Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Valentina Oana buda.
Since the prevalence of heart failure (HF) increases with age, HF is now one of the most common reasons for the hospitalization of elderly people. Although the treatment strategies and overall outcomes of HF patients have improved over time, hospitalization and mortality rates remain elevated, especially in developed countries where populations are aging.
- heart failure
- treatment strategies
- HF patients
1. Mechanisms of Action of the Classical Therapy in Chronic Heart Failure Patients
Beta-blockers bind to β adrenergic receptors (β1-receptors located in the heart and kidneys; β2-receptors located in the vessels, lungs, gastrointestinal tract, liver, uterus, and skeletal muscle; β3-receptors located in the adipocytes). By binding toβ1-receptors, they block the deleterious actions of catecholamines: noradrenaline and adrenaline [18][1]. As a result, the heart rate and the contractility decrease and thus, the cardiac output and blood pressure will also decrease. As the heart rate will decrease, this will allow a longer time for diastolic filling, without typically reducing the stroke volume. Moreover, certain beta-blockers (cardioselective ones) will also reduce rennin secretion (via the blockade of β1 receptors in the juxtaglomerular apparatus), thus decreasing the severity of angiotensin II-induced vasoconstriction and aldosterone-induced volume expansion [19][2]. They are classified into noncardioselective beta-blockers (e.g., propranolol, carvedilol, and labetalol) and cardioselective beta-blockers (β1-selective, e.g., atenolol, metoprolol, bisoprolol and nebivolol). Certain beta-blockers are associated with vasodilating properties (nebivolol improves nitric oxide release, whereas carvedilol and labetalol block the α1-receptor). The vasodilating properties are beneficial because they decrease the peripheral vascular resistance, thus improving stroke volume, left ventricular function and therefore, cardiac output [18][1].
ACEIs selectively inhibit the angiotensin-converting enzyme leading to decreased angiotensin II production and, therefore, limit its negative effects, such as vasoconstriction, antidiuretic hormones, and aldosterone secretion. Moreover, ACEIs will increase the levels of the potent vasoactive peptide bradykinin, an endogenous vasodilator. Thus, ACEIs will induce vasodilatation, decreasing the total peripheral resistance (both arterial and venous) and blood pressure. In this way, they decrease the left ventricular afterload, thus increasing cardiac output and decreasing filling pressures (both left and right), which will improve pulmonary and systemic venous congestion [20][3].
ARBs work on the same angiotensin pathway, the difference being the fact that they bind to AT1 receptors located on the vascular smooth muscle, as well as in other tissues (e.g., heart) and thus, they block the damaging actions of angiotensin II. They induce less vasoconstriction and antidiuretic hormone and aldosterone secretion and lower blood pressure. Therefore, as well as ACEIs, they prevent damage to the vasculature, heart and kidneys [20][3].
As in some cases, the ACEIs or ARBs do not suppress the excessive formation of aldosterone sufficiently, patients with moderate to severe heart failure can also benefit from aldosterone antagonists. MRAs work by competitively blocking the binding of aldosterone to the mineralocorticoid receptor, thus decreasing the reabsorption of sodium and water, as well as decreasing the excretion of potassium, leading to cardioprotective effects [21][4].
Loop diuretics act by inhibiting the luminal sodium-potassium chloride cotransporter located in the thick ascending limb of the loop of Henle, where approximately 20–30% of the filtration of sodium occurs. Therefore, compared with other diuretics, loop diuretics reduce the reabsorption of a much greater proportion of sodium, leading to the excretion of it, alongside water. This will decrease the plasma volume, cardiac workload, and oxygen demand, thereby relieving the signs and symptoms of volume excess. They are currently used to relieve symptoms associated with pulmonary congestion and peripheral edema in HF patients [22][5].
If the patient is intolerant to ACEIs/ARBs/ARNIs, other vasodilators can be used, such as isosorbide dinitrate or hydralazine. Isosorbide dinitrate acts by releasing nitric oxide into the vascular smooth muscle cell, which activates guanylyl cyclase (an enzyme that catalyzes the formation of cyclic guanosine monophosphate-cGMP from guanosine triphosphate–GTP). Therefore, the increased intracellular cGMP will activate a series of reactions, which will decrease the intracellular calcium and thus, the contractility of vascular smooth muscle, leading to smooth muscle relaxation and vasodilatation. Hydralazine also acts on the vascular smooth muscle, with multiple effects such as the stimulation of nitric oxide release from the vascular endothelium (with cGMP production and low-intracellular calcium concentration), opening of potassium channels and inhibition of calcium release from the sarcoplasmic reticulum, thus inducing smooth muscle relaxation and subsequent vasodilatation [23][6].
