Hydroxyapatite (HAp) is an attractive bioceramic from an environmental point of view. It mainly allows ion exchange between Ca2+ and other metal ions, making it an attractive material in the photodegradation of aquatic life effluents. Strategies for the performance of HAp-based functionalized material were reported, for example, doping, immobilization, deposition, incorporation, and support. Due to the production of stoichiometric defects capable of estimating response in the presence of light (UV, visible or solar) through charge carriers' interaction and/or mobility. Its favors photocatalytic performance and positive responses in the physicochemical properties to form an effective and sustainable photocatalyst.
- hydroxyapatite
- modification strategies
- photocatalytic degradation
- antibacterial applications
1. Introduction
The clean water is a challenging proposition worldwide, becoming one of the United Nations (U.N.) goal for sustainable development. The processes include the decontamination of natural resources have been widely investigated in recent decades. Although researchers' attention is focused on removing emerging pollutants [1][2][3], the presence of pathogens in wastewater has been seen as a severe problem of environmental contamination and with potential biological risk. 2020 is described in the history of humanity by Covid-19 [4], due to many deaths and environmental impacts since human interventions after SARS-CoV-2 [4][5]. Despite the efficiency in water treatment offered by the institutions responsible for the service, the impacts of viruses (and other biological agents) on biodiversity are still unknown [6][7]. Therefore, combating the contamination risks requires methods and management policies essential to prevent water pollution or minimize environmental impacts [8].
Environmentally viable technologies have aroused interest in directly ensuring a range of environmental benefits and global crise [9]. For example, heterogeneous photocatalysis is an advanced cleaning tool to combat the oxidation of organic pollutants, the removal of metal ions, combined with antibacterial and self-cleaning activity, and numerous other applications [10][11][12]. In addition, it has sustainable advantages (ease of operation and handling), oxidation energy savings compared to conventional techniques, speed in the conversion rate, and, above all, the complete remediation of pollutants [10].
The word "photocatalysis" is derived from Greek and consists of two components: photo (light) + catalysis (the process of chemical transformation of reagents) [13]. It is defined as a technology that involves a photoinduced reaction accelerated by the presence of a semiconductor driven by photons of light, which can be performed in the liquid and gas phases involving an application [11][13][14]. In addition, it varies in terms of reactions and mechanisms and can be described in stages [15][16]. Its general concept is understood by mechanisms of excitations and generations of hydroxyl radicals [15][17]. The fundamental characteristic of semiconductors is the energy discontinuity between the valence band (V.B. - lowest energy region) and the conduction band (C.B. - highest energy region), with the difference being called "bandgap" [18][19][20]. When the absorption of a photon (hυ) is equal to or greater than the energy of the Bandgap, electrons (e-) are promoted from V.B. to C.B., leaving a positive hole (h+) in V.B., thus creating an electron/hole pair, (e-/h+), or exciton [13][14][21][22].
Kabra et al. [18] describe photocatalytic reactions in the degradation of organic pollutants, and the hydroxyl radical (•OH) is considered the primary oxidant in the photocatalytic system. It is known that several properties affect the photocatalytic performance, such as the composition of the photocatalyst (crystallinity, chemical composition, bandgap energy, defect structure, morphology, surface area), as well as operational parameters that hinder or directly help the production of reactive oxygen species (ROS), transfer mechanisms between charge carriers and established physicochemical tests and parameters [14][18][23][24][25][26][27][28][29].
HAp activated by light (solar, visible, U.V.) has been considered promising in the degradation of pollutants. It allows chemical changes, adjustments in composition, and structural and morphological adaptations of great visibility in the economic point of view. and sustainable [29][30][31]. Important aspects such as the low cost, non-toxicity, slow biodegradation, high surface area, biocompatibility, thermal stability, and bioactive potential of HAp contribute to improving new properties and being titled recyclable heterogeneous effective photocatalyst in the mitigation of generated effluents [32][33]. Under visible irradiation, it gains primary prominence due to the possibility of surface modification to expand the absorption spectrum and consequently allow its successful use [32]. HAp has contributed to advances in several segments and daily applications, including pharmaceutical, biomedical, agriculture, and water treatment (adsorption, catalysis, photocatalysis) industries.[29][34], in particular, in the decontamination of dyes [35], drugs [36], pesticides [37], phenols [38], and also with antimicrobial efficacy against different bacteria [39][40][41], offering no risks to human health and environmental conflicts.
Due to the versatility and properties of chemical modifications, HAp-based compounds can come from natural and synthetic sources and have been prepared by different synthesis methods. The methods include precipitation, sol-gel, mechanochemical, solid-state, and hydrothermal synthesis, as Xu et al. [42]. Synthesis of HAp composites decorated with small amounts of ultrafine graphitic carbon nitride (gC3N4) for tetracycline photodegradation [29][42][43][44]. Rocha et al. [29] present details of synthesis, precursors, temperatures, and calcination times of several HAp compounds against specific degradation of dyes, drugs, and pesticides. HAp as photocatalyst is displayed in Figure 1. A possible degradation mechanism of the cited pollutants against the use and benefit of HAp as a basis for valuable and valuable photocatalysts. The mechanism is based on photocatalysis principles, which involve the charge carriers and their respective bands. The formation of intermediate radicals and the generation of hydroxyl radicals, as can be listed from the oxidation-reduction reactions involving the photoactivation of the photocatalyst ( HAp-compound) as described in the literature [45][46][47].
