Parkinson’s disease (PD) is a progressive neurodegenerative disorder that is characterized by a loss of dopaminergic neurons, leading to bradykinesia, rigidity, tremor at rest, and postural instability, as well as non-motor symptoms such as olfactory impairment, pain, autonomic dysfunction, impaired sleep, fatigue, and behavioral changes.
- Parkinson's
- Parkinson's pathology
- Lewy body
- Lewy neurites
- dopamine
- neurodegeneration
- oxidative stress
1. Introduction
MoreNote:All than two centuries ago, in 1817, James Parkinson first described the disease with his namesake in his essay, An Essay on the Shaking Palsy [1]. Parkins information’s disease (PD) is a slow progressive neurodegenerative disorder that predominately affects dopaminergic neurons in the substantia nigra pars compacta (SNpc). PD is a debilitating condition caused by a mixture of genetic and environmental factors affecting various neuroanatomical sites and begins years before the diagnosis can be made [2]in this draft can be edited by authors. The early disease caAn be divided into three stages: (i) Preclinical PD, the beginning of neurodegenerative processes but lack evident signs or symptoms; (ii) prodromal PD, the presence of signs and symptoms, however insufficient to define disease; and (iii) clinical PD, with diagnosis of PD based on the presence of classical motor signs [3] . Bradykind the entry will be onlinesia, rigidity, tremor at rest, and postural instability are the cardinal signs of PD [4].only after However, non-motor symptoms such as olfactory impairment, pain, autonomic dysfunction (orthostatic hypotension, GI dysfunctions), impaired sleep, fatigue, and behavioral changes (depression, anxiety, apathy) are also observed [5]uthors edit and submit it.
21. Prevalence and Incidence of ParkinsIntroduction’s Disease
The global burden of diseases study 2017 reported that the prevalence of PD globally was 8.52 (95% uncertainty interval (UI) 7.03–10.18) million and incidence was 1.02 (95% UI 0.85–1.22) million [6]. In 2017, 0.34 (95% UI 0.32–0.35) million people died from PD [7]. The male-to-female ratios of age-standardized prevalence rates of PD were 1.40 (95% UI 1.36–1.43) in 2016 and 1.37 (95% UI 1.34–1.40) in 1990 [8]. A combination of five separate cohort studies to estimate the prevalence of PD in North America shows overall prevalence to be 572 per 100,000 (95% confidence interval 537–614) among those aged ≥45 years and that that number is projected to increase to approximately 1,238,000 in 2030 [9]. In Korea, the annual incidence of PD was between 22.4–27.8 cases per 100,000 individuals, and the female-to-male ratio in the prevalence of PD was 1.6:1 while the incidence of PD was 1.4:1 [10]. Also, other PD prevalence studies show an increase in PD steadily with age and male gender [11][12][13].
2. Prevalence and Incidence of Parkinson’s Disease
3. Symptoms and Diagnostic Criteria
The movement disorder society (MDS) diagnostic criteria define supportive criteria, absolute exclusion criteria, and red flags. MDS uses a two-step approach, at first parkinsonism is defined, then checking whether parkinsonism is attributable to PD. In parkinsonism, bradykinesia must occur in combination with rest tremor, rigidity, or both. Parkinsonism caused by PD has a decline in either speed or amplitude as movements continue. A clinically established PD diagnosis requires the absence of absolute exclusion criteria, at least two supportive criteria, and no red flags. Absolute exclusion criteria include unequivocal cerebellar abnormalities (such as gait, limb ataxia, or cerebellar oculomotor abnormalities), downward vertical supranuclear gaze palsy, variable frontotemporal dementia, unequivocal cortical sensory loss, apraxia, or aphasia. It further includes parkinsonian features restricted to lower limbs for more than three years, anti-dopamine therapy, and absence of high-dose levodopa response. Supportive criteria are both motor and non-motor aspects. There should be a clear beneficial response to dopaminergic therapy, presence of levodopa-induced dyskinesia, rest tremor of a limb, and positive result on olfactory loss or cardiac sympathetic denervation. Red flags comprise early gait impairment, absence of progression of motor symptoms, early bulbar dysfunction, inspiratory dysfunction, severe autonomic failure in the first five years, unexplained pyramidal tract signs, and bilateral symmetric parkinsonism. Furthermore, included in these are recurrent falls due to impaired balance, disproportionate anterocollis, and absence of any common nonmotor features: Sleep dysfunction, autonomic dysfunction, hyposmia, or psychiatric dysfunction. This criterion does not include postural instability [14].
