithm as the calibration [81]. Both approaches use signal splitting into time frames (29 and 10 s, respectively), and parameterization of time courses also using the Teager–Kaiser energy operator (22 and 24 parameters), and calibration using a reference glucose device. The achieved maximum correlation coefficient of blood glucose determination is 0.91 and 0.86, respectively, which represents a standard prediction error of approximately 6.16 mg/dl.
2.3. Calculation of Respiration
The PPG signal is affected by several vital functions and physiological activities, mainly modulated by the heart and respiration. Hence, these activities can be determined by reverse demodulation. Monitoring cardiorespiratory activities using a PPG signal is a well-established non-invasive technique [44][72]. Researchers can use continuous wavelet transform and autoregressive modeling, or a method proposed by Chon et al., which uses variable-frequency complex demodulation and provides slightly better results [99][100]. Breathing manifests itself in PPG in three ways. Pulse wave amplitudes are affected by the blood vessel flexibility, the variation in pulse shape, and a decrease in intrathoracic pressure that leads to increased venous return during inspiration [101][102]. Therefore, researchers can extract the RR and amplitude of respiration [103][104][105]. PPG technology is used not only in hospitals in intensive care units, during respiratory diseases, or anesthesia, but today it is very often and successfully implemented in wearable health facilities for the general public [106].
2.4. Blood Pressure Estimation
Many algorithms are known where systolic and diastolic BP are estimated from the shape of the PPG curve [55][107]. It is possible to use progressive neural learning methods [108][109], FFT-based neural networks [110], or Poincare section analysis [111]. The reliability is continuously growing [112][113][114]. Today, devices that determine blood pressure from the delay of the PPG curve from the ECG signal, the so-called pulse transient time (PTT), have come into use [73][115][116][117]. The correlation of PTT with systolic and diastolic blood pressure is high. By involving multiple wavelengths in the determination, researchers can even increase accuracy. For example, in a study by Liu et al., where instead of using a single wavelength PPG, three wavelengths were used, the accuracy was increased about two times [78].
Great efforts and promising results are also shown in the extraction of BP without an ECG signal from the so-called local PTT, which is determined by the time difference between the different wavelengths of the PPG signal, for example, from the reflectance mode multi-wavelength PPG sensing at three different locations (fingertip, radial artery and dorsal surface of wrist) using 4 different wavelengths (green 525 nm, orange 595 nm, red 650 nm, and IR 870 nm) [77] or even using 15 different reflectance mode wavelengths and the cross-correlation method [74]. The combination of the described methods, PPG shape recognition and PTT delay methods, processed by deep neural networks, appears to be very promising in the future [66][118]. Today, optical blood pressure monitoring is a complete tool that can be built directly into the firmware or subsequent software, allowing it to be possible to continuously measure blood pressure without using a cuff for virtually any type of PPG sensor. In addition, these algorithms are adapted to analyze PPG signals acquired in different parts of the body and with different sensor topologies [119].
2.5. Others
There are also attempts to use PPG signals from wearables for carrier biometric authentication. They assume that the PPG signal is individual for each person and either uses classification algorithms extracting pulse features [120][121], or deep learning frameworks [122][123]. Monitoring absolute cerebral blood flow based on a combination of time-resolved dynamic contrast-enhanced near-infrared spectroscopy and diffuse correlation spectroscopy [124], which uses a configuration similar to multiwavelength (MW) PPG, looks very interesting. In Kooman et al. [125], there are even studies investigating the use of PPG wearables in hemodialysis patients, and Lima et.al. [126] monitored peripheral perfusion. Adhikari et al. [127] developed a multi-wavelength transmission mode PPG method to monitor drug delivery. They examined the concentration of therapeutic gold nanoparticles, quinine, and amphotericin B in mice possessing absorption peaks in the 350–1100 nm range.
A muscle contraction sensor has also been developed to measure signals from intact muscles, which can then be used in robotics and prosthetics [39]. The principle is that the sensor measures the amount of incident light backscattered from the muscles with the intensity of the light varying from whether the muscle is contracted or stretched. The myosin protein in muscle sarcomeres has liquid crystalline properties and is responsible for the anisotropic behavior of muscles [39]. During contraction, the muscle fibers slide along this myosin, and the muscle fiber becomes short and wide, causing anisotropic scattering of incident light. The light that propagates parallel to the muscle fibers is scattered differently to the light traveling perpendicular to the fibers. This anisotropy can be detected utilizing a surface light source and detectors placed longitudinally and perpendicular to the muscle fiber, which represents only a certain modification of the PPG sensor [79][128]. In this research, the authors specifically used a wavelength range from 610 to 700 nm.
