Wood modification is an excellent and increasingly used method to expand the application of woody materials. Traditional methods, such as chemical or thermal, have been developed for the targeted improvement of some selected properties, unfortunately typically at the expense of others. These methods generally alter the composition of wood, and thus its mechanical properties, and enhance dimensional stability, water resistance, or decrease its susceptibility to microorganisms. Although conventional methods achieve the desired properties, they require a lot of energy and chemicals, therefore research is increasingly moving towards more environmentally friendly processes. The advantage of modern methods is that in most cases, they only modify the surface and do not affect the structure and mechanical properties of the wood, while reducing the amount of chemicals used. Cold plasma surface treatment is one of the cheapest and easiest technologies with a limited burden on the environment
- wood modification
- cold plasma
- Plasma
1. Plasma
1.1. Atmospheric Plasma Treatment Applications
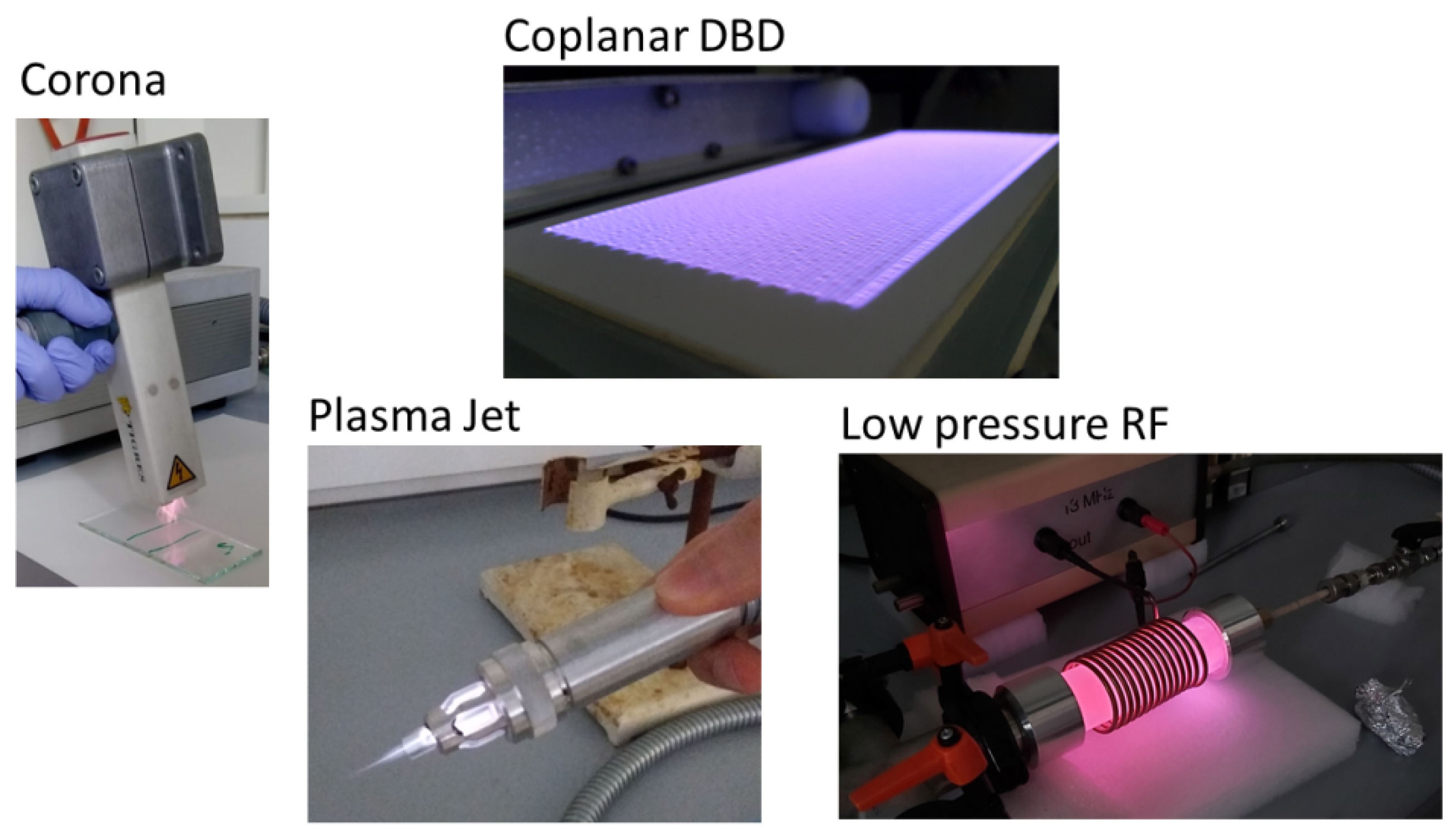
-
Corona
-
Dielectric Barrier Discharge (DBD)
-
Plasma jet
-
Radio frequency (RF) and microwave (MW) plasmas
1.2. Low-Pressure Plasma Treatment Applications
2. Plasma Surface Interaction
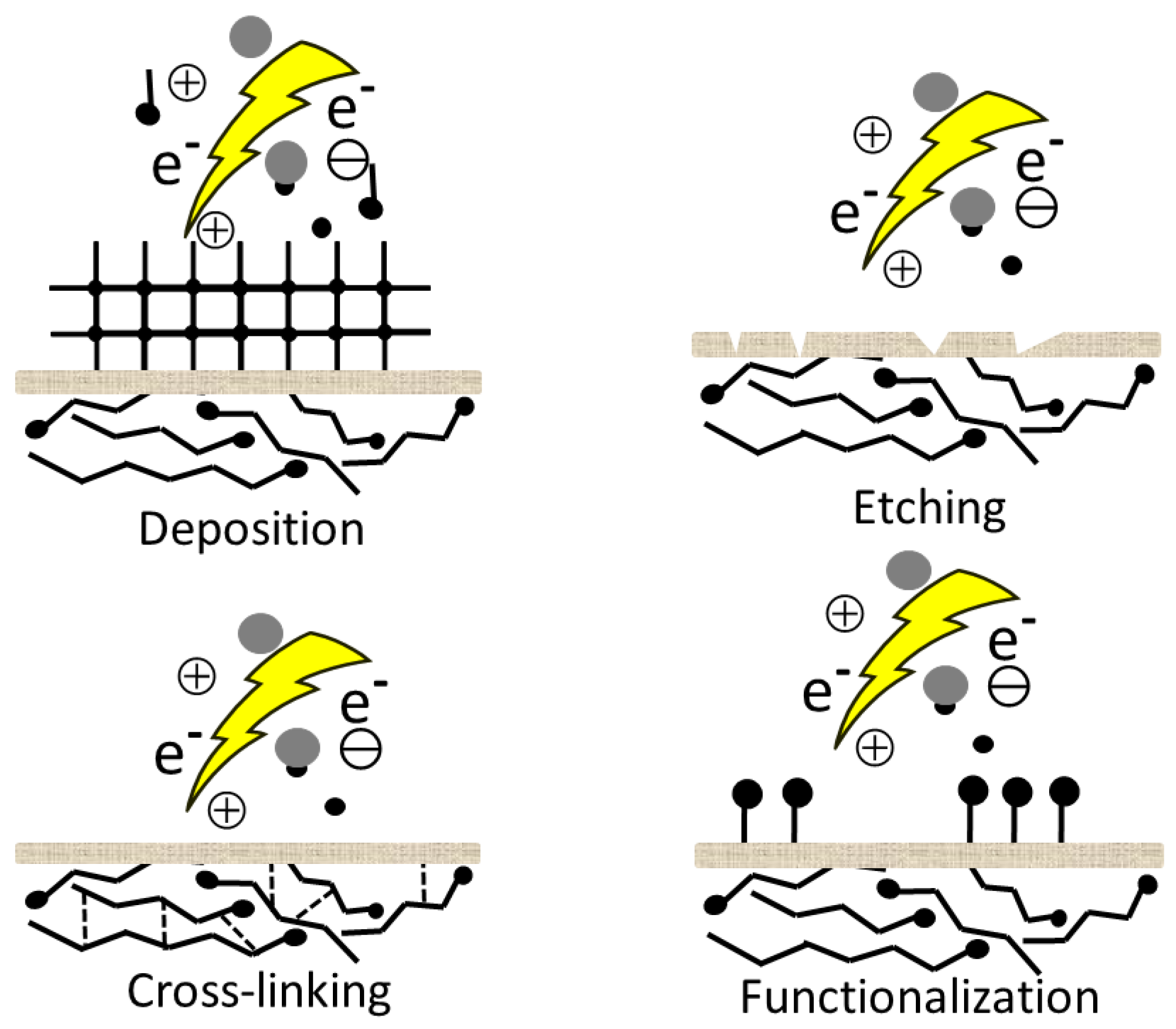
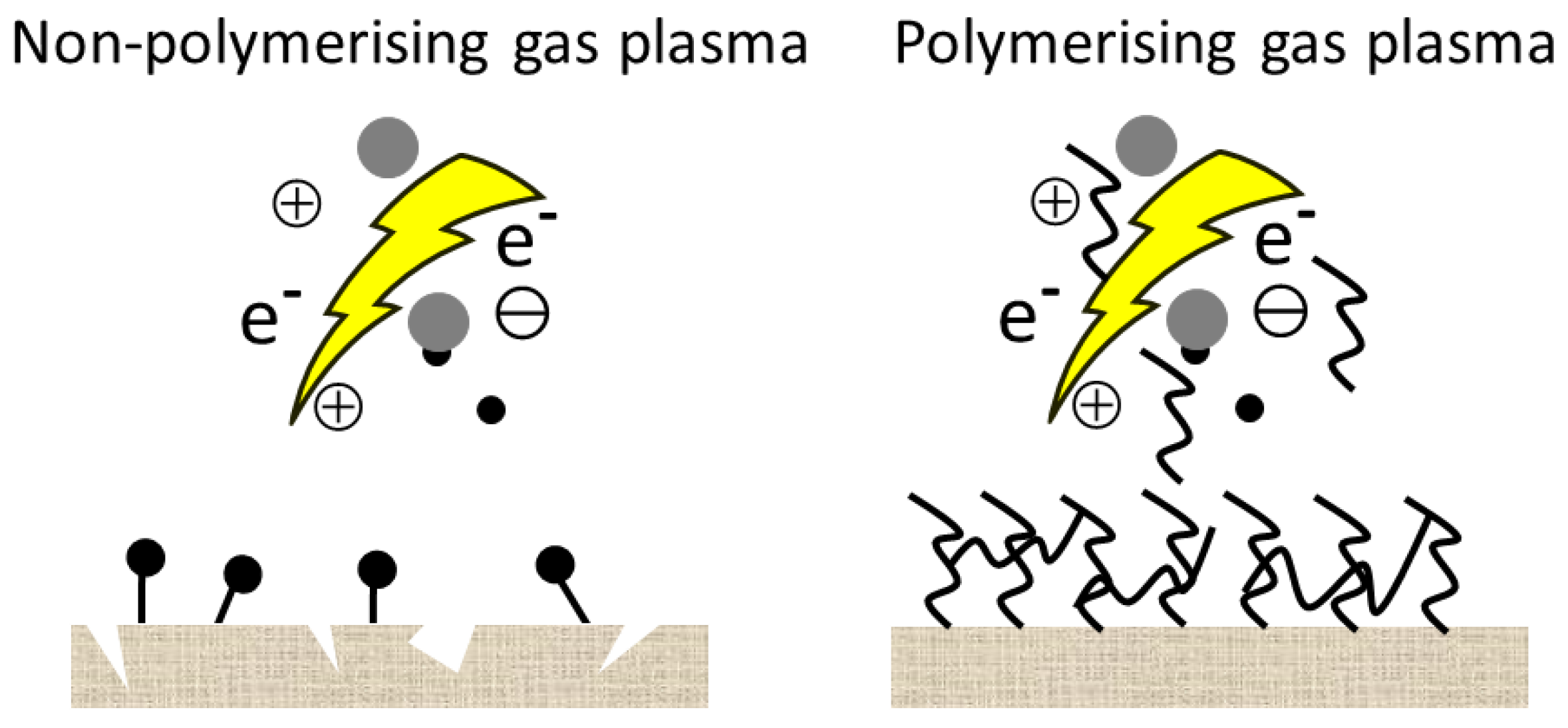
2.1. Surface Cleaning
2.2. Functionalization
2.3. Radicals, Double Bonds and Cross-Linking
2.4. Etching
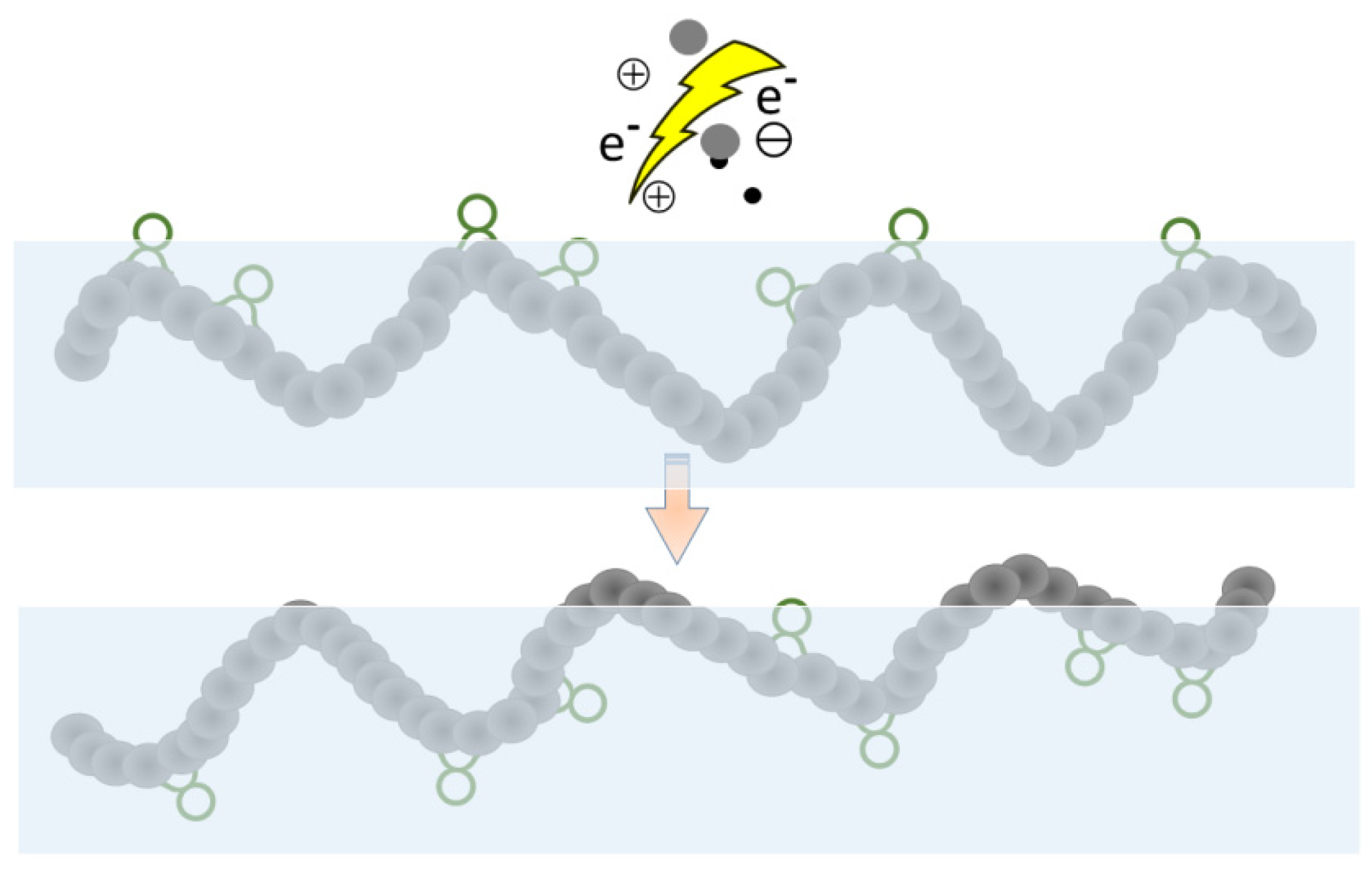