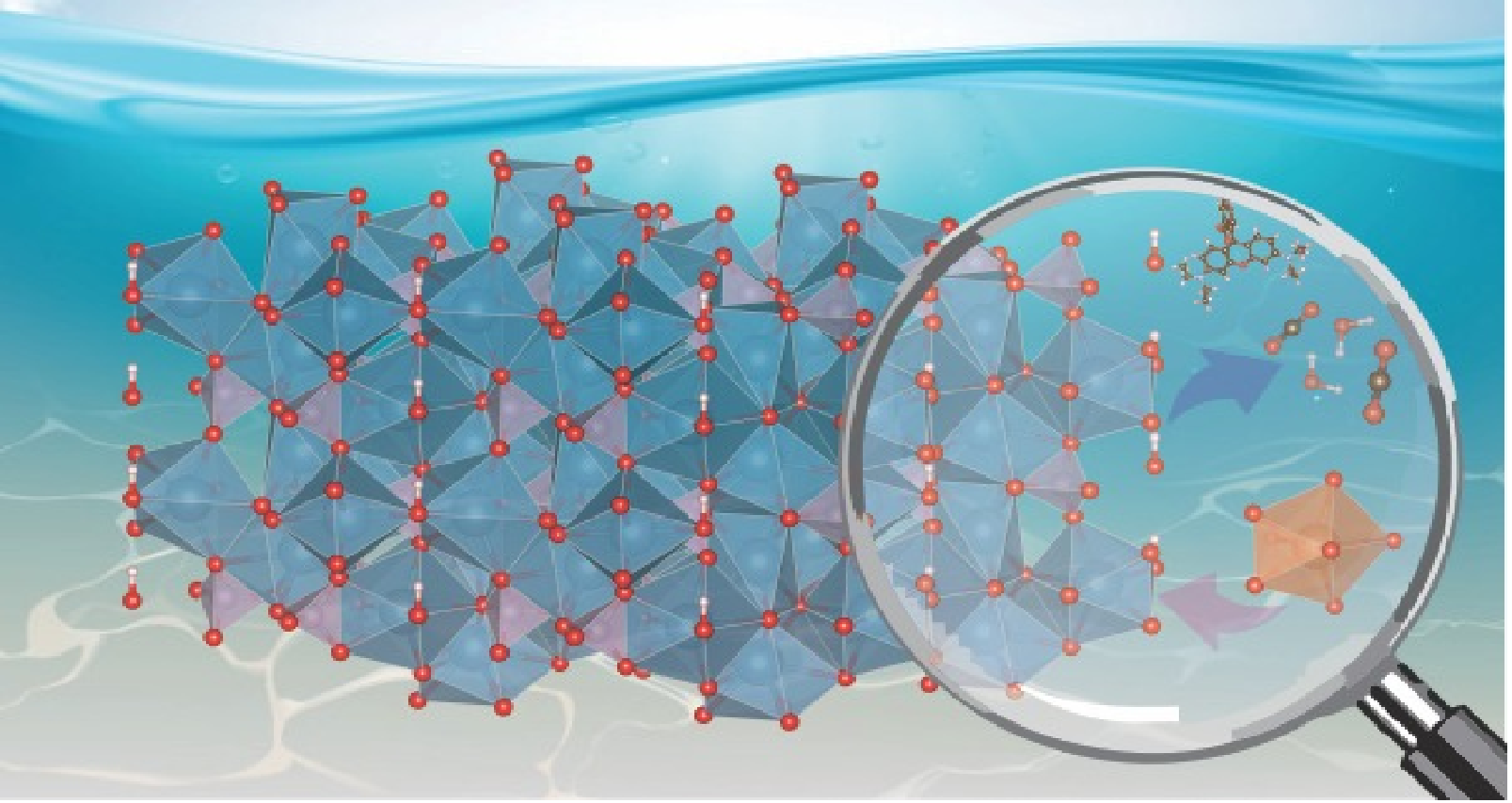
Figure 1. Main reaction when the hydroxyapatite is used as photocatalyst.