References
- Khan, Y.; Ostfeld, A.E.; Lochner, C.M.; Pierre, A.; Arias, A.C. Monitoring of Vital Signs with Flexible and Wearable Medical Devices. Adv. Mater. 2016, 28, 4373–4395.
- Pantelopoulos, A.; Bourbakis, N.G. A Survey on Wearable Sensor-Based Systems for Health Monitoring and Prognosis. IEEE Trans. Syst. Man. Cybern. Part C (Appl. Rev.) 2010, 40, 1–12.
- Kazanskiy, N.L.; Butt, M.A. Recent Advances in Wearable Optical Sensor Automation Powered by Battery versus Skin-like Battery-Free Devices for Personal Healthcare—A Review. Nanomaterials 2022, 12, 334.
- Fortune Business Insights Wearable Medical Devices Market Size Worth USD 195.57 Bn by 2027. With Stunning 26.4% CAGR. Available online: https://www.globenewswire.com/news-release/2022/02/03/2378221/0/en/Wearable-Medical-Devices-Market-Size-worth-USD-195-57-Bn-by-2027-With-stunning-26-4-CAGR.html (accessed on 8 March 2022).
- Yao, H.; Shum, A.J.; Cowan, M.; Lähdesmäki, I.; Parviz, B.A. A contact lens with embedded sensor for monitoring tear glucose level. Biosens. Bioelectron. 2011, 26, 3290–3296.
- Zhu, X.; Liu, W.; Shuang, S.; Nair, M.; Li, C.Z. Intelligent tattoos, patches, and other wearable biosensors. Med. Biosens. Point Care Appl. 2017, 133–150.
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371.
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A review of wearable sensors and systems with application in rehabilitation. J. Neuroeng. Rehabil. 2012, 9, 21.
- Guk, K.; Han, G.; Lim, J.; Jeong, K.; Kang, T.; Lim, E.K.; Jung, J. Evolution of wearable devices with real-time disease monitoring for personalized healthcare. Nanomaterials 2019, 9, 813.
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406.
- Yetisen, A.K.; Martinez-hurtado, J.L.; Khademhosseini, A. Wearables in Medicine. Adv. Mater. 2018, 30, 1706910.
- Kim, J.; Gutruf, P.; Chiarelli, A.M.; Heo, S.Y.; Cho, K.; Xie, Z.; Banks, A.; Han, S.; Jang, K.; Lee, J.W.; et al. Miniaturized Battery-Free Wireless Systems for Wearable Pulse Oximetry. Adv. Funct. Mater. 2017, 27, 1604373.
- Yokota, T.; Zalar, P.; Kaltenbrunner, M.; Jinno, H.; Matsuhisa, N.; Kitanosako, H.; Tachibana, Y.; Yukita, W.; Koizumi, M.; Someya, T. Ultraflexible organic photonic skin. Sci. Adv. 2016, 2, 1–8.
- Khan, Y.; Han, D.; Pierre, A.; Ting, J.; Wang, X.; Lochner, C.M.; Bovo, G.; Yaacobi-Gross, N.; Newsome, C.; Wilson, R.; et al. A flexible organic reflectance oximeter array. Proc. Natl. Acad. Sci. USA 2018, 115, E11015–E11024.
- Withings Thermo. Smart Temporal Thermometer. The Hottest Thermometer. The Coolest Technology. Available online: https://www.withings.com/sk/en/thermo (accessed on 8 March 2022).
- Bitanga, M. La Roche-Posay. My Skin Track UV Sensor. Available online: https://hiconsumption.com/la-roche-posay-my-skin-track-uv-sensor/ (accessed on 8 March 2022).
- Axonim Devices System That Makes Smart Glasses See Heat. Available online: https://axonim.com/works/thermal-imaging-device.html (accessed on 8 March 2022).
- Witt, J.; Narbonneau, F.; Schukar, M.; Krebber, K.; De Jonckheere, J.; Jeanne, M.; Kinet, D.; Paquet, B.; Depre, A.; D’Angelo, L.T.; et al. Medical Textiles with Embedded Fiber Optic Sensors for Monitoring of Respiratory Movement. IEEE Sens. J. 2012, 12, 246–254.
- Zhu, H.T.; Zhan, L.W.; Dai, Q.; Xu, B.; Chen, Y.; Lu, Y.Q.; Xu, F. Self-Assembled Wavy Optical Microfiber for Stretchable Wearable Sensor. Adv. Opt. Mater. 2021, 9, 1–7.