Digoxin increases cardiac muscle cells’ contractility by inhibiting Na+/K+/ATPaze pump in the cardiac muscle, a pump responsible for moving sodium ions out of the cells and bringing potassium ions into the cells. When sodium concentrations in the cardiac cell increases, another electrolyte mover known as the sodium-calcium exchanger pushes the excess of the sodium ions out, while bringing additional calcium ions in. Therefore, the intracellular calcium increases, which will later increase the force of contraction and thus the cardiac output. Cardiac output increases followed by a decrease in ventricular filling pressures. Moreover, it inhibits the atrio-ventricular node, by stimulating the parasympathetic nervous system. Therefore, it diminishes the electrical conduction in the AV node and thus the heart rate. However, it has not been shown to reduce mortality [24][7].
Ivabradine acts by blocking the If current channel, responsible for the cardiac peacemaker, which regulates the heart rate. In this way, it prolongs the diastolic time and decreases the heart rate without affecting myocardial contraction/relaxation or ventricular repolarization [25][8].
2. New Approaches in Heart Failure Pharmacological Treatment
2.1. Sacubitril/Valsartan
The combination of sacubitril and valsartan is the first from the class of angiotensin receptor–neprilysin inhibitors (ARNI). Agents in this new therapeutic class (sacubitril/valsartan) act at the level of RAAS and the neutral endopeptidase system. Sacubitril acts by inhibiting neprilysin and slowing down the degradation of natriuretic peptides, bradykinin, adrenomedullin, and other peptides [26][9]. It is indicated in chronic symptomatic heart failure with reduced ejection fraction [27][10].
Sacubitril/valsartan also improves symptom severity and heart functionality in patients with HFpEF, reducing the serum levels of the biomarker NT-pro BNP (and increasing BNP), an indicator of heart failure severity, and improves quality of life after 24 weeks [28][11].
One of the largest HF trials ever performed (PARADIGM-HF trial) compared enalapril with sacubitril/valsartan. In this trial, 8442 patients with HFrEF with FEVS ≤ 40% were enrolled and randomly received enalapril or sacubitril/valsartan twice daily. The trial was stopped early after 27 months because sacubitril/valsartan met the pre-specified stopping endpoint for an overwhelming benefit. All of the outcomes showed a 20% lower event rate in favor of sacubitril/valsartan; even the death rate from any cause was 16% lower in the group receiving sacubitril/valsartan [29][12]. ARNIs have been associated with improvements in diastolic function, left ventricular function, quality of life and decrease in ventricular arrythmias [30,31][13][14].
In the PROVE-HF and EVALUATE-HF trials, sacubitril/valsartan showed efficacy in improving the structural and functional changes that occur during heart failure. It improves cardiac remodeling and decreases the biomarker NT-pro BNP, so the drug reverses the damage to the heart in HFrEF patients [32][15].
Sacubitril/valsartan is recommended to replace ACE inhibitors when HFrEF patients are still symptomatic after optimal therapy. When initiating therapy with sacubitril/valsartan, there are some safety issues, including symptomatic hypotension, angioedema, and risk of hyperkalemia, so monitoring blood pressure levels, kidney function, and kalemia is extremely important [27,33][10][16]. Although the new combination was approved for the market starting from 2015, it is currently still underused, despite its proven benefits [34][17].
Figure 1 presents the mechanism of action of sacubitril/valsartan association and its consequences [27,28,29,30,31,32,33,34].
presents the mechanism of action of sacubitril/valsartan association and its consequences [10][11][12][13][14][15][16][17].