2. Multifunctionality of HAp in the photodegradation of pollutants
The concern for developing new compounds with photocatalytic attributes, HAp combined with various oxides (ZnO, TiO2, Fe3O4, graphene oxide) constitutes one of the main stabilization components of photoactive sites. Due to the flexibility to immobilize and offer high surface area, fit of acid-base properties, mobility between charge carriers, and good chemical stability [12][35][48], it has been used in a promising way in the treatment of effluents as it is an adsorption [49]. HAp does not show photocatalytic activity due to the high energy bandgap around 5.0 - 6.0 eV, making it impossible to be excited under a light source [50][51]. Once chemically modified from ion exchange processes, deposition, substitution, and/or metal ions (metal ion by Ca2+) in the crystal lattice generate stoichiometric defects capable of estimating response in light [51]. The main attribute of a photocatalyst is its ability to change, giving rise to new properties for apatite [52]. Basler et al. [35] investigated the lattice structure of HAp, which allows the ionic substitution in several crystallographic sites by different cations and phosphate positions, guaranteeing positive responses in the physicochemical properties and critical approaches from the photoluminescent point of view and the electronic excitation region (Bandgap). Karin et al. [22], collaborators reinforce that surface modification techniques can increase the photocatalytic performance, the photocatalyst's stability, and the specific surface area and the pore size being essential attributes to the formation of an efficient photocatalyst. [22]. The modifications are reported in the literature by process strategies: doping, immobilization, deposition, incorporation, and heterojunctions that entitle HAp to significant impact due to the strong interaction between fault engineering and transfer of charge carriers favored by mobility. Between charge separation, thus avoiding recombination [53]. Most works based on HAp are recent (last ten years according to Rocha et al.[29]) and are distinguished by the variety of syntheses, structural alterations, and the versatility of pollutants. Figure 2 shows how polluted water could be treated using modified hydroxyapatite compounds. Several studies focus on the degradation of synthetic dyes, drugs, and pesticides using HAp compounds. In recent review entitled "Light-Activated Hydroxyapatite Photocatalysts: New Environmentally-Friendly Materials to Mitigate Pollutants," treating preparation methods, chemical strategies, challenges, and future perspectives of the study.
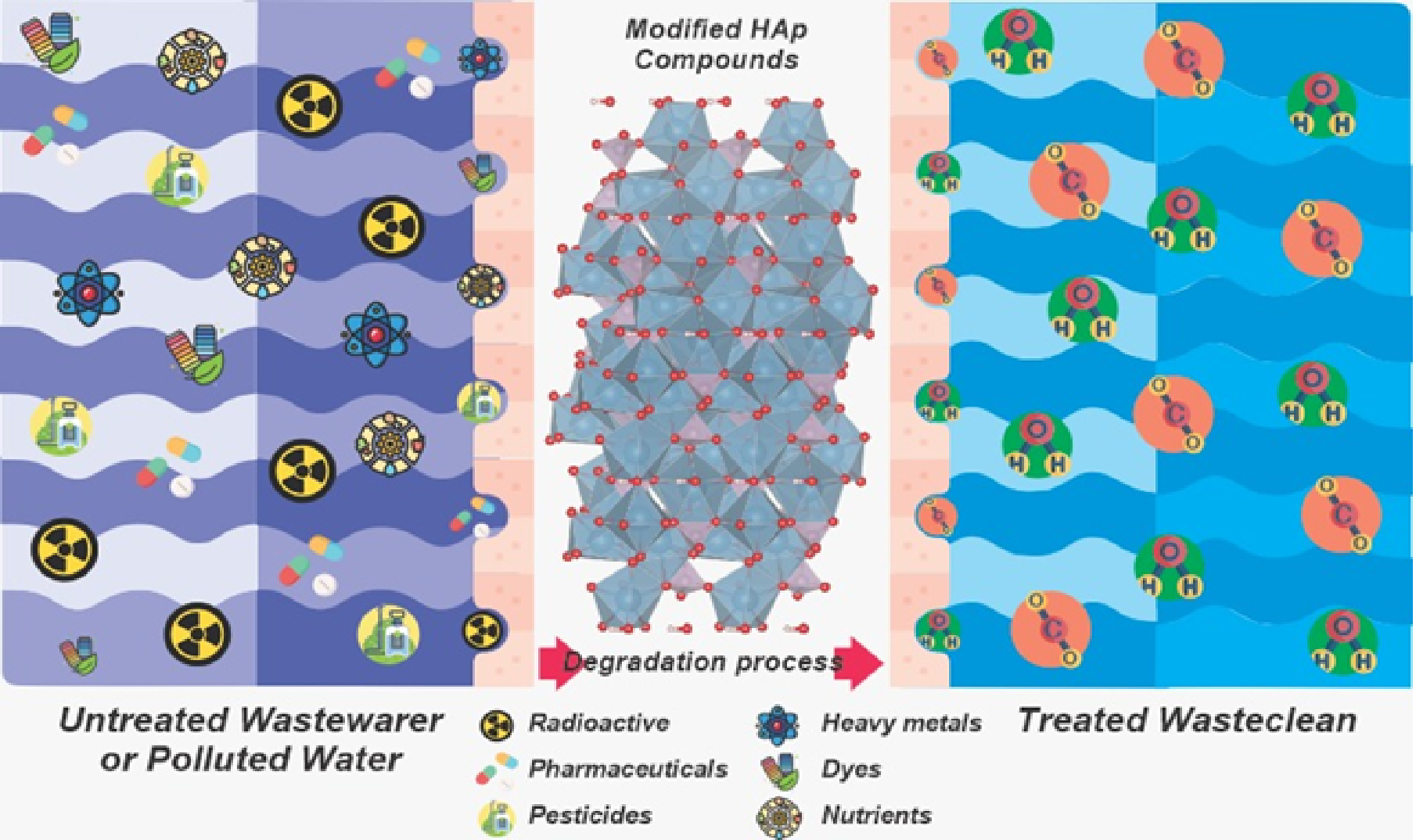
Figure 2. Polluted water could be treated using modified hydroxyapatite compounds.