- Li, X.; Liu, D.; Kumar, R.; Ng, W.P.; Fu, Y.Q.; Yuan, J.; Yu, C.; Wu, Y.; Zhou, G.; Farrell, G.; et al. A simple optical fiber interferometer based breathing sensor. Meas. Sci. Technol. 2017, 28, 035105.
- Bandodkar, A.J.; Gutruf, P.; Choi, J.; Lee, K.H.; Sekine, Y.; Reeder, J.T.; Jeang, W.J.; Aranyosi, A.J.; Lee, S.P.; Model, J.B.; et al. Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv. 2019, 5, 1–15.
- Elsherif, M.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Wearable Contact Lens Biosensors for Continuous Glucose Monitoring Using Smartphones. ACS Nano 2018, 12, 5452–5462.
- Sekine, Y.; Kim, S.B.; Zhang, Y.; Bandodkar, A.J.; Xu, S.; Choi, J.; Irie, M.; Ray, T.R.; Kohli, P.; Kozai, N.; et al. A fluorometric skin-interfaced microfluidic device and smartphone imaging module for: In situ quantitative analysis of sweat chemistry. Lab Chip 2018, 18, 2178–2186.
- Hayward, J. Wearable Sensors 2021–2031. Available online: http://www.idtechex.com/en/research-report/wearable-sensors-2021-2031/780 (accessed on 26 January 2022).
- Ballard, Z.S.; Ozcan, A. Wearable Optical Sensors. In Mobile Health; Rehg, J.M., Murphy, S.A., Kumar, S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 313–342. ISBN 978-3-319-51393-5.
- Kang, D.; Pikhitsa, P.V.; Choi, Y.W.; Lee, C.; Shin, S.S.; Piao, L.; Park, B.; Suh, K.-Y.; Kim, T.; Choi, M. Ultrasensitive mechanical crack-based sensor inspired by the spider sensory system. Nature 2014, 516, 222–226.
- Yin, D.; Feng, J.; Ma, R.; Liu, Y.F.; Zhang, Y.L.; Zhang, X.L.; Bi, Y.G.; Chen, Q.D.; Sun, H.B. Efficient and mechanically robust stretchable organic light-emitting devices by a laser-programmable buckling process. Nat. Commun. 2016, 7, 1–7.
- Miyamoto, A.; Lee, S.; Cooray, N.F.; Lee, S.; Mori, M.; Matsuhisa, N.; Jin, H.; Yoda, L.; Yokota, T.; Itoh, A.; et al. Inflammation-free, gas-permeable, lightweight, stretchable on-skin electronics with nanomeshes. Nat. Nanotechnol. 2017, 12, 907–913.
- Takei, K.; Takahashi, T.; Ho, J.C.; Ko, H.; Gillies, A.G.; Leu, P.W.; Fearing, R.S.; Javey, A. Nanowire active-matrix circuitry for low-voltage macroscale artificial skin. Nat. Mater. 2010, 9, 821–826.
- Larson, C.; Peele, B.; Li, S.; Robinson, S.; Totaro, M.; Beccai, L.; Mazzolai, B.; Shepherd, R. Highly stretchable electroluminescent skin for optical signaling and tactile sensing. Science 2016, 351, 1071–1074.
- Kim, Y.; Chortos, A.; Xu, W.; Liu, Y.; Oh, J.Y.; Son, D.; Kang, J.; Foudeh, A.M.; Zhu, C.; Lee, Y.; et al. A bioinspired flexible organic artificial afferent nerve. Science 2018, 360, 998–1003.
- Miller, D.A.B. Rationale and challenges for optical interconnects to electronic chips. Proc. IEEE 2000, 88, 728–749.
- Sessolo, M.; Khodagholy, D.; Rivnay, J.; Maddalena, F.; Gleyzes, M.; Steidl, E.; Buisson, B.; Malliaras, G.G. Easy-to-fabricate conducting polymer microelectrode arrays. Adv. Mater. 2013, 25, 2135–2139.
- Forrest, S.R. The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 2004, 428, 911–918.
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photonics 2012, 6, 153–161.
- Ryu, G.; Huang, J.; Hofmann, O.; Walshe, C.A.; Sze, J.Y.Y.; McClean, G.D.; Mosley, A.; Rattle, S.J.; Demello, J.C.; Demello, A.J.; et al. Highly sensitive fluorescence detection system for microfluidic lab-on-a-chip. Lab Chip 2011, 11, 1664–1670.
- Hofmann, O.; Wang, X.; DeMello, J.C.; Bradley, D.D.C.; DeMello, A.J. Towards microalbuminuria determination on a disposable diagnostic microchip with integrated fluorescence detection based on thin-film organic light emitting diodes. Lab Chip 2005, 5, 863–868.