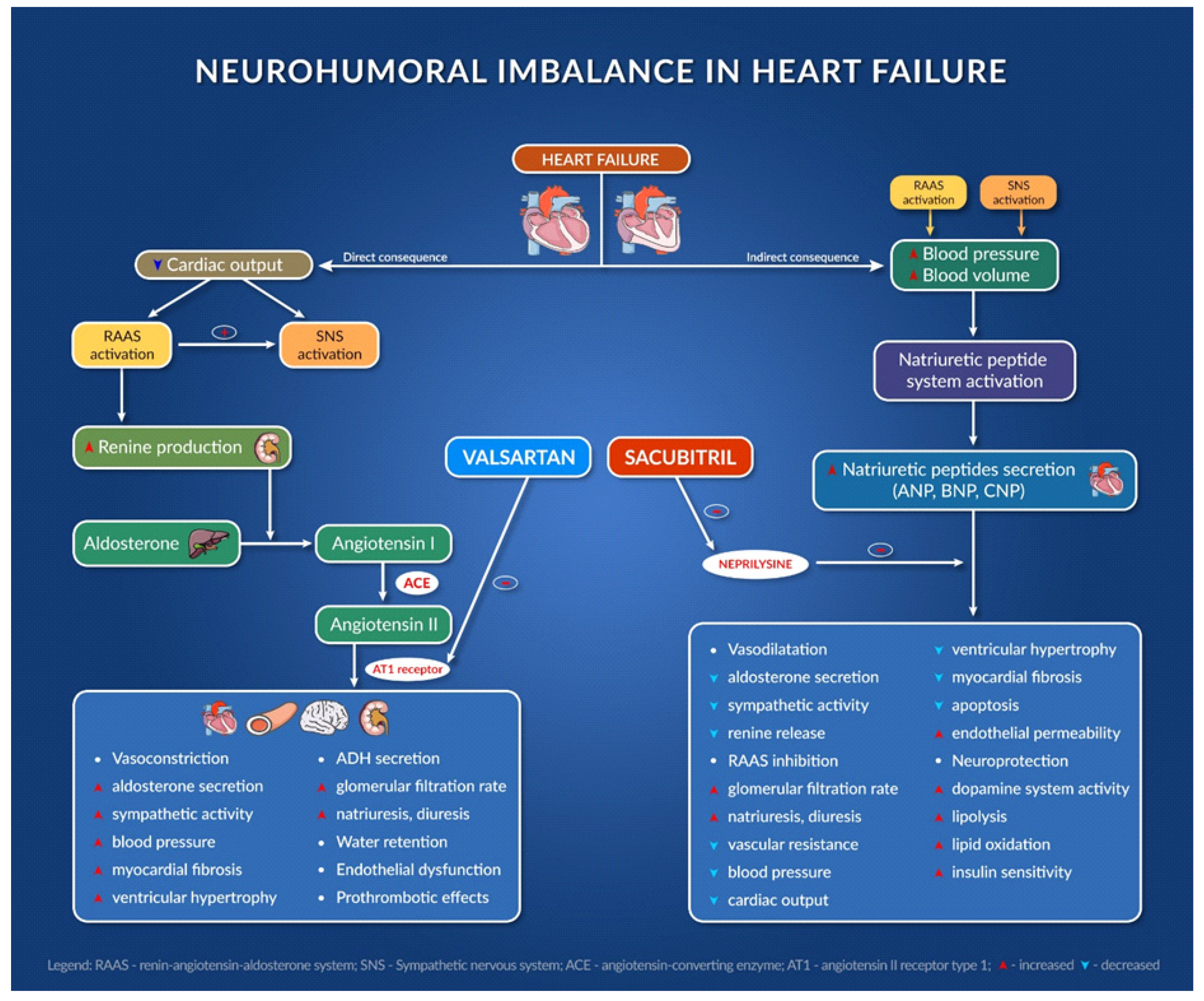
Figure 1.
Neurohumoral imbalance in heart failure.
2.2. Sodium Glucose Co-Transporter-2 Inhibitors
It is known that patients with type 2 diabetes mellitus (T2DM) are prone to developing cardiovascular events and heart failure, which can lead to high rates of hospitalization and premature mortality [35][18].
A new class of antidiabetics, sodium glucose co-transporter-2 (SGLT2) inhibitors, has also been found to have beneficial effects in patients with cardiac diseases [36,37][19][20]. The compounds in this class are represented by empagliflozin, dapagliflozin, canagliflozin, and ertugliflozin [38][21]. They act by inhibiting glucose transport in the proximal tube of the kidney, resulting in glucosuria and, as a result, lower blood glucose levels [35][18].
Aside from the direct mechanism of action on glucose control, other indirect mechanisms are taken into account regarding possible cardiovascular benefits [39][22].
In Figure 2, wresearchers summarize the possible mechanisms involved, their actions, and their effect on the heart [39,40][22][23].