3. Versatility of HAp compounds in antibacterial activity
A multifunctional compound, biocompatibility, osteointegration, osteoinductivity, high bioactivity, and low toxicity promoted hydroxyapatite as an attractive candidate in bone tissue engineering [40][54]. Martínez-Gracida et al. [55], studied hydroxyapatite has excellent biocompatibility, it does not have antibacterial properties. The authors reinforce the importance of circumventing these problems and directing antibacterial properties to HAp, for example, by accommodating various metallic cations in their chemical structure and replacing the calcium site. In the literature, metal cations associated with HAp that have antibacterial properties include mainly silver (Ag), copper (Cu), and zinc (Zn)[56][57][58][59][60][61], with the Ag+ cation being the most studied antibacterial or antifungal agent due to its mechanical and biocompatibility [60][62]. According to the scientific view, the role of Ag species and the understanding of the phenomena involved in substitution is concisely described by Khurshid et al. [63][63]. Notably, recent work has provided clues to mechanically explain the modification action in HAp compounds in the context of bioactivity against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) bacteria [54][60][64].
Thus, the biocompatible and bioactive nature of HAp with the antibacterial properties of silver can be designed with superior antibacterial properties [63]. In addition to possessing several essential properties for biomedical manipulation, HAp is attractive in maintaining antibacterial characteristics if properly incorporated/inserted [65]. This section describes studies in an attempt by researchers to include these substances in various forms of Hap [66][67][68][69].
Askarnia et al. [68] evaluated the deposition of ternary hydroxyapatite/chitosan/graphene oxide composite coating on magnesium alloy by the electrophoretic method. They studied vital points such as phase composition, surface morphology, hardness, corrosion behavior, bioactivity, and antibacterial of composite coatings. The authors observed that Escherichia coli and Staphylococcus aureus bacteria growth in broth medium after 24 h and OD600 results at 24 h post-inoculation for the 2%wt G.O. addition in coating [68]. Bhattacharjee and collaborators [66] studied the doping of cobalt in HAp hydroxyapatite and found that the substitution significantly increases the antibacterial properties (the zone of inhibition is 16.8 mm for the sample doped at 3% by weight against E. coli ) against E. coli and S. aureus because of availability of sufficient free. Sahoo et al. [69] synthesized a magnetic nanocomposite of HAp and evaluated its antibacterial characteristics against E. coli and Micrococcus luteus. As HAp increased, E. coli lost its structural stability from almost 87% to 97%, leading to a drop in cell viability. In the presence of M. luteus, these values ranged from 34% - to 80%, ensuring that the nanocomposite is active against Gram-positive and damaging cells. Incorporating metals into hydroxyapatite aids in antibacterial activity and environmental applications in the photocatalytic field [70][71][72][73][74].
4. Current environmental impacts of COVID-19
With the pandemic outbreak, the management of accumulated waste, for example, is one of the main concerns highlighted in aquatic ecosystems worldwide [75]. The inadequate disposal in industries and hospitals to combat the disease has led to risks of secondary infection in terms of sanitation that are evidence that proves the increase in pollution risks, requiring required management methods and policies to avoid and/or minimize such environmental impacts [8]. These data corroborate the studies by Urban et al. [76] and Azevedo et al. [77]. They pointed out that in Brazil, there was a considerable increase in the production of hospital waste between April and May 2020, with treatment management still ineffective.
The pandemic has emerged a direct relationship between the level of environmental pollution and economic activities in order to result in positive changes in the environment from various prevention strategies, such as well-organized public transport, effective waste management, use of ecological products, the composition of waste materials of a biodegradable nature, appropriate treatment of wastewater before releasing into the environment among other benefits [6][7][78]. Nevertheless, it is a fact that more systematic research is needed on environmental factors and COVID 19 so that effective prevention measures are agreed upon and implemented in specific populations in order to obtain positive results, with protection barriers mediated by environmental factors.
5. Final remarks and challenges
It is a fact that chemically modified HAp compounds have shown high potential in the photodegradation of contaminants. However, the crucial advantage of these compounds is based exclusively on surface modification strategies that improve physicochemical properties and allow a clear understanding of the benefits. Discussions of photocatalytic principles regarding reactive oxidation species, photodegradation mechanism, and research challenges. The emerging issue regarding the development of an environmentally friendly photocatalyst. In addition, it is known the importance of the availability of active sites favored by the high surface area of HAp. However, the interactions in the reaction medium and the post-degradation effects are not yet clearly reported. The effluent treatments focus on synthesized compounds of a non-toxic nature and appropriate combinations to be efficient and cost-effective in the preparation method. Finally, valuable multi-observations and multi-strategies are presented on the versatility of these compounds in photocatalytic targeting and the investigation of the control and elimination of bacterial growth.