- Ramuz, M.; Leuenberger, D.; Bürgi, L. Optical biosensors based on integrated polymer light source and polymer photodiode. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 80–87.
- Bansal, A.K.; Hou, S.; Kulyk, O.; Bowman, E.M.; Samuel, I.D.W. Wearable Organic Optoelectronic Sensors for Medicine. Adv. Mater. 2015, 27, 7638–7644.
- Guo, J.; Yang, C.; Dai, Q.; Kong, L. Soft and stretchable polymeric optical waveguide-based sensors for wearable and biomedical applications. Sensors 2019, 19, 3771.
- Mannsfeld, S.C.B.; Tee, B.C.K.; Stoltenberg, R.M.; Chen, C.V.H.H.; Barman, S.; Muir, B.V.O.; Sokolov, A.N.; Reese, C.; Bao, Z. Highly sensitive flexible pressure sensors with microstructured rubber dielectric layers. Nat. Mater. 2010, 9, 859–864.
- Ernst, G. Heart-Rate Variability—More than Heart Beats? Front. Public Health 2017, 5, 1–12.
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 1–17.
- Dias, D.; Paulo Silva Cunha, J. Wearable Health Devices—Vital Sign Monitoring, Systems and Technologies. Sensors 2018, 18, 2414.
- Ray, D.; Collins, T.; Woolley, S.; Ponnapalli, P. A Review of Wearable Multi-wavelength Photoplethysmography. IEEE Rev. Biomed. Eng. 2021, 1.
- Ozana, N.; Arbel, N.; Beiderman, Y.; Mico, V.; Sanz, M.; Garcia, J.; Anand, A.; Javidi, B.; Epstein, Y.; Zalevsky, Z. Improved noncontact optical sensor for detection of glucose concentration and indication of dehydration level. Biomed. Opt. Express 2014, 5, 1926.
- Alexeev, V.L.; Das, S.; Finegold, D.N.; Asher, S.A. Photonic crystal glucose-sensing material for noninvasive monitoring of glucose in tear fluid. Clin. Chem. 2004, 50, 2353–2360.
- Elmesmari, R.; Martin, A.; Reilly, J.J.; Paton, J.Y. Comparison of accelerometer measured levels of physical activity and sedentary time between obese and non-obese children and adolescents: A systematic review. BMC Pediatr. 2018, 18, 1–22.
- Robertson, W.; Stewart-Brown, S.; Wilcock, E.; Oldfield, M.; Thorogood, M. Utility of accelerometers to measure physical activity in children attending an obesity treatment intervention. J. Obes. 2011, 2011.
- Manchanda, S.; Ehsanullah, M. Suspected Cardiac Syncope in Elderly Patients: Use of the 12-Lead Electrocardiogram to Select Patients for Holter Monitoring. Gerontology 2001, 47, 195–197.
- Gokalp, H.; Clarke, M. Monitoring Activities of Daily Living of the Elderly and the Potential for Its Use in Telecare and Telehealth: A Review. Telemed. e-Health 2013, 19, 910–923.
- Shelley, K.H. Photoplethysmography: Beyond the Calculation of Arterial Oxygen Saturation and Heart Rate. Anesth. Analgesia 2007, 105, 31–36.
- Lee, C.; Sik Shin, H.; Lee, M. Relations between ac-dc components and optical path length in photoplethysmography. J. Biomed. Opt. 2011, 16, 077012.
- Utami, N.; Setiawan, A.W.; Zakaria, H.; Mengko, T.R.; Mengko, R. Extracting blood flow parameters from Photoplethysmograph signals: A review. In Proceedings of the 2013 3rd International Conference on Instrumentation, Communications, Information Technology and Biomedical Engineering (ICICI-BME), Bandung, Indonesia, 7–8 November 2013; pp. 403–407.
- Elgendi, M.; Fletcher, R.; Liang, Y.; Howard, N.; Lovell, N.H.; Abbott, D.; Lim, K.; Ward, R. The use of photoplethysmography for assessing hypertension. NPJ Digit. Med. 2019, 2, 1–11.
- Marzorati, D.; Bovio, D.; Salito, C.; Mainardi, L.; Cerveri, P. Chest Wearable Apparatus for Cuffless Continuous Blood Pressure Measurements Based on PPG and PCG Signals. IEEE Access 2020, 8, 55424–55437.
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017, 32, 1909–1918.
- Anderson, R.R.; Parrish, J.A. The optics of human skin. J. Invest. Dermatol. 1981, 77, 13–19.