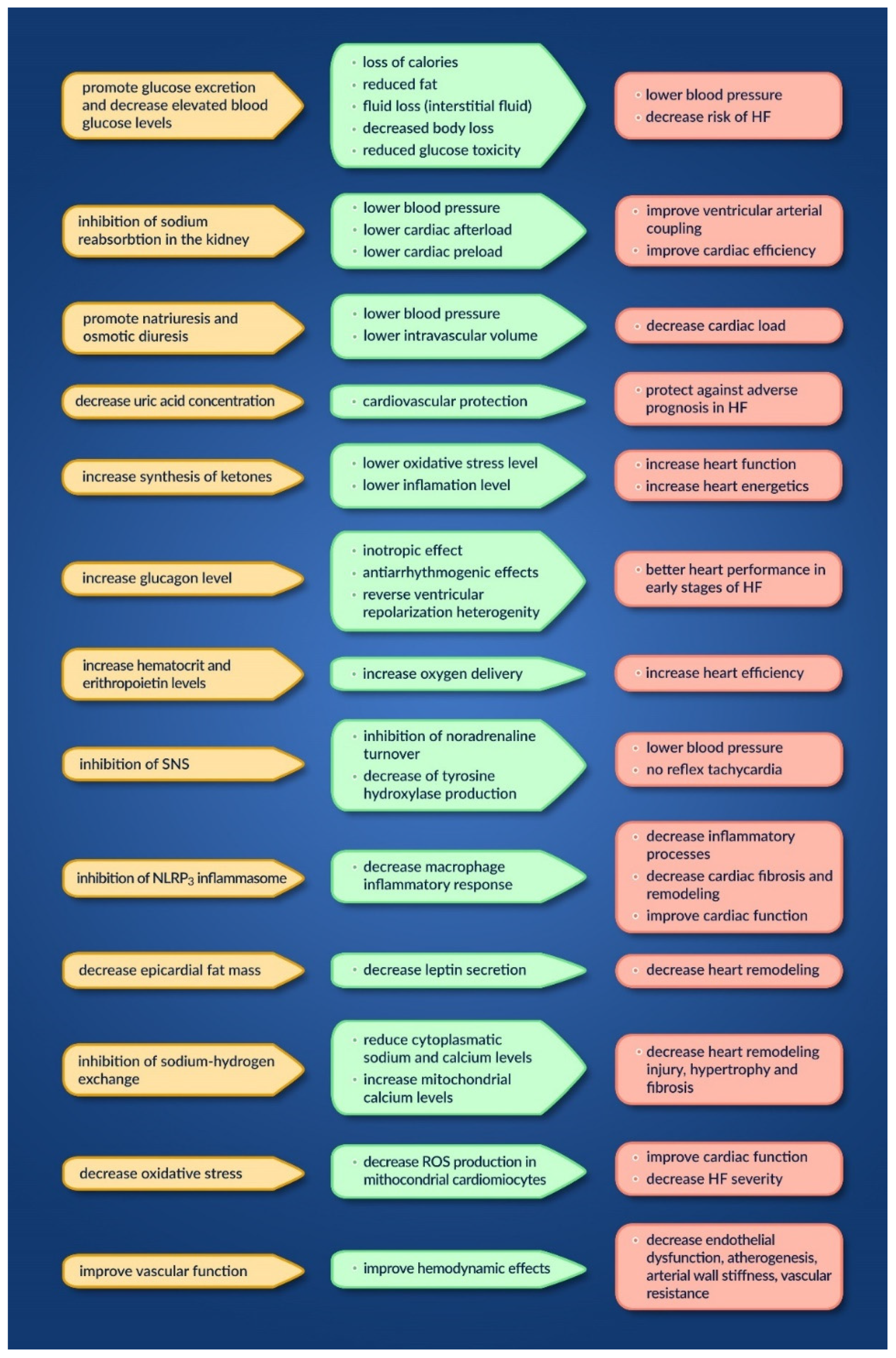
Figure 2.
Mechanisms, actions and effects of SGLT2 inhibitors on the heart.
The main trials reporting the benefits of SGLT2 inhibitors in HF patients with reduced EF, more precisely of dapagliflozin and empagliflozin, are as follows: DAPA-HF [41][24], DEFINE-HF [42][25] and EMPEROR-reduced [43][26].
The DAPA-HF trial evaluated the long-term effects of dapagliflozin on the incidence of cardiovascular death or HF hospitalization, regardless of the presence of diabetes. It was a phase 3 randomized placebo-controlled study, enrolling 4744 patients suffering from chronic HFrEF (NYHA class II-IV, LVEF ≤ 40% in addition to the recommended HF therapy, NT-proBNP high and eGFR ≥ 30 mL/min/1.73 m2) and having a median period of 18 months. The obtained results were as follows: a reduction in all-cause mortality and HF symptom aggravation, and the improvement in physical condition and overall quality of life. Their excellent benefits were seen very soon after starting the treatment with dapagliflozin. Regarding the incidence of adverse effects, they were attributed to volume depletion, renal dysfunction or hypoglycemia, but they did not differ between the studied groups [41][24].