References
- Solís-Casados, D.A.; Escobar-Alarcón, L.; Gómez-Oliván, L.M.; Haro-Poniatowski, E.; Klimova, T. Photodegradation of pharmaceutical drugs using Sn-modified TiO2 powders under visible light irradiation. Fuel 2017, 198, 3–10.
- Fiorenza, R.; Mauro, A. Di; Cantarella, M.; Privitera, V. Journal of Photochemistry & Photobiology A : Chemistry Selective photodegradation of 2 , 4-D pesticide from water by molecularly imprinted TiO2. J. Photochem. Photobiol. A Chem. 2019, 380, 111872.
- Vinod, V.T.P.; Sashidhar, R.B.; Sivaprasad, N.; Sarma, V.U.M.; Satyanarayana, N.; Kumaresan, R.; Rao, T.N.; Raviprasad, P. Bioremediation of mercury (II) from aqueous solution by gum karaya (Sterculia urens): A natural hydrocolloid. Desalination 2011, 272, 270–277.
- Patel, M.; Chaubey, A.K.; Pittman, C.U.; Mlsna, T.; Mohan, D. Coronavirus (SARS-CoV-2) in the Environment: Occurrence, Persistence, Analysis in Aquatic Systems and Possible Management. Sci. Total Environ. 2020, 142698.
- Singhal, T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286.
- Singh, V.; Mishra, V. Bioresource Technology Reports Environmental impacts of coronavirus disease 2019 ( COVID-19 ). 2021, 15.
- Wang, Y.; Xue, Q. The implications of COVID-19 in the ambient environment and psychological conditions. NanoImpact 2021, 21.
- Fadare, O.O.; Okoffo, E.D. Covid-19 face masks: A potential source of microplastic fibers in the environment. Sci. Total Environ. 2020, 737.
- Paumo, H.K.; Dalhatou, S.; Katata-Seru, L.M.; Kamdem, B.P.; Tijani, J.O.; Vishwanathan, V.; Kane, A.; Bahadur, I. TiO2 assisted photocatalysts for degradation of emerging organic pollutants in water and wastewater. J. Mol. Liq. 2021, 331, 115458.
- Li, X.; Chen, Y.; Tao, Y.; Shen, L.; Xu, Z.; Bian, Z.; Li, H. Challenges of photocatalysis and their coping strategies. Chem Catal. 2022, 1–31.
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P.; Li, X. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chinese J. Catal. 2022, 43, 178–214.
- Yao, Z.; Wang, X.; Hu, M.; Yao, Z.; Liu, X.; Ma, L.; He, Z.; Wang, X. Enhancement mechanism of hydroxyapatite for photocatalytic degradation of gaseous formaldehyde over TiO2/hydroxyapatite. J. Taiwan Inst. Chem. Eng. 2018, 85, 91–97.
- Saravanan, R.; Gracia, F.; Stephen, Basic principles, mechanism, and challenges of photocatalysis. In: Nanocomposites for visible light-induced photocatalysis. Springer, Cham, 2017. p. 19-40.ISBN 978-3-319-62445-7.
- Honorio, L.M.C.; Trigueiro, P.A.; Viana, B.C.; Ribeiro, A.B.; Osajima, J.A. Nanostructured Materials for the Photocatalytic Degradation of Organic Pollutants in Water. In Nanostructured Materials for Treating Aquatic Pollution; 2019; pp. 65–90 ISBN 9783030337445.
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248.
- Zhu, S.; Wang, D. Photocatalysis: Basic Principles, Diverse Forms of Implementations and Emerging Scientific Opportunities. Adv. Energy Mater. 2017, 7, 1700841.
- Henderson, M.A. A surface science perspective on TiO2 photocatalysis. Surf. Sci. Rep. 2011, 66, 185–297.
- Kabra, K.; Chaudhary, R.; Sawhney, R.L. Treatment of Hazardous Organic and Inorganic Compounds through Aqueous-Phase Photocatalysis: A Review. Ind. Eng. Chem. Res. 2004, 43, 7683–7696.
- Wei, Z.; Spinney, R.; Ke, R.; Yang, Z.; Xiao, R. Effect of pH on the sonochemical degradation of organic pollutants. Environ. Chem. Lett. 2016, 14, 163–182.
- Qian, R.; Zong, H.; Schneider, J.; Zhou, G.; Zhao, T.; Li, Y.; Yang, J.; Bahnemann, D.W.; Pan, J.H. Charge carrier trapping, recombination and transfer during TiO2 photocatalysis: An overview. Catal. Today 2019, 335, 78–90.
- Teixeira, C.P.D.A.B.; Jardim, W.D.F. Processos Oxidativos Avançados: conceitos teóricos. Campinas, SP: Universidade Estadual de Campinas (UNICAMP), 2004, v. 3, 83 p. Cad. Temático 2004, 03, 83.