- Young, A.R. Chromophores in human skin. Phys. Med. Biol. 1997, 42, 789–802.
- Lee, J.; Kim, M.; Park, H.-K.; Kim, I.Y. Motion Artifact Reduction in Wearable Photoplethysmography Based on Multi-Channel Sensors with Multiple Wavelengths. Sensors 2020, 20, 1493.
- Sandberg, M.; Zhang, Q.; Styf, J.; Gerdle, B.; Lindberg, L.G. Non-invasive monitoring of muscle blood perfusion by photoplethysmography: Evaluation of a new application. Acta Physiol. Scand. 2005, 183, 335–343.
- Perpetuini, D.; Chiarelli, A.M.; Vinciguerra, V.; Vitulli, P.; Rinella, S.; Cardone, D.; Bianco, F.; Perciavalle, V.; Gallina, S.; Fallica, G.; et al. Integrated Multi-channel PPG and ECG System for Cardiovascular Risk Assessment. Proceedings 2019, 27, 8.
- Elsamnah, F.; Bilgaiyan, A.; Affiq, M.; Shim, C.H.; Ishidai, H.; Hattori, R. Reflectance-based organic pulse meter sensor for wireless monitoring of photoplethysmogram signal. Biosensors 2019, 9, 87.
- Finnerty, R. How to Design a Better Pulse Oximeter: Implementation. Available online: https://www.embedded.com/how-to-design-a-better-pulse-oximeter-implementation/ (accessed on 26 January 2022).
- Khan, Y.; Han, D.; Ting, J.; Ahmed, M.; Nagisetty, R.; Arias, A.C. Organic Multi-Channel Optoelectronic Sensors for Wearable Health Monitoring. IEEE Access 2019, 7, 128114–128124.
- Rundo, F.; Conoci, S.; Ortis, A.; Battiato, S. An Advanced Bio-Inspired PhotoPlethysmoGraphy (PPG) and ECG Pattern Recognition System for Medical Assessment. Sensors 2018, 18, 405.
- Maxim Integrated Products. Guidelines to Enhancing the Heart-Rate Monitoring Performance of Biosensing Wearables. Available online: https://www.maximintegrated.com/en/design/technical-documents/app-notes/6/6768.html (accessed on 26 January 2022).
- Budidha, K.; Kyriacou, P.A. In vivo investigation of ear canal pulse oximetry during hypothermia. J. Clin. Monit. Comput. 2018, 32, 97–107.
- Constant, N.; Douglas-Prawl, O.; Johnson, S.; Mankodiya, K. Pulse-Glasses: An unobtrusive, wearable HR monitor with Internet-of-Things functionality. In Proceedings of the 2015 IEEE 12th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Cambridge, MA, USA, 9–12 June 2015; pp. 1–5.
- Kang, M.H.; Lee, G.J.; Yun, J.H. NFC-Based Wearable Optoelectronics Working with Smartphone Application for Untact Healthcare. Sensors 2021, 21, 878.
- Polat, E.O.; Mercier, G.; Nikitskiy, I.; Puma, E.; Galan, T.; Gupta, S.; Montagut, M.; Piqueras, J.J.; Bouwens, M.; Durduran, T.; et al. Flexible graphene photodetectors for wearable fitness monitoring. Sci. Adv. 2019, 5, 1–9.
- Scully, C.G.; Lee, J.; Meyer, J.; Gorbach, A.M.; Granquist-Fraser, D.; Mendelson, Y.; Chon, K.H. Physiological parameter monitoring from optical recordings with a mobile phone. IEEE Trans. Biomed. Eng. 2012, 59, 303–306.
- Liu, W.; Fang, X.; Chen, Q.; Li, Y.; Li, T. Reliability analysis of an integrated device of ECG, PPG and pressure pulse wave for cardiovascular disease. Microelectron. Reliab. 2018, 87, 183–187.
- Chang, C.C.; Wu, C.T.; Choi, B.I.; Fang, T.J. MW-PPG sensor: An on-chip spectrometer approach. Sensors 2019, 19, 3698.
- Blanos, P.; Hu, S.; Mulvaney, D.; Alharbi, S. An applicable approach for extracting human heart rate and oxygen saturation during physical movements using a multi-wavelength illumination optoelectronic sensor system. In Proceedings of the Design and Quality for Biomedical Technologies XI; Raghavachari, R., Liang, R., Pfefer, T.J., Eds.; SPIE: San Francisco, CA, USA, 2018; p. 27.