3. Characterization of the Effectiveness of Plasma Treatment
3.1. X-Ray PShotoelectron Spectroscopy, Fourier Transform Infrared Spectroscopy (FTIR)
3.2. Scanning Electron Microscopy
3.3. Imaging X-Ray PShotoelectron Spectroscopy and Scanning AEuger Electron Spectroscopy
3.4. Atomic Force Microscopy
References
- Bárdos, L.; Baránková, H. Cold atmospheric plasma: Sources, processes, and applications. Thin Solid Films 2010, 518, 6705–6713.
- Aggelopoulos, C.A. Recent advances of cold plasma technology for water and soil remediation: A critical review. Chem. Eng. J. 2022, 428, 131657.
- Rane, R.; Ranjan, M.; Mukherjee, S. Basics of plasma and its industrial applications in textiles. In Plasma Technologies for Textile and Apparel; Nema, S.K., Jhala, P.B., Eds.; Taylor & Francis: New Delhi, India, 2015; ISBN 9789380308951.
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: A review. Biomacromolecules 2009, 10, 2351–2378.
- Chu, P. Plasma-surface modification of biomaterials. Mater. Sci. Eng. R Rep. 2002, 36, 143–206.
- Bogaerts, A.; Neyts, E.; Gijbels, R.; Van der Mullen, J. Gas discharge plasmas and their applications. Spectrochim. Acta-Part B At. Spectrosc. 2002, 57, 609–658.
- Kócs, L.; Késmárki, A.; Klébert, S.; Madarász, J.; Hórvölgyi, Z. Plasma-assisted template removal and consolidation of silica coatings on polycarbonate. Thin Solid Films 2021, 738, 138976.
- Károly, Z.; Románszki, L.; Weltz, G.; Mohai, M.; Móczó, J.; Klébert, S. Comparison of dielectric barrier discharge and radio-frequency plasma processing of carbon fibers. Express Polym. Lett. 2021, 15, 1004–1017.
- Gomez, E.; Rani, D.A.; Cheeseman, C.R.; Deegan, D.; Wise, M.; Boccaccini, A.R. Thermal plasma technology for the treatment of wastes: A critical review. J. Hazard. Mater. 2009, 161, 614–626.
- Okumoto, M.; Mizuno, A. Conversion of methane for higher hydrocarbon fuel synthesis using pulsed discharge plasma method. Catal. Today 2001, 71, 211–217.
- Plattfaut, I.; Besser, M.; Severing, A.L.; Stürmer, E.K.; Opländer, C. Plasma medicine and wound management: Evaluation of the antibacterial efficacy of a medically certified cold atmospheric argon plasma jet. Int. J. Antimicrob. Agents 2021, 57, 106319.
- Demir, A. Atmospheric plasma advantages for mohair fibers in textile applications. Fibers Polym. 2010, 11, 580–585.