- Karim, A. V; Krishnan, S.; Shriwastav, A. An overview of heterogeneous photocatalysis for the degradation of organic compounds: A special emphasis on photocorrosion and reusability. J. Indian Chem. Soc. 2022, 99, 100480.
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529.
- García-López, E.I.; Palmisano, L. Fundamentals of photocatalysis: The role of the photocatalysts in heterogeneous photo-assisted reactions. In Materials Science in Photocatalysis; Elsevier, 2021; Vol. 4, pp. 3–9 ISBN 2013206534.
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59.
- Rezaei, M.; Habibi-Yangjeh, A. Simple and large scale refluxing method for preparation of Ce-doped ZnO nanostructures as highly efficient photocatalyst. Appl. Surf. Sci. 2013, 265, 591–596.
- Reza, K.M.; Kurny, A.; Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: a review. Appl. Water Sci. 2017, 7, 1569–1578.
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268.
- Rocha, R.L.P.; Honorio, L.M.C.; Bezerra, R.D.D.S.; Trigueiro, P.; Duarte, T.M.; Fonseca, M.G.; Silva-Filho, E.C.; Osajima, J.A. Light-Activated Hydroxyapatite Photocatalysts: New Environmentally-Friendly Materials to Mitigate Pollutants. Minerals 2022, 12, 525.
- Campisi, S.; Castellano, C.; Gervasini, A. Tailoring structural and morphological properties of hydroxyapatite materials to enhance the capture efficiency towards copper(II) and lead(II) ions New J. Chem. 2018, 42, 4520–4530.
- Ibrahim, M.; Labaki, M.; Giraudon, J.-M.; Lamonier, J.-F. Hydroxyapatite, a multifunctional material for air, water and soil pollution control: A review. J. Hazard. Mater. 2020, 383, 121139.
- Mariappan, A.; Pandi, P.; Rajeswarapalanichamy, R.; Neyvasagam, K.; Sureshkumar, S.; Gatasheh, M.K.; Hatamleh, A.A. Bandgap and visible-light-induced photocatalytic performance and dye degradation of silver doped HAp/TiO2 nanocomposite by sol-gel method and its antimicrobial activity. Environ. Res. 2022, 113079.
- Das, K.C.; Dhar, S.S.; Thakurata, D.G.; Das, J. Sn(II) inserted on hydroxyapatite encapsulated nickel ferrite (NiFe2O4@HAp-Sn2+): A novel nanocomposite for the effective photo-degradation of rhodamine B dye. J. Clean. Prod. 2021, 290, 125172.
- Kalita, J.; Das, B.; Dhar, S.S. Synergistic effect of iron and copper in hydroxyapatite nanorods for Fenton-like oxidation of organic dye. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 643, 128750.
- Basfer, N.M.; Mansour, S.F.; Ahmed, M.K. Physicochemical properties of hydroxyapatite modified with vanadium ions for degradation of methylene blue. J. Mol. Struct. 2021, 1240, 130562.
- Bekkali, C. El; Bouyarmane, H.; Karbane, M. El; Masse, S.; Saoiabi, A.; Coradin, T.; Laghzizil, A. Zinc oxide-hydroxyapatite nanocomposite photocatalysts for the degradation of ciprofloxacin and ofloxacin antibiotics. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 539, 364–370.
- Anirudhan, T.S.; Shainy, F.; Sekhar, V.C.; Athira, V.S. Highly efficient photocatalytic degradation of chlorpyrifos in aqueous solutions by nano hydroxyapatite modified CFGO/ZnO nanorod composite. J. Photochem. Photobiol. A Chem. 2021, 418, 113333.
- Chahkandi, M.; Zargazi, M.; Hajizadeh, A.; Tayebee, R. In situ incorporation of Bi2O3 nanorods and Ag metal plasmonic surface into crystalline HAp nanosheets: Efficient visible light degradation of phenol. J. Alloys Compd. 2022, 902, 163737.
- Piccirillo, C.; Dunnill, C.W.; Pullar, R.C.; Tobaldi, D.M.; Labrincha, J.A.; Parkin, I.P.; Pintado, M.M.; Castro, P.M.L. Calcium phosphate-based materials of natural origin showing photocatalytic activity. J. Mater. Chem. A 2013, 1, 6452.
- Erdem, U.; Dogan, M.; Metin, A.U.; Baglar, S.; Turkoz, M.B.; Turk, M.; Nezir, S. Hydroxyapatite-based nanoparticles as a coating material for the dentine surface: An antibacterial and toxicological effect. Ceram. Int. 2020, 46, 270–280.
- Mariappan, A.; Pandi, P.; Beula Rani, K.R.; Rajeswarapalanichamy; Neyvasagam, K. Study of the photocatalytic and antibacterial effect of Zn and Cu doped hydroxyapatite. Inorg. Chem. Commun. 2022, 136, 109128.
- Xu, T.; Zou, R.; Lei, X.; Qi, X.; Wu, Q.; Yao, W.; Xu, Q. New and stable g-C3N4/HAp composites as highly efficient photocatalysts for tetracycline fast degradation. Appl. Catal. B Environ. 2019, 245, 662–671.