- Alharbi, S.; Hu, S.; Mulvaney, D.; Barrett, L.; Yan, L.; Blanos, P.; Elsahar, Y.; Adema, S. Oxygen Saturation Measurements from Green and Orange Illuminations of Multi-Wavelength Optoelectronic Patch Sensors. Sensors 2018, 19, 118.
- Pasta, S.; Blanos, P.; Yan, L.; Hu, S.; Scardulla, F.S.; D’Acquisto, L.; Barrett, L. A novel multi-wavelength procedure for blood pressure estimation using opto-physiological sensor at peripheral arteries and capillaries. In Proceedings of the Design and Quality for Biomedical Technologies XI; Raghavachari, R., Liang, R., Pfefer, T.J., Eds.; SPIE: San Francisco, CA, USA, 2018; p. 39.
- Liu, J.; Ping-yen Yan, B.; Dai, W.; Ding, X.; Zhang, Y.; Zhao, N.; Baker, W.B.; Parthasarathy, A.B.; Busch, D.R.; Mesquita, R.C.; et al. Multi-wavelength photoplethysmography method for skin arterial pulse extraction. Biomed. Opt. Express. 2016, 7, 4313–4326.
- Chianura, A.; Giardini, M.E. An electrooptical muscle contraction sensor. Med. Biol. Eng. Comput. 2010, 48, 731–734.
- Gupta, S.S.; Hossain, S.; Haque, C.A.; Kim, K.-D. In-Vivo Estimation of Glucose Level Using PPG Signal. In Proceedings of the 2020 International Conference on Information and Communication Technology Convergence (ICTC), Jeju, Korea, 21–23 October 2020; pp. 733–736.
- Rachim, V.P.; Chung, W.-Y. Wearable-band type visible-near infrared optical biosensor for non-invasive blood glucose monitoring. Sensors Actuators B Chem. 2019, 286, 173–180.
- Lindberg, L.G.; Tamura, T.; Öberg, P.Å. Photoplethysmography. Med. Biol. Eng. Comput. 1991, 29, 40–47.
- Kyriacou, P.A.; May, J.M. Photoplethysmography: New trends and future directions. In Photoplethysmography; Elsevier: Amsterdam, The Netherlands, 2022; pp. 469–487.
- König, V.; Huch, R.; Huch, A. Reflectance pulse oximetry—Principles and obstetric application in the Zurich system. J. Clin. Monit. Comput. 1998, 14, 403–412.
- Mendelson, Y.; Ochs, B.D. Noninvasive Pulse Oximetry Utilizing Skin Reflectance Photoplethysmography. IEEE Trans. Biomed. Eng. 1988, 35, 798–805.
- Lee, H.; Ko, H.; Lee, J. Reflectance pulse oximetry: Practical issues and limitations. ICT Express 2016, 2, 195–198.
- Kumar, S.; Buckley, J.L.; Barton, J.; Pigeon, M.; Newberry, R.; Rodencal, M.; Hajzeraj, A.; Hannon, T.; Rogers, K.; Casey, D.; et al. A Wristwatch-Based Wireless Sensor Platform for IoT Health Monitoring Applications. Sensors 2020, 20, 1675.
- Cui, W.; Ostrander, L.E.; Lee, B.Y. In vivo reflectance of blood and tissue as a function of light wavelength. IEEE Trans. Biomed. Eng. 1990, 37, 632–639.
- Lai, P.H.; Kim, I. Lightweight wrist photoplethysmography for heavy exercise: Motion robust heart rate monitoring algorithm. Healthc. Technol. Lett. 2015, 2, 6–11.
- Mendelson, Y.; Duckworth, R.J.; Comtois, G. A Wearable Reflectance Pulse Oximeter for Remote Physiological Monitoring. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 912–915.
- Tarar, A.A.; Mohammad, U.; Srivastava, S.K. Wearable skin sensors and their challenges: A review of transdermal, optical, and mechanical sensors. Biosensors 2020, 10, 56.
- Bent, B.; Goldstein, B.A.; Kibbe, W.A.; Dunn, J.P. Investigating sources of inaccuracy in wearable optical heart rate sensors. npj Digit. Med. 2020, 3, 1–9.
- L’Her, E.; N’Guyen, Q.T.; Pateau, V.; Bodenes, L.; Lellouche, F. Photoplethysmographic determination of the respiratory rate in acutely ill patients: Validation of a new algorithm and implementation into a biomedical device. Ann. Intensive Care 2019, 9, 11.