- Pavliňák, D.; Galmiz, O.; Pavliňáková, V.; Poláček, P.; Kelar, J.; Stupavská, M.; Černák, M. Application of dielectric barrier plasma treatment in the nanofiber processing. Mater. Today Commun. 2018, 16, 330–338.
- Pykönen, M.; Johansson, K.; Dubreuil, M.; Vangeneugden, D.; Ström, G.; Fardim, P.; Toivakka, M. Evaluation of plasma-deposited hydrophobic coatings on pigment-coated paper for reduced dampening water absorption. J. Adhes. Sci. Technol. 2010, 24, 511–537.
- Radetić, M.; Marković, D. A review on the role of plasma technology in the nano-finishing of textile materials with metal and metal oxide nanoparticles. Plasma Process. Polym. 2022, e2100197.
- Laroque, D.A.; Seó, S.T.; Valencia, G.A.; Laurindo, J.B.; Carciofi, B.A.M. Cold plasma in food processing: Design, mechanisms, and application. J. Food Eng. 2022, 312, 110748.
- Černák, M.; Černáková, L.; Hudec, I.; Kováčik, D.; Zahoranová, A. Diffuse coplanar surface barrier discharge and its applications for in-line processing of low-added-value materials. EPJ Appl. Phys. 2009, 47, 22806.
- Galmiz, O.; Tucekova, Z.K.; Kelar, J.; Zemanek, M.; Stupavska, M.; Kovacik, D.; Cernak, M. Effect of atmospheric pressure plasma on surface modification of paper. AIP Adv. 2019, 9, 105013.
- Lata, S.; Chakravorty, S.; Mitra, T.; Pradhan, P.K.; Mohanty, S.; Patel, P.; Jha, E.; Panda, P.K.; Verma, S.K.; Suar, M. Aurora Borealis in dentistry: The applications of cold plasma in biomedicine. Mater. Today Bio 2022, 13, 100200.
- Puligundla, P.; Mok, C. Microwave- and radio-frequency-powered cold plasma applications for food safety and preservation. In Advances in Cold Plasma Applications for Food Safety and Preservation; Bermudez-Aguirre, D., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 309–329.
- Riedl, B.; Angel, C.; Prégent, J.; Blanchet, P.; Stafford, L. Effect of wood surface modification by atmospheric pressure plasma on waterborne coating adhesion. BioResources 2014, 9, 4908–4923.
- Dilks, A. Polymer surfaces. Anal. Chem. 1981, 53, 802A–816A.
- Lafleur, T.; Schulze, J.; Donkó, Z. Plasma-surface interactions. Plasma Sources Sci. Technol. 2019, 28, 040201.
- Kale, K.H.; Desai, A.N. Atmospheric pressure plasma treatment of textiles using non-polymerising gases. Indian J. Fibre Text. Res. 2011, 36, 289–299.
- George, J.; Sreekala, M.S.; Thomas, S. A review on interface modification and characterization of natural fiber reinforced plastic composites. Polym. Eng. Sci. 2001, 41, 1471–1485.
- Karaca, B.; Csiszár, E.; Bozdogan, F. Effects of atmospheric plasma pretreatments on pectinase efficiency in bioscouring of linen fabrics. Plasma Chem. Plasma Process. 2011, 31, 623–633.
- Dimitrakellis, P.; Gogolides, E. Atmospheric plasma etching of polymers: A palette of applications in cleaning/ashing, pattern formation, nanotexturing and superhydrophobic surface fabrication. Microelectron. Eng. 2018, 194, 109–115.
- Alanis, A.; Valdés, J.H.; María Guadalupe, N.V.; Lopez, R.; Mendoza, R.; Mathew, A.P.; Díaz De León, R.; Valencia, L. Plasma surface-modification of cellulose nanocrystals: A green alternative towards mechanical reinforcement of ABS. RSC Adv. 2019, 9, 17417–17424.