The DEFINE-HF trial assessed the effect of dapagliflozin on the symptoms and biomarker plasmatic concentration of HFrEF patients (NYHA class II-III, LVEF ≤ 40%, eGFR ≥ 30 mL/min/1.73 m2 and with elevated natriuretic peptides). In total, 263 patients were included (taking either dapagliflozin 10 mg/once, daily, or a placebo, for a period of 12 weeks, in addition to the recommended HF therapy). Dapagliflozin induced an improvement in the patients’ health conditions or in their natriuretic peptides’ plasmatic concentrations [42][25].
The EMPEROR-reduced clinical trial evaluated the outcome of empagliflozin in patients with chronic HFrEF (NYHA class II-IV, LVEF ≤ 40%, eGFR ≥ 20 mL/min/1.73 m2). It was a double-blind clinical trial involving 3730 patients who received either empagliflozin (10 mg/once daily) or a placebo, in addition to the recommended HF therapy, for a median period of 18 months. Cardiovascular death and hospitalization rates (due to the worsening of HF) were reduced by empagliflozin, regardless of the presence of diabetes mellitus. The annual decline in the renal filtration rate was reduced, as well as the severity of renal complications. Non-complicated fungal infections of the genital tract were reported more often in patients taking empagliflozin [43][26].
Therefore, both substances are included as recommend treatments for HFrEF patients by the American and European guidelines [1,17][27][28].
The EMPEROR-preserved study assessed the effects of empagliflozin in patients with chronic HFpEF (NYHA class II-IV, LVEF ≥ 40%, eGFR ≥ 20 mL/min/1.73 m2). In total, 5988 patients were included, who were randomized 1:1 and received either empagliflozin (10 mg/once daily) or a placebo, in addition to their classical HF therapy. Over a period of 26.2 months, the primary outcome was obtained (decreased risk of hospitalization in HF patients, regardless of the presence/absence of diabetes). Beneficial effects were also seen in eGFR, without considering the renal outcomes by themselves. It is important to note the fact that the most used medicines for HFrEF have not shown benefits in patients with HFpEF; therefore, empagliflozin is superior in improving HF outcomes even in patients with HFpEF, which are symptomatic and stable [44,45][29][30].
In Table 1, reseachers summarize the indications, contra-indications and cautions worth considering for ARNI and SGLT2 inhibitors [17][28].
Table 1.
The indications, contra-indications and cautions for ARNI and SGLT2 inhibitors.
| ARNI | SGLT2 Inhibitors | |
|---|---|---|
| Indications | ▪ HFrEF (≤40%) ▪ NYHA class II-IV ▪ Alternative of ACEI/ARB |
▪ HFrEF (≤40%) ± diabetes mellitus ▪ NYHA class II-IV |
| Contra-indications | - hypersensitivity to the active substances - history of angioedema - severe hepatic impairment - ≤36 h of the last ACEI dose |
- hypersensitivity to the active substance - type I diabetes - dialysis - eGFR < 30 mL/min/1.73 m2 (dapagliflozin) - eGFR < 20 mL/min/1.73 m2 (empagliflozin) |
| Cautions | ◊ severe renal impairment (starting dose: 24/26 mg × 2/day) ◊ moderate hepatic impairment (starting dose: 24/26 mg × 2/day) ◊ SBP < 100 mmHg ◊ volume depletion ◊ renal artery stenosis ◊ pregnancy/lactation |
◊ high risk of genital infections (especially mycotic) and urinary infections ◊ hypovolemia ◊ ketoacidosis ◊ acute renal impairment ◊ necrotizing fasciitis of the perineum (Fournier gangrene) ◊ bladder cancer ◊ pregnancy |
3. Treatment Strategies in Heart Failure Patients
For the treatment of heart failure with preserved ejection fraction or with mildly reduced ejection fraction (LVEF ≥ 50% or LVEF between 40 and 49), the guidelines recommend the prescription of diuretics, as first line therapy [1][27]. The other drugs (ACEI or ARB, beta-blockers or MRA) may be considered as a second alternative [1][27].