- Arokiasamy, P.; Al Bakri Abdullah, M.M.; Abd Rahim, S.Z.; Luhar, S.; Sandu, A.V.; Jamil, N.H.; Nabiałek, M. Synthesis methods of hydroxyapatite from natural sources: A review. Ceram. Int. 2022, 48, 14959–14979.
- Agbeboh, N.I.; Oladele, I.O.; Daramola, O.O.; Adediran, A.A.; Olasukanmi, O.O.; Tanimola, M.O. Environmentally sustainable processes for the synthesis of hydroxyapatite. Heliyon 2020, 6.
- Rauf, M.A.; Meetani, M.A.; Hisaindee, S. An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 2011, 276, 13–27.
- Hasanpour, M.; Hatami, M. Photocatalytic performance of aerogels for organic dyes removal from wastewaters: Review study. J. Mol. Liq. 2020, 309.
- Rauf, M.A.; Ashraf, S.S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 2009, 151, 10–18.
- Mishra, J.; Pattanayak, D.S.; Das, A.A.; Mishra, D.K.; Rath, D.; Sahoo, N.K. Enhanced photocatalytic degradation of cyanide employing Fe-porphyrin sensitizer with hydroxyapatite palladium doped TiO2 nano-composite system. J. Mol. Liq. 2019, 287, 110821.
- Bouyarmane, H.; El Bekkali, C.; Labrag, J.; Es-saidi, I.; Bouhnik, O.; Abdelmoumen, H.; Laghzizil, A.; Nunzi, J.M.; Robert, D. Photocatalytic degradation of emerging antibiotic pollutants in waters by TiO2/Hydroxyapatite nanocomposite materials. Surfaces and Interfaces 2021, 24, 101155.
- Tsukada, M.; Wakamura, M.; Yoshida, N.; Watanabe, T. Chemical Band gap and photocatalytic properties of Ti-substituted hydroxyapatite : Ti-HAP. J. Mol. Catal. A. Chem. 2011, 338, 18–23.
- Nishikawa, M.; Tan, L.H.; Nakabayashi, Y.; Hasegawa, T.; Shiroishi, W.; Kawahara, S.; Saito, N.; Nosaka, A.; Nosaka, Y. Visible light responsive vanadium-substituted hydroxyapatite photocatalysts. J. Photochem. Photobiol. A Chem. 2015, 311, 30–34.
- Shalika, T.; Perera, H.; Han, Y.; Lu, X.; Wang, X.; Dai, H.; Li, S. Rare Earth Doped Apatite Nanomaterials for Biological Application. J. Nanomater. 2015, 2015.
- Reddy, M.P.; Venugopal, A.; Subrahmanyam, M. Hydroxyapatite photocatalytic degradation of calmagite (an azo dye) in aqueous suspension. Appl. Catal. B Environ. 2007, 69, 164–170.
- Alinavaz, S.; Mahdavinia, G.R.; Jafari, H.; Hazrati, M.; Akbari, A. Hydroxyapatite (HA)-based hybrid bionanocomposite hydrogels: Ciprofloxacin delivery, release kinetics and antibacterial activity. J. Mol. Struct. 2021, 1225, 129095.
- Martínez-Gracida, N.O.; Esparza-González, S.C.; Castillo-Martínez, N.A.; Serrano-Medina, A.; Olivas-Armendariz, I.; Campos-Múzquiz, L.G.; Múzquiz-Ramos, E.M. Synergism in novel silver-copper/hydroxyapatite composites for increased antibacterial activity and biocompatibility. Ceram. Int. 2020, 46, 20215–20225.
- Hidalgo-Robatto, B.M.; López-Álvarez, M.; Azevedo, A.S.; Dorado, J.; Serra, J.; Azevedo, N.F.; González, P. Pulsed laser deposition of copper and zinc doped hydroxyapatite coatings for biomedical applications. Surf. Coatings Technol. 2018, 333, 168–177.
- Ofudje, E.A.; Adeogun, A.I.; Idowu, M.A.; Kareem, S.O. Synthesis and characterization of Zn-Doped hydroxyapatite: scaffold application, antibacterial and bioactivity studies. Heliyon 2019, 5, e01716.
- Hadidi, M.; Bigham, A.; Saebnoori, E.; Hassanzadeh-Tabrizi, S.A.; Rahmati, S.; Alizadeh, Z.M.; Nasirian, V.; Rafienia, M. Electrophoretic-deposited hydroxyapatite-copper nanocomposite as an antibacterial coating for biomedical applications. Surf. Coatings Technol. 2017, 321, 171–179.
- Wang, J.; Gong, X.; Hai, J.; Li, T. Synthesis of silver–hydroxyapatite composite with improved antibacterial properties. Vacuum 2018, 152, 132–137.
- Zhou, Q.; Wang, T.; Wang, C.; Wang, Z.; Yang, Y.; Li, P.; Cai, R.; Sun, M.; Yuan, H.; Nie, L. Synthesis and characterization of silver nanoparticles-doped hydroxyapatite/alginate microparticles with promising cytocompatibility and antibacterial properties. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 585, 124081.