- Li, K.H.C.; White, F.A.; Tipoe, T.; Liu, T.; Wong, M.C.; Jesuthasan, A.; Baranchuk, A.; Tse, G.; Yan, B.P. The Current State of Mobile Phone Apps for Monitoring Heart Rate, Heart Rate Variability, and Atrial Fibrillation: Narrative Review. JMIR mHealth uHealth 2019, 7, e11606.
- Horton, J.F.; Stergiou, P.; Fung, T.S.; Katz, L. Comparison of Polar M600 Optical Heart Rate and ECG Heart Rate during Exercise. Med. Sci. Sport. Exerc. 2017, 49, 2600–2607.
- Ishikawa, T.; Hyodo, Y.; Miyashita, K.; Yoshifuji, K.; Komoriya, Y.; Imai, Y. Wearable Motion Tolerant PPG Sensor for Instant Heart Rate in Daily Activity. In Proceedings of the 10th International Joint Conference on Biomedical Engineering Systems and Technologies, SCITEPRESS—Science and Technology Publications. Porto, Portugal, 21–23 February 2017; pp. 126–133.
- Tison, G.H.; Sanchez, J.M.; Ballinger, B.; Singh, A.; Olgin, J.E.; Pletcher, M.J.; Vittinghoff, E.; Lee, E.S.; Fan, S.M.; Gladstone, R.A.; et al. Passive detection of atrial fibrillation using a commercially available smartwatch. JAMA Cardiol. 2018, 3, 409–416.
- Faulkner, C. What is NFC? Everything You Need to Know. Available online: https://www.techradar.com/news/what-is-nfc (accessed on 29 March 2022).
- Chon, K.H.; Dash, S.; Ju, K. Estimation of Respiratory Rate from Photoplethysmogram Data Using Time–Frequency Spectral Estimation. IEEE Trans. Biomed. Eng. 2009, 56, 2054–2063.
- Ghamari, M. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int. J. Biosens. Bioelectron. 2018, 4, 195.
- Akbar, U.; Akbari, A.; Alinia, P.; Amato, F.; Amendola, S.; Balaban, E.; Beach, C.; Bertschi, M.; Braun, F.; Caldani, L.; et al. Wearable Sensors: Fundamentals, Implementation and Applications. In Wearable Sensors; Elsevier: Amsterdam, The Netherlands, 2021; pp. xi–xiii. ISBN 9780128192467.
- Madhav, K.V.; Ram, M.R.; Krishna, E.H.; Komalla, N.R.; Reddy, K.A. Estimation of respiration rate from ECG, BP and PPG signals using empirical mode decomposition. In Proceedings of the 2011 IEEE International Instrumentation and Measurement Technology Conference, Hangzhou, China, 10–12 May 2011; pp. 1–4.
- Karlen, W.; Raman, S.; Ansermino, J.M.; Dumont, G.A. Multiparameter Respiratory Rate Estimation from the Photoplethysmogram. IEEE Trans. Biomed. Eng. 2013, 60, 1946–1953.
- Nemati, S.; Malhotra, A.; Clifford, G.D. Data Fusion for Improved Respiration Rate Estimation. EURASIP J. Adv. Signal Process. 2010, 2010, 926305.
- Shokoueinejad, M.; Fernandez, C.; Carroll, E.; Wang, F.; Levin, J.; Rusk, S.; Glattard, N.; Mulchrone, A.; Zhang, X.; Xie, A.; et al. Sleep apnea: A review of diagnostic sensors, algorithms, and therapies. Physiol. Meas. 2017, 38, R204–R252.
- Lei, R.; Ling, B.W.K.; Feng, P.; Chen, J. Estimation of heart rate and respiratory rate from ppg signal using complementary ensemble empirical mode decomposition with both independent component analysis and non-negative matrix factorization. Sensors 2020, 20, 3238.
- Hosanee, M.; Chan, G.; Welykholowa, K.; Cooper, R.; Kyriacou, P.A.; Zheng, D.; Allen, J.; Abbott, D.; Menon, C.; Lovell, N.H.; et al. Cuffless Single-Site Photoplethysmography for Blood Pressure Monitoring. J. Clin. Med. 2020, 9, 723.
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, June 27–30 2016; Volume 2016-Decem, pp. 770–778.
- Hardoon, D.R.; Szedmak, S.; Shawe-Taylor, J. Canonical correlation analysis: An overview with application to learning methods. Neural Comput. 2004, 16, 2639–2664.
- Xing, X.; Sun, M. Optical blood pressure estimation with photoplethysmography and FFT-based neural networks. Biomed. Opt. Express 2016, 7, 3007.
- Shoeibi, F.; Najafiaghdam, E.; Ebrahimi, A. Poincaré’s section analysis of Photoplethysmography signals for cuff-less non-invasive blood pressure measurement. Preprint 2021.