The treatment strategy also focuses on treating co-morbidities such as: hypertension, atrial fibrillation, cardiac ischemic disease, pulmonary hypertension, diabetes mellitus, chronic kidney disease, COPD (chronic obstructive pulmonary disease), anemia and obesity. The optimal management of co-morbidities has been shown to improve symptoms and to improve the patient’s quality of life [2][31].
In the case of congestion, diuretics will be very effective and will improve the symptomatology. There is proof that nebivolol, candesartan, digoxin and spironolactone might reduce hospitalization for patients with HFpEF in sinus rhythm [46][32]. Moreover, besides empagliflozin, none of other drugs consistently met their primary endpoint in the clinical trials that were performed, and none reduced mortality and morbidity [44,45][29][30].
For patients in atrial fibrillation, the prescription of an anticoagulant is very important for reducing thrombo-embolic events [47][33]. For the control of heart rate, the use of digoxin, beta-blockers or verapamil/diltiazem is recommended, targeting an optimal rate control between 60 and 100 bpm [48][34].
Amiodarone and non-dihydropyridine calcium-channel blockers (CCB) are able to reduce heart rate, but due to their adverse effects profile, they should be replaced, if possible. In the case of a fast ventricular rate and symptoms, it might be appropriate to consider AV node ablation, and if there are indications for ICD (implantable cardioverter-defibrillator), AV node ablation with the implantation of CRT-D (cardiac resynchronization therapy–defibrillator) might be preferred. The rhythm control strategy has not been shown to be superior to the rate control strategy. Urgent cardioversion is indicated if atrial fibrillation is life threatening [49][35].
Regarding HFrEF treatment, the evidence base for drug treatment in HF is for HFrEF. Either an ACEI/ARB/ARNI or a beta-blocker should be started (sometimes also ACEI/ARB/ARNI and beta-blocker at the same time), with doses up-titrated to the maximum tolerated/targeted dose every 2 weeks. ACEI, beta-blockers and MRA proved to improve survival and are recommended for the treatment of every patient with HFrEF. The new ARNI (sacubitril/valsartan) has been shown to be superior to ACEI in reducing the risk of death and hospitalization. Thus, ARNI is recommended to replace ACEI in cases of HFrEF patients if they are symptomatic despite optimal therapy [26][9].
In the case of decompensated patients, beta-blockers should not be initiated or if already initiated but patients develop worsening of HF symptoms (e.g., fatigue, dyspnea, dizziness or erectile dysfunction) caution should be applied regarding their prescription. Moreover, in the case of frailty or other complications (e.g., marginal hemodynamics), a longer period of time may be required for dose up-titration [17][28].
ARNI can be prescribed as an alternative to ACEI/ARB intolerance (e.g., angioedema) or in the absence of hypotension, electrolyte or renal imbalance. It is recommended to avoid the association of an ARNI with an ACEI and if previously administered ACEI, to ensure a 36 h washout period before the initiation of an ARNI, due to the high risk of angioedema [50][36]. This delay is not required when switching from ARB to ARNI. When up-titrating ARNI/ACEI/ARB (every 2 weeks or more), the monitoring of the potassium level, renal function and blood pressure is required. Lower loop diuretic doses may be necessary for the optimal titration of ARNI/ACEI/ARB and caution regarding the potassium concentration is required, as well as the dietary restriction of/supplementation with potassium, as the kaliuretic effect of loop diuretics might no longer be present [17][28].
If the patients have LVEF ≤ 35%, the guidelines recommend the use of MRAs to reduce mortality and hospitalization. MRAs (e.g., spironolactone or eplerenone) are added in patients with symptomatic chronic HFrEF, as a triple therapy (ACEI/ARB/ARNI + beta-blockers + MRA), in the absence of contra-indications. It is essential to achieve the targeted dose of other drugs before initiating the treatment with an aldosterone antagonist and to monitor the potassium levels and renal function under the treatment [17][28].
SGLT2 inhibitors can also be added, as part of the quadruple therapy (ACEI/ARB/ARNI + beta-blocker + MRA + SGLT2 inhibitor), in the absence of contra-indications. There is no need to achieve targeted doses of other drugs before adding SGLT2 inhibitors, although the loop diuretic dose might require adjustments based on the close monitoring of symptoms and weight [17][28].
Isosorbide dinitrate/Hydralazine could be prescribed especially for African American patients once the targeted dose of ACEI/ARB/ARNI + beta-blockers + MRA has been achieved [17][28].