- Riaz, M.; Zia, R.; Ijaz, A.; Hussain, T.; Mohsin, M.; Malik, A. Synthesis of monophasic Ag doped hydroxyapatite and evaluation of antibacterial activity. Mater. Sci. Eng. C 2018, 90, 308–313.
- Sri Devi, P.; Vijayalakshmi, K.A. Analysis of antibacterial activity and cytotoxicity of silver oxide doped hydroxyapatite exposed to DC glow discharge plasma. Mater. Today Proc. 2019, 26, 3604–3608.
- Khurshid, Z.; Zafar, M.S.; Hussain, S.; Fareed, A.; Yousaf, S.; Sefat, F. Silver-substituted hydroxyapatite. In Handbook of Ionic Substituted Hydroxyapatites; Elsevier, 2020; pp. 237–257 ISBN 9780081028346.
- Ali Al-Ahmed, Z.; Al-Radadi, N.S.; Ahmed, M.K.; Shoueir, K.; Elkemary, M. Dye removal, antibacterial properties, and morphological behavior of hydroxyapatite doped with Pd ions. Arab. J. Chem. 2020.
- Varadavenkatesan, T.; Vinayagam, R.; Pai, S.; Kathirvel, B.; Pugazhendhi, A.; Selvaraj, R. Synthesis, biological and environmental applications of hydroxyapatite and its composites with organic and inorganic coatings. Prog. Org. Coatings 2021, 151, 106056.
- Bhattacharjee, A.; Gupta, A.; Verma, M.; Anand, M.P.; Sengupta, P.; Saravanan, M.; Manna, I.; Balani, K. Antibacterial and magnetic response of site-specific cobalt incorporated hydroxyapatite. Ceram. Int. 2020, 46, 513–522.
- Fierascu, I.; Raditoiu, V.; Nicolae, C.A.; Raditoiu, A.; Somoghi, R.; Raduly, M.; Trica, B.; Fierascu, R.C.; Ditu, L.M. Analytical Characterization and Potential Antimicrobial and Photocatalytic Applications of Metal-Substituted Hydroxyapatite Materials. Anal. Lett. 2019, 52, 2332–2347.
- Askarnia, R.; Fardi, S.R.; Sobhani, M.; Staji, H. Ternary hydroxyapatite/chitosan/graphene oxide composite coating on AZ91D magnesium alloy by electrophoretic deposition. Ceram. Int. 2021.
- Sahoo, J.K.; Konar, M.; Rath, J.; Kumar, D.; Sahoo, H. Magnetic hydroxyapatite nanocomposite: Impact on eriochrome black-T removal and antibacterial activity. J. Mol. Liq. 2019, 294.
- Sathiyavimal, S.; Vasantharaj, S.; LewisOscar, F.; Selvaraj, R.; Brindhadevi, K.; Pugazhendhi, A. Natural organic and inorganic–hydroxyapatite biopolymer composite for biomedical applications. Prog. Org. Coatings 2020, 147, 105858.
- Mushtaq, A.; Zhao, R.; Luo, D.; Dempsey, E.; Wang, X.; Iqbal, M.Z.; Kong, X. Magnetic hydroxyapatite nanocomposites: The advances from synthesis to biomedical applications. Mater. Des. 2021, 197, 109269.
- Piccirillo, C.; Pinto, R.A.; Tobaldi, D.M.; Pullar, R.C.; Labrincha, J.A.; Pintado, M.M.E.; Castro, P.M.L. Light induced antibacterial activity and photocatalytic properties of Ag/Ag3PO4 -based material of marine origin. J. Photochem. Photobiol. A Chem. 2015, 296, 40–47.
- Zou, R.; Xu, T.; Lei, X.; Wu, Q.; Xue, S. Novel design of porous hollow hydroxyapatite microspheres decorated by reduced graphene oxides with superior photocatalytic performance for tetracycline removal. Solid State Sci. 2020, 99, 106067.
- Melnikov, P.; Teixeira, A.R.; Malzac, A.; Coelho, M. de B. Gallium-containing hydroxyapatite for potential use in orthopedics. Mater. Chem. Phys. 2009, 117, 86–90.
- Zambrano-Monserrate, M.A.; Ruano, M.A.; Sanchez-Alcalde, L. Indirect effects of COVID-19 on the environment. Sci. Total Environ. 2020, 728, 138813.
- Urban, R.C.; Nakada, L.Y.K. COVID-19 pandemic: Solid waste and environmental impacts in Brazil. Sci. Total Environ. 2021, 755, 142471.
- Impacto da pandemia de COVID-19 na epidemiologia pediátrica.
- Cheval, S.; Adamescu, C.M.; Georgiadis, T.; Herrnegger, M.; Piticar, A.; Legates, D.R. Observed and potential impacts of the covid-19 pandemic on the environment. Int. J. Environ. Res. Public Health 2020, 17, 1–25.