4. Pathology
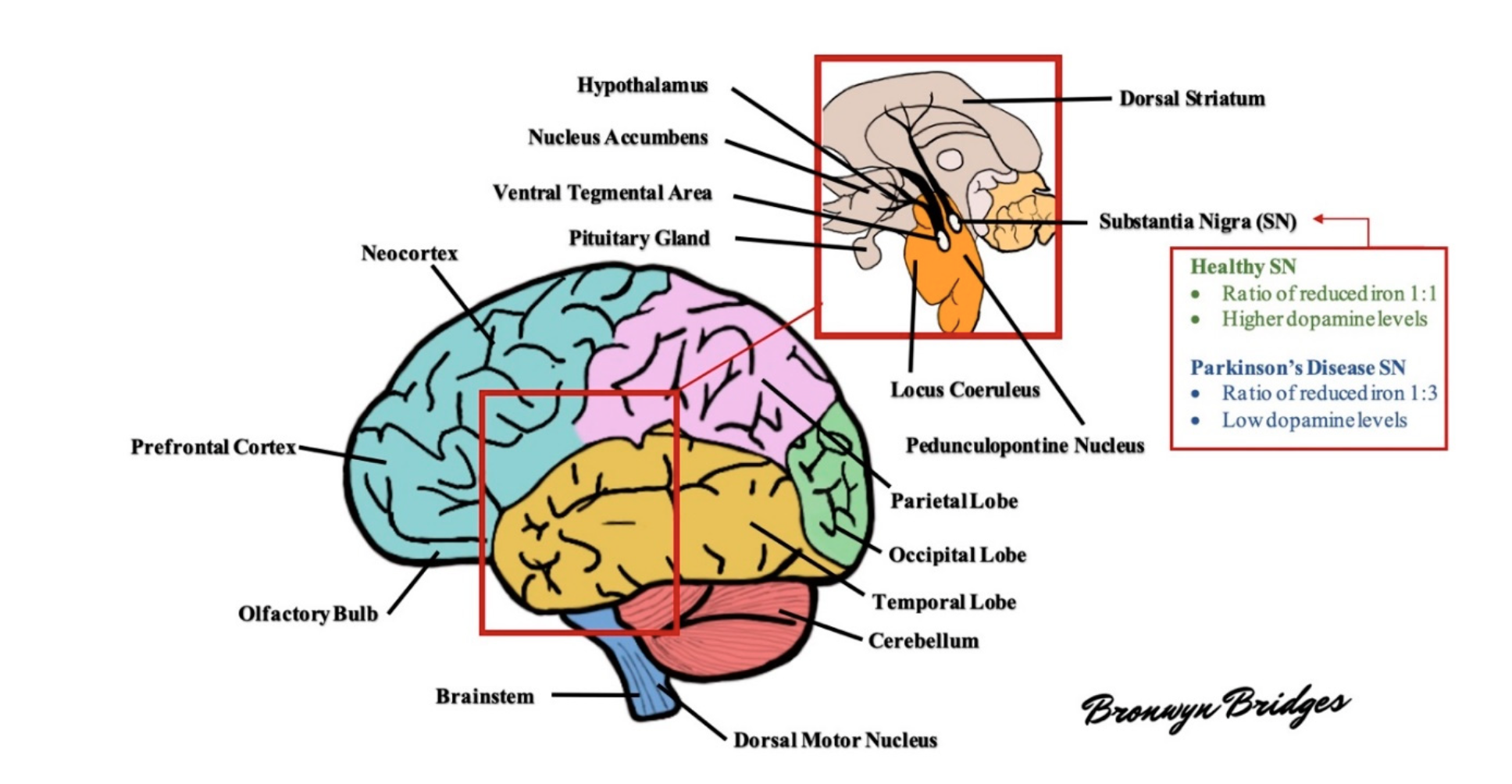
Figure 1: Schematic diagram of the human brain. Several areas of the brain are adversely affected in Parkinson's disease. For example, the substantia nigra exhibits a profound loss of dopaminergic neurons and altered levels of reduced iron, likely as a result of increased oxidative stress. As the disease progresses, other areas of the brain develop lesions, including the dorsal motor nucleus, neocortex, prefrontal cortex, locus coeruleus, amygdala, and more (see text for further details).
Major pathological hallmarks of PD include the degeneration and death of melanin-containing neurons of SN and Lewy pathology. Lewy pathology comprises the formation of intracytoplasmic Lewy bodies (LB) with inclusions containing mainly α-synuclein and ubiquitin and Lewy neurites (LN), which are the neuronal projections of similar inclusions [16].
Braak and colleagues in 2003 staged PD pathology based on a semiquantitative assessment of LB distribution at postmortem, which revealed that LB pathology spreads rostrocaudally throughout the brain in a chronologically predictable sequence [17]. At Braak stages 1 and 2, LB lesions are seen in the dorsal motor nucleus (IX/X), reticular formation, and anterior olfactory nucleus where patients are considered asymptomatic/presymptomatic. Few non-motor features such as autonomic dysfunctions (constipation), olfactory dysfunction, and sleep-related dysfunction occur at these stages. The disease progression to Stage 3 involves SNpc with LB pathology, and neuronal loss is seen at melanized neurons. The pathology further extends to locus coeruleus and amygdala, ultimately reaching the temporal limbic cortex at Stage 4. During these two stages, typical clinical motor features start to appear. There is involvement of the entire neocortex and areas, including the prefrontal cortex and primary sensory and motor areas in stages 5 and 6 [17][18]. This system is criticized for being based only on the distribution of Lewy pathology but not on neuronal loss [19].
After the motor symptoms appear, nigral DA neuron loss increases to 60% or higher and strongly correlates with the severity of motor features and disease duration. This remarkable cell loss is the denervation of the nigrostriatal pathway, leading to diminished dopamine levels in the striatum. The reduction of dopaminergic signaling is considered responsible for the appearance of the cardinal motor symptoms in PD. Apart from the SNpc, widespread cell loss can be found in several subcortical nuclei, including the locus coeruleus, the nucleus basalis of Meynert, the dorsal motor nucleus of the vagus nerve, the pedunculopontine nucleus, the raphe nuclei, the hypothalamus, and the olfactory bulb [20]. Multiple non-dopaminergic neurotransmitter systems are affected, such as the cholinergic, glutamatergic, GABAergic, noradrenergic, serotonergic, and histaminergic systems [21].