The If channel inhibitor ivabradine is recommended in patients with symptomatic HFrEF or LVEF ≤ 35%, in sinus rhythm and heart rate ≥ 70 bpm, and in patients that have been hospitalized for HF in the last year, despite receiving beta-blockers at the maximum tolerated dose, ACEI and an MRA. The titration of the dose should be performed every 2 weeks in order to decrease the heart rate. In the case of patients ≥ 75 years old or in those with a history of conduction defects, the recommended initial dose is 2.5 mg twice daily, administered with meals [17][28].
References
- Oliver, E.; Mayor, F., Jr.; D’Ocon, P. Beta-blockers: Historical Perspective and Mechanisms of Action. Rev. Española Cardiol. 2019, 72, 853–862, (In English and Spanish).
- Bie, P.; Mølstrøm, S.; Wamberg, S. Normotensive sodium loading in conscious dogs: Regulation of renin secretion during beta-receptor blockade. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 296, R428–R435.
- Sayer, G.; Bhat, G. The renin-angiotensin-aldosterone system and heart failure. Cardiol. Clin. 2014, 32, 21–32.
- Bruno, N.; Sinagra, G.; Paolillo, S.; Bonomi, A.; Corrà, U.; Piepoli, M.; Veglia, F.; Salvioni, E.; Lagioia, R.; Metra, M.; et al. Mineralocorticoid receptor antagonists for heart failure: A real-life observational study. ESC Heart Fail. 2018, 5, 267–274.
- Casu, G.; Merella, P. Diuretic Therapy in Heart Failure-Current Approaches. Eur. Cardiol. 2015, 10, 42–47.
- Levine, T.B. Role of vasodilators in the treatment of congestive heart failure. Am. J. Cardiol. 1985, 55, 32A–35A.
- David, M.N.V.; Shetty, M. Digoxin Toxicity. In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556025/ (accessed on 25 January 2022).
- Reed, M.; Kerndt, C.C.; Nicolas, D. Ivabradine. In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507783/ (accessed on 25 January 2022).
- Yancy, C.; Jessup, M.; Butler, J.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161.
- Sauer, A.J.; Cole, R.; Jensen, B.C.; Pal, J.; Sharma, N.; Yehya, A.; Vader, J. Practical guidance on the use of sacubitril/valsartan for heart failure. Heart Fail. Rev. 2019, 24, 167–176.
- Chandra, A.; Lewis, E.; Claggertt, B.; Desai, A.S.; Packer, M.; Zile, M.R.; Swedberg, K.; Rouleau, J.L.; Shi, V.C.; Lefkowitz, M.P.; et al. The Effects of Sacubitril/Valsartan on Physical and Social Activity Limitations in Heart Failure Patients: The PARADIGM-HF Trial. JAMA Cardiol. 2018, 3, 498–505.
- Velazquez, E.; Morrow, D.; DeVore, A.; Duffy, C.I.; Ambrosy, A.P.; McCague, K.; Rocha, R.; Braunwald, E.; PIONEER-HF Investigators. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N. Engl. J. Med. 2019, 380, 539–548.
- Myhre, P.L.; Vaduganathan, M.; Claggett, B.; Packer, M.; Desai, A.S.; Rouleau, J.L.; Zile, M.R.; Swedberg, K.; Lefkowitz, M.; Shi, V.; et al. B-type natriuretic peptide during treatment with sacubitril/valsartan: The PARADIGM-HF trial. J. Am. Coll. Cardiol. 2019, 73, 1264–1272.
- Lewis, E.F.; Claggett, B.L.; McMurray, J.J.V.; Packer, M.; Lefkowitz, M.P.; Rouleau, J.L.; Liu, J.; Shi, V.C.; Zile, M.R.; Desai, A.S.; et al. Health-related quality of life outcomes in PARADIGM-HF. Circ. Heart Fail. 2017, 10, e003430.
- Seferovic, P.; Ponikowski, P.; Anker, S.; Bauersachs, J.; Chioncel, O.; Cleland, J.G.F.; de Boer, R.A.; Drexel, H.; Ben Gal, T.; Hill, L.; et al. Clinical practice update on heart failure 2019: Pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of The Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 1169–1186.
- Gillette, M.; Bozkurt, B. Ins and Outs: Perspectives of Inpatient Prescribing for Sacubitril/Valsartan. Ann. Pharmacother. 2020, 55, 805–813.
- Byrne, D.; Fahey, T.; Moriarty, F. Efficacy and safety of sacubitril/valsartan in the treatment of heart failure: Protocol for a systematic review incorporating unpublished clinical study reports. HRB Open Res. 2020, 3, 5.