- Rundo, F.; Ortis, A.; Battiato, S.; Conoci, S. Advanced bio-inspired system for noninvasive cuff-less blood pressure estimation from physiological signal analysis. Computation 2018, 6, 46.
- Liang, Y.; Chen, Z.; Ward, R.; Elgendi, M. Photoplethysmography and deep learning: Enhancing hypertension risk stratification. Biosensors 2018, 8, 101.
- Slapničar, G.; Mlakar, N.; Luštrek, M. Blood Pressure Estimation from Photoplethysmogram Using a Spectro-Temporal Deep Neural Network. Sensors 2019, 19, 3420.
- Jeon, G.-R.; Jung, D.-K.; Kim, G.-R.; Shin, B.-J. The Development of Integrated Sensor System for Measuring Simultaneously ECG, PPG and PPW. J. Korea Acad. Coop. Soc. 2009, 10, 992–999.
- Yoon, Y.; Cho, J.H.; Yoon, G. Non-constrained blood pressure monitoring using ECG and PPG for personal healthcare. J. Med. Syst. 2009, 33, 261–266.
- Shin, W.; Cha, Y.D.; Yoon, G. ECG/PPG integer signal processing for a ubiquitous health monitoring system. J. Med. Syst. 2010, 34, 891–898.
- Liu, S.-H.; Liu, L.-J.; Pan, K.-L.; Chen, W.; Tan, T.-H. Using the Characteristics of Pulse Waveform to Enhance the Accuracy of Blood Pressure Measurement by a Multi-Dimension Regression Model. Appl. Sci. 2019, 9, 2922.
- Sola, J.; Bertschi, M.; Krauss, J. Measuring Pressure. Available online: https://www.embs.org/pulse/articles/optical-blood-pressure-monitoring/ (accessed on 26 January 2022).
- Reşit Kavsaoglu, A.; Polat, K.; Recep Bozkure, M.; Muthusamy, H. Fotopletismografi sinyalleri ile biyometrik tanlmaya yönelik özellilk çlkarlml. In Proceedings of the 2013 21st Signal Processing and Communications Applications Conference (SIU), Haspolat, Turkey, 24–26 April 2013.
- Al Sidani, A.; Cherry, A.; Hajj-Hassan, H.; Hajj-Hassan, M. Comparison between K-Nearest Neighbor and Support Vector Machine Algorithms for PPG Biometric Identification. In Proceedings of the 2019 5th International Conference on Advances in Biomedical Engineering (ICABME), Tripoli, Lebanon, 17–19 October 2019; pp. 19–22.
- Biswas, D.; Everson, L.; Liu, M.; Panwar, M.; Verhoef, B.-E.; Patki, S.; Kim, C.H.; Acharyya, A.; Van Hoof, C.; Konijnenburg, M.; et al. CorNET: Deep Learning Framework for PPG-Based Heart Rate Estimation and Biometric Identification in Ambulant Environment. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 282–291.
- Hwang, D.Y.; Taha, B.; Hatzinakos, D. PBGAN: Learning PPG Representations from GAN for Time-Stable and Unique Verification System. IEEE Trans. Inf. Forensics Secur. 2021, 16, 5124–5137.
- He, L.; Baker, W.B.; Milej, D.; Kavuri, V.C.; Mesquita, R.C.; Busch, D.R.; Abramson, K.; Jiang, J.Y.; Diop, M.; Lawrence, K.S.; et al. Noninvasive continuous optical monitoring of absolute cerebral blood flow in critically ill adults. Neurophotonics 2018, 5, 1.
- Kooman, J.P.; Wieringa, F.P.; Han, M.; Chaudhuri, S.; Van Der Sande, F.M.; Usvyat, L.A.; Kotanko, P. Wearable health devices and personal area networks: Can they improve outcomes in haemodialysis patients? Nephrol. Dial. Transplant. 2020, 35, II43–II50.
- Lima, A.; Bakker, J. Noninvasive monitoring of peripheral perfusion. Intensive Care Med. 2005, 31, 1316–1326.
- Adhikari, P.; Magaña, I.B.; O’Neal, P.D. Multi-Wavelength Pulse Plethysmography for Real-Time Drug Delivery Monitoring; Coté, G.L., Ed.; SPIE: San Francisco, CA, USA, 2014; 89510p.
- Huxley, A.F.; Niedergerke, R. Structural Changes in Muscle During Contraction: Interference Microscopy of Living Muscle Fibres. Nature 1954, 173, 971–973.
More