- Bertóti, I.; Mohai, M.; Tóth, A.; Ujvári, T. Nitrogen-PBII modification of ultra-high molecular weight polyethylene: Composition, structure and nanomechanical properties. Surf. Coat. Technol. 2007, 201, 6839–6842.
- Fridman, A. Plasma Chemistry; Cambridge University Press: New York, NY, USA, 2008.
- Bormashenko, E.; Chaniel, G.; Grynyov, R. Towards understanding hydrophobic recovery of plasma treated polymers: Storing in high polarity liquids suppresses hydrophobic recovery. Appl. Surf. Sci. 2013, 273, 549–553.
- Kuo, Y.L.; Kung, F.C.; Ko, C.L.; Okino, A.; Chiang, T.C.; Guo, J.Y.; Chen, S.Y. Tailoring surface properties of polyethylene terephthalate by atmospheric pressure plasma jet for grafting biomaterials. Thin Solid Films 2020, 709, 138152.
- de Farias, J.G.G.; Cavalcante, R.C.; Canabarro, B.R.; Viana, H.M.; Scholz, S.; Simão, R.A. Surface lignin removal on coir fibers by plasma treatment for improved adhesion in thermoplastic starch composites. Carbohydr. Polym. 2017, 165, 429–436.
- Altgen, D.; Avramidis, G.; Viöl, W.; Mai, C. The effect of air plasma treatment at atmospheric pressure on thermally modified wood surfaces. Wood Sci. Technol. 2016, 50, 1227–1241.
- Szabo, O.E.; Csiszar, E.; Koczka, B.; Toth, A.; Klebert, S. Enhancing the accessibility of starch size and cellulose to enzymes in raw cotton woven fabric by air-plasma pretreatment. Text. Res. J. 2016, 86, 868–877.
- Kaur, A.; Kale, D.P.; Bansal, A.K. Surface characterization of pharmaceutical solids. Trends Anal. Chem. 2021, 138, 116228.
- Bertóti, I.; Mohai, M.; László, K. Surface modification of graphene and graphite by nitrogen plasma: Determination of chemical state alterations and assignments by quantitative X-ray photoelectron spectroscopy. Carbon 2015, 84, 185–196.
- Tóth, A.; Černáková, L.; Černák, M.; Kunovská, K. Surface analysis of groundwood paper treated by diffuse coplanar surface barrier discharge (DCSBD) type atmospheric plasma in air and in nitrogen. Holzforschung 2007, 61, 528–531.
- Tang, C.Y.; Kwon, Y.N.; Leckie, J.O. Probing the nano- and micro-scales of reverse osmosis membranes-A comprehensive characterization of physiochemical properties of uncoated and coated membranes by XPS, TEM, ATR-FTIR, and streaming potential measurements. J. Memb. Sci. 2007, 287, 146–156.
- Vandencasteele, N.; Reniers, F. Plasma-modified polymer surfaces: Characterization using XPS. J. Electron Spectros. Relat. Phenom. 2010, 178–179, 394–408.
- Pejić, B.M.; Kramar, A.D.; Obradović, B.M.; Kuraica, M.M.; Žekić, A.A.; Kostić, M.M. Effect of plasma treatment on chemical composition, structure and sorption properties of lignocellulosic hemp fibers (Cannabis sativa L.). Carbohydr. Polym. 2020, 236, 116000.
- Hansmann, C.; Weichslberger, G.; Gindl, W. A two-step modification treatment of solid wood by bulk modification and surface treatment. Wood Sci. Technol. 2005, 39, 502–511.
- Ţălu, Ş. Micro and Nanoscale Characterization of Three Dimensional Surfaces: Basics and Applications; Napoca Star: Cluj-Napoca, Romania, 2015.
- Baldelli, A.; Trivanovic, U.; Sipkens, T.A.; Rogak, S.N. On determining soot maturity: A review of the role of microscopy- and spectroscopy-based techniques. Chemosphere 2020, 252, 126532.