- Scheen, A.J. Sodium-glucose cotransporter type 2 inhibitors for the treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 556–577.
- Al Hamed, F.A.; Elewa, H. Potential Therapeutic Effects of Sodium Glucose-linked Cotransporter 2 Inhibitors in Stroke. Clin. Ther. 2020, 42, e242–e249.
- Ling, A.W.; Chan, C.C.; Chen, S.W.; Kao, Y.W.; Huang, C.Y.; Chan, Y.H.; Chu, P.H. The risk of new-onset atrial fibrillation in patients with type 2 diabetes mellitus treated with sodium glucose cotransporter 2 inhibitors versus dipeptidyl peptidase-4 inhibitors. Cardiovasc. Diabetol. 2020, 19, 188.
- Lee, H.C.; Shiou, Y.L.; Jhuo, S.J.; Chang, C.Y.; Liu, P.L.; Jhuang, W.J.; Dai, Z.K.; Chen, W.Y.; Chen, Y.F.; Lee, A.S. The sodium-glucose co-transporter 2 inhibitor empagliflozin attenuates cardiac fibrosis and improves ventricular hemodynamics in hypertensive heart failure rats. Cardiovasc. Diabetol. 2019, 18, 45.
- GrubićRotkvić, P.; CigrovskiBerković, M.; Bulj, N.; Rotkvić, L. Minireview: Are SGLT2 inhibitors heart savers in diabetes? Heart Fail. Rev. 2020, 25, 899–905.
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl. Sci. 2020, 5, 632–644.
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008.
- Nassif, M.E.; Windsor, S.L.; Tang, F.; Khariton, Y.; Husain, M.; Inzucchi, S.E.; McGuire, D.K.; Pitt, B.; Scirica, B.M.; Austin, B.; et al. Dapagliflozin Effects on Biomarkers, Symptoms, and Functional Status in Patients with Heart Failure with Reduced Ejection Fraction: The DEFINE-HF Trial. Circulation 2019, 140, 1463–1476.
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424.
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726.
- Writing Committee; Maddox, T.M.; Januzzi, J.L., Jr.; Allen, L.A.; Breathett, K.; Butler, J.; Davis, L.L.; Fonarow, G.C.; Ibrahim, N.E.; Lindenfeld, J.; et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure with Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 77, 772–810.
- Anker, S.D.; Butler, J.; Filippatos, G.; Shahzeb Khan, M.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; EMPEROR-Preserved Trial Committees and Investigators; et al. Baseline characteristics of patients with heart failure with preserved ejection fraction in the EMPEROR-Preserved trial. Eur. J. Heart Fail. 2020, 22, 2383–2392.
- Packer, M.; Butler, J.; Zannad, F.; Filippatos, G.; Ferreira, J.P.; Pocock, S.J.; Carson, P.; Anand, I.; Doehner, W.; Haass, M.; et al. Effect of Empagliflozin on Worsening Heart Failure Events in Patients with Heart Failure and Preserved Ejection Fraction: EMPEROR-Preserved Trial. Circulation 2021, 144, 1284–1294.
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975.
- Gazewood, J.D.; Turner, P.L. Heart failure with preserved ejection fraction: Diagnosis and management. Am. Fam. Physician 2017, 96, 582–588.
- Mulder, B.A.; Schnabel, R.B.; Rienstra, M. Predicting the future in patients with atrial fibrillation: Who develops heart failure? Eur. J. Heart Fail. 2013, 15, 366–367.
- Ouyang, A.J.; Lv, Y.N.; Zhong, H.L.; Wen, J.H.; Wei, X.H.; Peng, H.W.; Zhou, J.; Liu, L.L. Meta-analysis of digoxin use and risk of mortality in patients with atrial fibrillation. Am. J. Cardiol. 2015, 115, 901–906.
- Van Gelder, I.C.; Haegeli, L.M.; Brandes, A.; Heidbuchel, H.; Aliot, E.; Kautzner, J.; Szumowski, L.; Mont, L.; Morgan, J.; Willems, S.; et al. Rationale and current perspective for early rhythm control therapy in atrial fibrillation. Europace 2011, 13, 1517–1525.
- Packer, M.; Califf, R.M.; Konstam, M.A.; Krum, H.; McMurray, J.J.; Rouleau, J.L.; Swedberg, K. Comparison of omapatrilat and enalapril in patients with chronic heart failure: The Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation 2002, 106, 920–926.
More
